Abstract
Experimental data indicate that several pharmacological agents that have long been used for the management of various diseases unrelated to cancer exhibit profound in vitro and in vivo anticancer activity. This is of major clinical importance, since it would possibly aid in reassessing the therapeutic use of currently used agents for which clinicians already have experience. Further, this would obviate the time-consuming process required for the development and the approval of novel antineoplastic drugs. Herein, both pre-clinical and clinical data concerning the antineoplastic function of distinct commercially available pharmacological agents that are not currently used in the field of oncology, ie, nonsteroidal anti-inflammatory drugs, antihypertensive agents, and anti-human immunodeficiency virus agents inhibiting viral protease, are reviewed. The aim is to provide integrated information regarding not only the molecular basis of the antitumor function of these agents but also the applicability of the reevaluation of their therapeutic range in the clinical setting.
Introduction
Research advances have largely “molecularized” medicine and other life sciences, not only at the theoretical but also at the practical level.Citation1 Inevitably, pharmacology has been transformed into molecular pharmacology, a basic medical science that continuously surprises researchers with data being accrued daily opening novel perspectives. Consistent with this, numerous pharmacological agents that are already available in the market and have gained approval for the management of diseases other than neoplasia are being characterized as potent anticancer compounds.Citation2,Citation3 Therefore, the possible expansion of the therapeutic uses of already prescribed pharmaceuticals could be harnessed in the field of cancer therapeutics, in order to save time and money from bench to bedside. Moreover, this would be advantageous over launching newly characterized agents, due to the preexisting clinical experience. From a theoretical point of view, however, this also highlights functional pleiotropy as a prominent feature in intracellular signaling routes and their components.Citation4–Citation6
This review aims to present the current knowledge regarding the anticancer function of certain non-antineoplastic agents gathered from pre-clinical and clinical experimentation. These include nonsteroidal anti-inflammatory drugs (NSAIDs), human immunodeficiency virus (HIV) agents falling into the category of protease inhibitors (PIs), and finally different types of antihypertensive drugs. Although many other non-antineoplastic agents available in the market are also known to exhibit anticancer properties,Citation7–Citation9 the afore mentioned pharmaceuticals were chosen for many purposes: first, NSAIDs have attracted appreciable scientific interest as potent anticancer agents.Citation10,Citation11 Second, antihypertensive and anti-HIV medication is primarily prescribed to elderly people and to HIV patients, respectively, with both of these population categories being at high risk of developing neoplasia. In fact, cancer is considered an age-related pathology,Citation12,Citation13 while HIV patients commonly develop such tumors as Kaposi’s sarcoma and non-Hodgkin’s lymphoma.Citation14,Citation15 Considerations for using and repositioning them are also presented here in an attempt to compel the reassessment of the clinical utility of the aforementioned agents. This could possibly enable the better management of tumorigenesis by virtue of novel therapeutic schemes that are less toxic and more efficacious than conventional chemotherapy.
NSAIDs
Current clinical use
NSAIDs are commonly prescribed pharmacological agents that principally serve as selective or non-selective inhibitors of cyclooxygenase (COX)-mediated pathway(s).Citation16,Citation17 Prostaglandins are the main COX-derived pro-inflammatory, tumor-promoting eicosanoids that act via binding to their cognate G protein-coupled receptors, most importantly EP1–EP4.
The most widely known and the oldest NSAID in use is aspirin. Depending on its concentration, low (≤100 mg/day) or high, aspirin inhibits COX-1 and both COX-1/2, respectively, thereby blocking the formation of prostanoids. COX-2, however, can be selectively targeted by a specific class of NSAIDs including celecoxib and etoricoxib, the so-called coxibs. Etodolac and meloxicam also display selectivity toward COX-2, whereas NSAIDs, such as sulindac and ibuprofen, mediate non-selective COX-1/2 blockage.Citation17,Citation18 Currently, the main therapeutic indication of NSAIDs is for the management of different types of acute or chronic pain and inflammation.Citation16,Citation19,Citation20 In addition, such NSAIDs as aspirin and sulindac find application in the prevention of thrombosis and preeclampsiaCitation21,Citation22 or as antipyretic and tocolytic agents.Citation23,Citation24 This further emphasizes the versatility of NSAIDs as therapeutics aside their potency as antitumor drugs, as presented in the following section.
The possible therapeutic repositioning of NSAIDs: mechanistic basis, pre-clinical, clinical, and epidemiological data
Although NSAIDs have been approved for the treatment of the aforementioned pathological conditions (ie, inflammation and pain), the existence of a mechanistic link among inflammation/COX signaling and tumorigenesis and pre-clinical data points to the future harnessing of NSAIDs in oncology.Citation16 More importantly, the ample clinical as well as epidemiological evidence that is presented in the next paragraphs encourages the use of COX inhibitors in tumor therapeutics or even in the prevention of tumorigenesis.
In fact, it is well established that chronic inflammation and aberrant COX-2 expression is causally linked to cancer,Citation25 while NSAID-mediated inhibition of COX-1/2 is known to exert protective effects against the development of several malignancies, especially the gastrointestinal ones.Citation26,Citation27 Additionally, the protumorigenic function of prostaglandin E2 (PGE2) and its receptors EP1–EP4 has been firmly demonstrated in animal models of colon carcinogenesis.Citation28–Citation30 Intriguingly, in colon cancer cell lines, experimental data suggest crosstalk between PGE2- and β-catenin-dependent pathways, strongly arguing for a mechanistic interconnection among the APC- and the PGE2-driven carcinogenesis. Apart from colon cancer, the COX-2/PGE2/EP1–EP4 signaling axis, fostering angiogenesis, tumor growth and metastasis, involving both tumor and stromal cells, has been incriminated in multiple types of solid tumors, such as non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma, and breast cancer.Citation31–Citation34
The COX-2 inhibitor etodolac has shown remarkable anti-metastatic function in animal models by virtue of its ability to downregulate the expression of matrix metalloproteinase (MMP)-9 as well as to interfere with the formation and the tone of lymphatic vessels.Citation35–Citation37 The COX-2 selective inhibitor meloxicam has been shown to trigger apoptosis in both COX-2-dependent and COX-2-independent routes in hepatocellular carcinoma cells.Citation38 Similarly, meloxicam can act either in a COX-2-dependent or COX-2-independent fashion in osteosarcoma at multiple levels.Citation39
The chemopreventive role of the coxibs against the emergence, malignant progression, or recurrence of colorectal polyps has been clinically confirmed, and clinical data regarding combinational schemes of COX-2 inhibitors with conventional chemotherapy for the treatment of various solid tumors are quite encouraging. In fact, APC mutation-positive familial adenomatous polyposis (FAP) patients receiving COX-2 inhibitors experience a marked numerical reduction and shrinkage of adenomas, thereby being less likely to develop colorectal cancer.Citation25,Citation40 Similar data for marketed NSAIDs that non-selectively inhibit COX-1/2, such as aspirin and ibuprofen, point to the chemopreventive role of these agents in smoking-induced lung cancer and skin cancer.Citation41,Citation42
Interestingly, the chemopreventive and/or antitumor therapeutic potency of non-selective COX inhibitors, such as aspirin, sulindac, and ibuprofen, may go beyond the realm of COX regulation. This notion is supported by in vitro experimentation, epidemiological data, or data from clinical studies.Citation43–Citation48 Aspirin or other NSAIDs results in the reduction of the risk of developing prostate cancer, as well as the risk of high-grade prostate cancer in men, irrespectively of the concurrent use of the antiandrogen agent dutasteride. This was evidenced by a study in which there were enrolled subjects with cancer-negative biopsies prior to initiation of the experimentation.Citation49 Moreover, aspirin may not only serve as an adjuvant agent in FAP patients who have been subjected to prophylactic colectomy but also in patients suffering from Lynch syndrome. The latter are highly prone to develop malignancies in the gastrointestinal tract, as well as in other organs. In fact, Lynch syndrome patients who are chronic users of aspirin (>10 years) are significantly protected from developing cancers related to their genetic disorder.Citation50 Hopefully, there is evidence that long-term usage of sulindac (≥5 months) can also act in a prophylactic manner, even in FAP patients who have not been colectomized for prophylactic purposes.Citation51 Several ongoing clinical trials (eg, NCT01187901 and NCT00468910) will shed more light on this issue. Of note, there have been designed phosphoderivatives of various NSAIDs that display marked anticancer function.Citation52–Citation54 However, the antitumor potency of these agents is not discussed here, because these agents are not commercially available, at least so far.
Considerations for using and repositioning NSAIDs
Although coxibs seem promising anticancer agents, gastrointestinal side effects (ranging from irritation of the gastrointestinal mucosa to gastrointestinal bleeding), renal toxicity, and cardiovascular complications (thrombotic events, myocardial infraction, and ischemic episodes) raised serious concerns about the safety of this class of drugs, which is still a point of controversy, culminating in the withdrawal of distinct coxibs from the market.Citation43,Citation55–Citation61 Consequently, there is much skepticism regarding the applicability of coxibs in the field of oncology.
Theoretically, cancer patients would suffer more than non-cancer subjects from the unwanted effects of these NSAIDs, given the hypercoagulable state associated with malignancy, as well as the cardiotoxicity of some anticancer drugs that they might receive.Citation62,Citation63 Hypersensitivity reactions to NSAIDs and NSAID-induced central nervous system toxicity are additional issues of concern,Citation64,Citation65 given that commonly used chemotherapeutic drugs may also be “provocative” to the immune system and induce hypersensitivity reactions or they may exhibit neurotoxicity.Citation66,Citation67
On the other hand, a severe limitation of the potential usage of NSAIDs in the field of oncology is that their anti-platelet function might increase the risk of hemorrhage.Citation68 Notably, there are tumors such as gastrointestinal stromal tumors, which are commonly associated with gastrointestinal bleeding.Citation69 In addition, one should also consider the probability of intratumoral hemorrhage. Certain types of tumors, eg, intracranial tumors, are not associated with a high risk for intratumoral bleeding, at least spontaneously.Citation70,Citation71 In any case, given that bleeding is a severe, life-threatening complication, ongoing and future clinical trials evaluating the antitumor function of NSAIDs in histologically different types of malignancies are necessary to address this issue.
Another issue is that non-selective COX inhibitors exemplified by aspirin can cause gastrointestinal irritation. Therefore, these drugs should cautiously be administered in subjects suffering from peptic ulcers. However, in the latter case, these unwanted effects can be satisfactorily managed by drugs protecting the digestive tract mucosa, such as the proton pump inhibitor omeprazole.Citation72 Another promising option to avoid toxicity is nanoparticle formulation, something that allows successful usage of much lower concentrations, at least in the case of some NSAIDs.Citation73 Using NSAIDs in the field of oncology without causing major health problems is a big future challenge.
Drugs blocking the renin–angiotensin system
Current clinical use
Agents targeting the renin–angiotensin system (RAS) are common active ingredients of combination drugs used for the management of hypertension and other health issues that may arise for hypertensive individuals, such as proteinuria.Citation74,Citation75 They are typically combined with a thiazide diuretic or even both with a thiazide and a calcium channel blocker into a single formulation. The vasoconstrictory effects of angiotensin II are mediated by the angiotensin II type 1 receptor (AT1R).Citation76 Consequently, pharmacological agents targeting RAS interfere either with AT1R-dependent signaling or with the cleavage of angiotensin I into angiotensin II. Anti-hypertensives acting as AT1R blockers are termed sartans, whereas ACE inhibitors (ACEIs) prevent the formation of angiotensin II.
But why is the pharmacological control of RAS so important? The answer lies in the physiological significance of this system. RAS is a signaling circuit that critically modulates the volume of extracellular fluids and arterial blood pressure. It is a hormonal route that principally relies on the sensory function of the juxtaglomerular cells in kidneys, which secrete the aspartyl protease renin into the bloodstream in response to various stimuli, including decreased blood pressure or signals deriving from the sympathetic nervous system. Consequently, renin converts angiotensinogen into angiotensin I. The latter is eventually cleaved into the vasoactive peptide angiotensin II via the ACE.Citation76,Citation77 More importantly, the RAS is molecularly linked to the pathogenesis of cancer, as referred to in the following section.
Possible therapeutic repositioning of drugs blocking the renin–angiotensin system
Overview
The theoretical cornerstone of redirecting agents that block the RAS into tumor therapeutics is the fact that the RAS has been incriminated in carcinogenesis. In addition, angiotensin II exerts pleiotropic cellular effects. It acts not only as a vasoactive peptide but also as a powerful mitogenic and as an angiogenic agent.Citation78,Citation79 In fact, angiotensin II receptors are commonly expressed in human cancers,Citation80–Citation82 while AT1R blockage gains ground as a novel antitumor approach.Citation83,Citation84 Moreover, it is well-documented that ACEI-receiving hypertensive patients are somehow protected from developing cancer, and clinical data also support the beneficial effects of sartans in the performance status of hormone-refractory prostate cancer patients.Citation85,Citation86
Taking into account that some sartans exhibit antiangiogenic activity, as is discussed in the following sections, such a future application would possibly help in obviating the necessity of administering antiangiogenic drugs, such as anti-VEGF agents, which may exacerbate hypertension.Citation87 Since hypertension and cancer commonly coexist in aged subjects, the therapeutic reevaluation of sartans and ACEIs would be feasible. The expansion of the clinical use of these antihypertensive drugs in the field of oncology is further corroborated by numerous pre-clinical and clinical data, as presented in the following sections.
Pre-clinical data regarding sartans
The AT1R blocker olmesartan was found to mitigate the growth of tumors developed in nude mice upon the concurrent injection of a pancreatic cancer cell line and pancreatic stellate cells.Citation88 The latter cells constitute a specific pancreatic cell subpopulation that exhibits profibrotic effects and fuels pancreatic cancer, due to interactions both with malignant cells and the cancer-promoting stromal elements in pancreas.Citation89
Candesartan has been demonstrated to exert remarkable in vivo antiangiogenic activity. In fact, it reduces the occurrence of renal cancer lung metastases and inhibits VEGF production in androgen-independent human prostate cancer mouse xenografts, along with suppression of tumor growth and reduction of serum prostate-specific antigen (PSA).Citation90 In urogenital cancers, inhibition of angiogenesis is considered the predominant antitumor mechanism of AT1R blockage.Citation82 Accordingly, a clinically relevant dosing scheme of candesartan exhibits antitumor activity in human bladder cancer murine xenografts via suppressing angiogenesis without a direct pro-apoptotic effect.Citation91
Candesartan also suppresses the production of VEGF and the invasiveness of AT1R-positive SKOV-3 human ovarian carcinoma cells. Additionally, candesartan reduces tumor angiogenesis in vivo and the ability of SKOV-3 cells to disseminate into the peritoneum in mice. Therefore, given that in clinical samples from patients with invasive ovarian cancer, AT1R immunoreactivity is associated with increased VEGF expression and microvascular density, candesartan is a candidate pharmacological tool not only for male-specific neoplasias (prostate cancer) but also for gynecological cancers (ovarian lesions).Citation81 In xenografted prostate tumors, candesartan also acts as an antiangiogenic agent.Citation92 Further, candesartan displays antifibrotic and antiproliferative activity in gastric cancer.Citation93
Losartan has been shown to cause tumor shrinkage and apoptosis in rats with C6 glioma.Citation80,Citation94 Moreover, losartan exerts synergistic cancer cell-killing or antiproliferative effects when it is coadministered with other potent anticancer agents, specifically the angiotensin II type 2 receptor agonist CGP42112A in ovarian carcinomaCitation95 and anti-miR-155 in endometrial cancer cells, respectively.Citation96 Therefore, losartan may find use in the treatment of neurological as well as gynecological tumors. Of note, another possible application of this AT1R blocker is its ability to potentiate the therapeutic value of tumor-targeting nanoparticles; either these are oncolytic herpes viruses or liposomal doxorubicin conjugated with polyethylene glycol. This is achieved owing to the ability of losartan to impair the deposition of collagen in various different models of desmoplasia.Citation97 Conceivably, losartan may not only be a valuable antitumor agent per se but may also open the road for increasing the efficacy of other antitumor factors.
The high-affinity AT1R antagonist telmisartan, which is known to exhibit the longest plasma half-life among all the sartans, triggers apoptosis in prostate cancer cells in a concentration-dependent fashion without affecting normal prostate stromal cells. Still, it is postulated that the cytotoxic activity of telmisartan is attributed to its PPARγ agonism rather than its AT1R-blocking activity.Citation98 In addition, telmisartan restrains the EGFR-dependent proliferation of colon cancer cells in response to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate by preventing the nuclear translocation of the C-terminal fragment of the EGF family member HB-EGF.Citation99
Pre-clinical data regarding ACEIs
On the basis of the structural similarity of ACE with MMPs, the ACEI captopril inhibits MMP-mediated gelatinolysis like MMP inhibitors do. In this way, captopril suppresses the invasiveness of HT1080 fibrosarcoma cells and T98G glioma cells in vitro and impedes the growth of human gastric adenocarcinoma cells in a mouse xenograft model, either when it is administered alone or in synergy with cisplatin.Citation100,Citation101 A short-term clinically relevant dosing scheme (2.8 mg) of captopril displays remarkable antitumor activity against lung cancer growth and lymph node metastasis in mouse xenografts though evoking an apoptotic response without impinging on tumor neoangiogenesis,Citation102 although there is evidence that this ACEI negatively regulates the chemotactic behavior of capillary endothelial cells and neovascularization.Citation103 Moreover, captopril has been shown to serve as a free sulfhydryl-group donor for the plasmin-mediated generation of angiostatin and to display synergistic anticancer properties in human melanoma xenografts with tissue-type plasminogen activator (a protease participating in the conversion of plasminogen to plasmin).Citation104 Captopril also attenuates renal cancer cell growth, possibly via sensitizing renal cancer cells to the cytostatic activities of TGFβCitation105 and inhibits renal carcinogenesis in a mouse model.Citation106 The in vitro antiproliferative properties of captopril in human mammary ductal carcinoma are possibly attributed to its ability to interfere with the expression of sex-steroid receptors and key biosynthetic routes (ribonucleic acid/protein synthesis),Citation107 further perplexing its biological activity.
The process of liver regeneration and tumor recurrence after partial hepatectomy performed in colorectal cancer patients with liver metastases is associated with an increase in the intrahepatic levels of ACE in mouse models. On the contrary, administration of captopril hinders tumor angiogenesis and triggers tumor apoptotic death.Citation108 Aside from metastatic colorectal cancer, this ACEI has been suggested to be effective for the pharmacological management of recurrent glioblastoma in a mixture with other medicines, collectively termed CUSP9*.Citation109
Of note, captopril is able to exert remarkable antimitotic activity in cancer cells that are devoid of functional RASCitation110 and to counteract endothelial cell migration irrespectively of ACE inhibition.Citation103 Therefore, there is evidence that this ACEI can impede carcinogenesis irrespective of the link among RAS and cancer and its mechanistic basis. This warrants further investigation.
The ACEI perindopril has been shown to inhibit hepatocellular carcinogenesis in mice and to suppress tumor neovascularization at the clinically achievable dose of 2 mg/kg/day. The profound antiangiogenic activity of perindopril in vivo and its biologically active metabolite perindoprilat in vitro, as evidenced by the impediment of the formation of endothelial cell-derived tubular structures and the reduction of CD31 immunoreactivity within tumors, are possibly attributed to its ability to shut off VEGF gene transcription. The antitumor activity of perindopril does not seem to be dependent on the blockage of AT1R signaling, since neither losartan nor candesartan, even at higher doses, could suppress hepatocellular carcinoma development.Citation111,Citation112 Given the fact that perindopril displays virtually no cytotoxicityCitation112 and is generally well tolerated,Citation113 it is a promising drug against liver cancer. Of note, in rodent models of hepatocellular carcinoma, perindopril exhibits a remarkable synergism in the suppression of tumorigenesis and chemoprevention with IFN-β and vitamin K2, respectively.Citation114–Citation116 This further consolidates the notion of its anticancer exploitation alone or in combination with other agents already clinically used. In addition, perindopril is a potent antiangiogenic agent in head and neck squamous cell carcinoma, as evidenced by in vitro and in vivo experimentation.Citation117
Clinical/epidemiological data regarding drugs blocking the renin–angiotensin system
A pilot clinical study assessing the benefits of 8 mg candesartan (total daily clinically relevant dose to treat hypertension ranges from 8 mg to 32 mg) in combination with antiandrogens in PSA expression and performance status in hormone-refractory prostate cancer patientsCitation118 yielded encouraging results. In addition, there is clinical evidence supporting that the antihypertensive treatment with ACEIs or sartans prolongs life expectancy of advanced lung cancer patients receiving conventional platinum drugs.Citation119 A Phase II clinical trial indicated remarkable benefits of receiving low-dose (4 mg) candesartan or perindopril in combination with IFN-α, the COX-2 inhibitor meloxicam, and cimetidine, namely the “I-CCA therapy”, as first-line treatment in advanced renal cell carcinoma patients, with trifling toxicity.Citation120 Though oral administration of candesartan (16 mg) in combination with intravenously infused gemcitabine was reported to be well tolerated in advanced pancreatic cancer patients, it does not seem to be an effective combinational therapeutic scheme for this type of malignancy.Citation121,Citation122
Noteworthy, according to epidemiological data the ACE genotype DD, which is associated with high ACE enzymatic activity, both predisposes carriers to breast cancer development and increases their responsiveness to the antitumor function of ACEIs or sartans.Citation123 Ongoing clinical trials assessing the antitumor activity of antihypertensive agents that target RAS components (eg, NCT00077064) will aid in the repositioning of these drugs beyond the field of cardiovascular therapeutics. Taking into account the polymorphisms in the enrolled patients at loci which are critical for RAS and its targeting, would be of great predictive importance.
Considerations for using and repositioning drugs blocking the renin– angiotensin system
In general, sartans are devoid of major side effects, and they are well tolerated by the majority of hypertensive patients.Citation124 However, considerable caution should be taken regarding the putative application of captopril or other ACEIs in oncology, given the pulmonary toxicity of this class of drugs: ACEIs may lead to bronchospasm, dyspnea, or the provocation of persistent dry cough, due to the drug-induced increase in bradykinin levels. This happens because ACE is also responsible for the catabolism of bronchoconstrictive kinins.Citation125–Citation127 This is a significant limiting factor, especially in cancer patients concomitantly suffering from respiratory diseases, lung cancer patients with compromised respiratory function, or even cancer patients with irradiation-induced pulmonary fibrosis and lung malfunction.
Patients suffering from advanced cancer may experience hypotension. This could be ascribed to cancer-associated deregulated function of the autonomous nervous system.Citation128 Alternatively, drop in blood pressure may be iatrogenic.Citation129 This can be the case when patients are treated with IFN, given that a decrease in blood pressure is a well-known adverse effect of IFN.Citation130 Hopefully, however, most of the available clinical data stem from studies assessing the antitumor potency of drugs targeting the RAS in cancer in which there were enrolled patients with advanced-stage solid tumors.Citation118–Citation120 In these studies, agents blocking the RAS were well tolerated by the recruited patients, even when a sartan or ACEI was combined with IFN-α.Citation120 However, in the case that agents targeting the RAS would induce hypotension upon the concomitant administration of cytokines, this could be managed either with melatoninCitation129 or with conventional medication that is indicated for the treatment of hypotension, such as etilefrine. Importantly, the administration of this sympathomimetic agent would possibly yield multiple beneficial effects in cancer patients with concomitant hypotension and chylothorax.Citation131,Citation132
HIV protease inhibitors
Current clinical use
HIV aspartyl PIs gained US Food and Drug Administration (FDA) approval and entered the anti-HIV cocktail market in the early 1990s.Citation133 Actually, saquinavir was the first drug of this class to be approved by the FDA in 1995 through a relatively rapid process of only 3 months. Saquinavir and other PIs gained approval for the control of HIV infection as well as to offer HIV-infected individuals a better quality of life and increased survival rates. PIs target viral protease, an enzyme that is crucial for HIV replication.Citation134 This class of antiviral drugs comprises both peptidomimetic agents, like the prototype drug saquinavir, and non-peptidic drugs, such as nelfinavir. The latter was launched in the late 1990s and was the first PI to be approved for pediatric use.Citation135
The possible therapeutic repositioning of HIV PIs: pre-clinical and clinical data
PIs are currently employed only in the management of HIV infection. Surprisingly, however, there is ample evidence highlighting their antitumor function. As a matter of fact, PI-receiving HIV patients are less likely to develop infection-associated tumors, such as non-Hodgkin’s lymphomas and Kaposi’s sarcoma (KS),Citation136 or may even experience KS regression.Citation137 Further, there are numerous in vitro and in vivo experiments clearly demonstrating that PIs inhibit the growth of many non-HIV-related human cancer models. Let us note that aside from PIs, the anti-HIV nucleoside analog reverse-transcriptase inhibitor azidothymidine has also been reported to exert antitumor activity. Still, only the antitumor properties of PIs are presented in the following paragraphs, since the in vitro anticancer potency of azidothymidine does not correlate with in vivo evidence,Citation138 thereby attracting no more research interest.
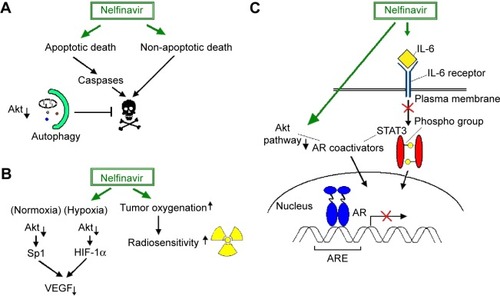
The antiretroviral agent nelfinavir was found to exhibit a wide range of antitumor activities in several cancer cell lines, including chemoresistant ones, as well as in NSCLC mouse xenografts, at clinically attainable doses. Mechanistically, the cytotoxic effects of nelfinavir are associated with both caspase-dependent apoptotic and non-apoptotic cell death that are overall mitigated by a prosurvival autophagic response that coincides with Akt inhibition.Citation139 Similar anti-growth and pro-apoptotic function of nelfinavir along with Akt-pathway inhibition by this drug has been reported by other researchers as well. In fact, there has been observed a chemosensitizing effect of nelfinavir in NSCLC cells and IL-6/STAT3 and androgen receptor (AR) signaling in prostate cancer cells, which hinders their proliferation. Suppression of the IL-6/STAT3 pathway occurs either at the level of STAT3 phosphorylation stimulated by IL-6 or at the level of STAT3 binding to deoxyribonucleic acid (DNA).Citation140,Citation141 AR blockage results from the fact that Akt and STAT3 function as coactivators for AR. Yang et al combined the in vitro evidence of nelfinavir’s activity against prostate cancer with in vivo data in LNCaP-xenografted mice that received small short-term doses of nelfinavir (60 mg/kg five times a week) with excellent tolerability.Citation140 The therapeutic potency of nelfinavir in prostate cancer is also supported by a more recent publication, wherein this PI triggered ER stress and apoptosis in castration-resistant prostate cancer cells. In this case, apoptosis was actually triggered due to blockage of site-2 protease, which mediates the transcriptional activation of SREBP-1 and ATF6 through regulated intramembrane proteolysis.Citation142 A similar anticancer mechanism has been reported in liposarcoma cells also.Citation143
Another study underscores the possible utility of nelfinavir in multiple myeloma, where this anti-HIV agent displayed anti-proteasomal and pro-apoptotic activity.Citation144 Further, nelfinavir may also exert anti-glioblastoma activity by virtue of its property to block catalysis mediated by MMP-2 and -9.Citation145 In melanoma cells, nelfinavir triggers apoptosis and ceases the cell cycle via decreasing CDK2 activity through stimulating the proteasomal degradation of CDC25A.Citation146 Another study demonstrating the pro-apoptotic and cytostatic activity of nelfinavir in ovarian cancer cells offers further impetus toward the rapid clinical testing of this PI for oncological purposes.Citation147
Interestingly, nelfinavir also blunts the transcriptional upregulation of VEGF by Sp1 and HIF-1α under normoxic and hypoxic conditions, respectively, presumably via inhibiting Akt (). VEGF downregulation is functionally associated with in vitro and in vivo perturbation of angiogenesis. In addition, nelfinavir exhibits in vivo radiosensitizing effects, through inducing tumor reoxygenation via an as-yet-unidentified mechanism.Citation148 Since pO2 critically determines radiosensitivity, this would be of major clinical importance, particularly in hypoxic solid tumors that are resistant to radiotherapy.
Figure 1 Nelfinavir-regulated signaling pathways which affect tumor cell biology or determine the effectiveness of antitumor therapy.
Abbreviations: IL, interleukin; STAT, Signal transducer and activator of transcription; AR, androgen receptor; SP, specificity protein; ARE, androgen response element; HIF, hypoxia-inducible factor; VEGF, vascular endothelial growth factor.
Apart from nelfinavir, other PIs also exhibit antitumor activity, including ritonavir in breast cancer cells, ritonavir and saquinavir in ovarian cancer cells,Citation149–Citation151 lopinavir in cervical cancer cells,Citation152 and amprenavir in hepatocarcinoma xenografts.Citation153 In breast cancer cells, ritonavir-mediated growth inhibition partially depends on disrupting the assembly of the Akt/Hsp90 complex,Citation149 which had been previously shown to dampen ASK1-dependent apoptosis.Citation154 Noticeably, although ER stress response has been reported to induce autophagy that eventually counteracts nelfinavir-induced cell death, the ability of atazanavir and nelfinavir to kill malignant glioma cells seems to rely on an active ER stress response/caspase 4 pathway.Citation155 This is not surprising, given that ER stress is known to result in apoptosis via multiple pathways.Citation156 Saquinavir and indinavir display in vitro antiangiogenic properties comparable to those of taxol and promote the regression of KS-like lesions in murine disease models.Citation137 Saquinavir, like nelfinavir, inhibits the proteasome.Citation157 The latter molecular event is functionally associated with the induction of apoptosis and potentiation of the cytotoxic effects of ionizing radiation.
In a small, Phase I clinical trial, the clinically relevant dose of 1,250 mg twice daily of nelfinavir combined with conventional chemotherapy (gemcitabine and cisplatin) and radiotherapy yielded satisfying antitumor response, as evidenced with positron emission tomography in pancreatic cancer patients. In detail, there was a complete response and stabilization of disease progression in five and two of nine patients, respectively, with minor toxicity.Citation158 Interestingly, in glioblastoma multiforme patients, a recent Phase I study reported that 1,250 mg twice daily was the maximally tolerated dose of nelfinavir when combined with radiotherapy and temozolomide.Citation159 Hopefully, nelfinavir is well tolerated even at a dosing scheme that exceeds the dose that has gained FDA approval for the management of HIV infection by 2.5 times, as a Phase I trial reported.Citation160 Another Phase I study showed that 750 mg of nelfinavir twice daily yields encouraging preoperative results in locally advanced rectal cancer patients when combined with chemotherapy and radiation therapy.Citation161 A Phase I trial demonstrated that up to 1,250 mg of nelfinavir twice a day combined with chemotherapy and radiotherapy was well tolerated in patients suffering from advanced, unresectable lung cancer.Citation162 More importantly, the results regarding the clinical response of the patients enrolled in the latter study were satisfactory enough. However, nelfinavir at 1,250 mg twice daily does not significantly impact on progression-free survival of patients with recurrent adenoid cystic carcinoma, as a Phase II trial showed.Citation163 Further clinical evaluation of HIV PIs in cancer therapeutics is needed.
Considerations for using and repositioning HIV PIs
Given the multi-year clinical experience of nelfinavir administration in HIV patients and its broad-spectrum anticancer activity, scientists have envisioned the introduction of this drug in the field of oncology as a promising cancer-fighting strategy. This is also the case for other PIs as well, such as ritonavir.Citation150 Unfortunately, PIs commonly cause disturbances in the glycemic and lipidemic profile. However, these unwanted effects can be pharmacologically managed in acquired immunodeficiency syndrome (AIDS) patients. In fact, there are certain medications that are recommended for patients receiving antiviral therapy. It is also important for clinicians to take into account each patient’s individual physiology before prescribing a glucose- and/or lipid-lowering agent to AIDS patients.Citation164–Citation166 Such a cautious, individualized management of the aforementioned clinical conditions would allow the safe use of PIs in oncology. In turn, this could hopefully pave the road for the design of more efficacious anticancer modalities, circumventing the laggard process of new drug approval.Citation139,Citation147,Citation167
Conclusion and future prospects
The non-antitumor pharmaceuticals with anticancer properties presented here reflect the functional redundancy characterizing the molecules and/or signaling pathways targeted by these drugs. Moreover, the potent multiple utility of a given pharmacological agent is not a novel phenomenon. For instance, the lysosomotropic agent hydroxychloroquine is currently used both as an antimalarial drug and in the treatment of various inflammatory diseases, including rheumatoid arthritis and systemic lupus erythematosus.Citation168 Additionally, experimental evidence indicates that hydroxychloroquine could also be therapeutically used in the field of oncology.Citation169 In general, repositioning of drugs is an emerging concept that gains ground in light of novel data.Citation170,Citation171
Herein, there was provided evidence for the antitumor activity of three different categories of non-antineoplastic drugs that are already commercially available. The fact that only data stemming from pre-clinical experimentation and that Phase I or II clinical trials were reviewed should be considered as a limitation. To the best of our knowledge, no Phase III clinical trials have been conducted in order to assess the clinical value of the aforementioned marketed drugs in cancer therapeutics so far. However, the evidence presented herein could achieve its goal, ie, the conceptual corroboration of the repositioning of these marketed drugs, the compelling of the prioritization of basic or clinical research toward this direction, and the instigation of further experimentation.
Taking into account the highly interlocked intracellular pathways, the therapeutic utility of many agents that are currently available in the market is rather underrated. Repositioning of the aforementioned non-antitumor drugs may offer clinicians the opportunity to fight cancer through therapeutic schemes with a safer toxicological profile. The latter is a major challenge, inasmuch as targeted therapeutic agents, such as monoclonal antibodies, were found to have serious adverse effects, such as drug-induced hypertension,Citation172 that raise concerns, especially in elderly people, who are most prone to tumorigenesis. Many clinical studies that are under way (eg, NCT01485731, NCT01729923) will hopefully aid in the exploitation of the antineoplastic function of non-antitumor agents, such as NSAIDs, antihypertensive drugs, and HIV PIs, thereby opening new avenues for the development of safer and perhaps more efficacious alternative anticancer medications.
Disclosure
The authors report no conflicts of interest in this work.
References
- QuirkeVGaudillièreJPThe era of biomedicine: science, medicine, and public health in Britain and France after the Second World WarMed Hist200852444145218958248
- BernhardEJBrunnerTBProgress towards the use of HIV protease inhibitors in cancer therapyCancer Biol Ther20087563663718421253
- KastREBoockvarJABrüningAA conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma CareOncotarget20134450253023594434
- LaPorteSLJuoZSVaclavikovaJMolecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 systemCell2008132225927218243101
- BeharMBarkenDWernerSLHoffmannAThe dynamics of signaling as a pharmacological targetCell2013155244846124120141
- LangBTWangJFilousARAuNPMaCHShenYPleiotropic molecules in axon regeneration and neuroinflammationExp Neurol2014258172325017884
- FangZTangYFangJSimvastatin inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK and JAK2/STAT3 pathwayPLoS One201385e6282323690956
- AgostiniMAlmeidaLYBastosDCThe fatty acid synthase inhibitor orlistat reduces the growth and metastasis of orthotopic tongue oral squamous cell carcinomasMol Cancer Ther201413358559524362464
- HiroshimaYMaawyAHassaneinMKThe tumor-educated-macrophage increase of malignancy of human pancreatic cancer is prevented by zoledronic acidPLoS One201498e10338225116261
- HuangLWongCCMackenzieGGPhospho-aspirin (MDC-22) inhibits breast cancer in preclinical animal models: an effect mediated by EGFR inhibition, p53 acetylation and oxidative stressBMC Cancer20141414124575839
- BowersLWMaximoIXBrennerAJNSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactionsCancer Res201474164446445725125682
- AhmadABanerjeeSWangZKongDMajumdarAPSarkarFHAging and inflammation: etiological culprits of cancerCurr Aging Sci20092317418619997527
- DjansugurovaLBPerfilyevaAVZhunusovaGSDjantaevaKBIksanOAKhussainovaEMThe determination of genetic markers of age-related cancer pathologies in populations from KazakhstanFront Genet201347023675381
- GibsonTMMortonLMShielsMSClarkeCAEngelsEARisk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based studyAIDS201428152313231825111081
- NelwanEJPramonoLALubisAMDjoerbanZKaposi sarcoma of the eye in an HIV patient well-responded to HAARTActa Med Indones201446325325525348189
- HiľovskáLJendželovskýRFedoročkoPPotency of non-steroidal anti-inflammatory drugs in chemotherapyMol Clin Oncol20153131225469262
- KarnezisTShayanRFoxSAchenMGStackerSAThe connection between lymphangiogenic signalling and prostaglandin biology: a missing link in the metastatic pathwayOncotarget20123889390623097685
- BaigentCPatronoCSelective cyclooxygenase 2 inhibitors, aspirin, and cardiovascular disease: a reappraisalArthritis Rheum2003481122012528099
- LynchMEWatsonCPThe pharmacotherapy of chronic pain: a reviewPain Res Manag2006111113816511612
- ChouRHuffmanLHMedications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guidelineAnn Intern Med2007147750551417909211
- BujoldERobergeSLacasseYPrevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysisObstet Gynecol20101162 Pt 140241420664402
- FloydCNFerroAMechanisms of aspirin resistancePharmacol Ther20141411697823993980
- BachertCChuchalinAGEisebittRNetayzhenkoVZVoelkerMAspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging studyClin Ther2005277993100316154478
- ParkSLeeNRLeeKEParkJYKimYJGwakHSEffects of single-nucleotide polymorphisms of FMO3 and FMO6 genes on pharmacokinetic characteristics of sulindac sulfide in premature laborDrug Metab Dispos2014421404324173915
- BrownJRDuBoisRNCOX-2: a molecular target for colorectal cancer preventionJ Clin Oncol200523122840285515837998
- HarrisREBeebe-DonkJDossHBurr DossDAspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockadeOncol Rep200513455958315756426
- GhoshNChakiRMandalVMandalSCCOX-2 as a target for cancer chemotherapyPharmacol Rep201062223324420508278
- SonoshitaMTakakuKSasakiNAcceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(delta 716) knockout miceNat Med2001791048105111533709
- Hansen-PetrikMBMcEnteeMFJullBShiHZemelMBWhelanJProstaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) miceCancer Res200262240340811809688
- HullMAKoSCHawcroftGProstaglandin EP receptors: targets for treatment and prevention of colorectal cancer?Mol Cancer Ther2004381031103915299086
- HidaTYatabeYAchiwaHIncreased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomasCancer Res19985817376137649731479
- LiuCHChangSHNarkoKOverexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic miceJ Biol Chem200127621185631856911278747
- ChangSHAiYBreyerRMLaneTFHlaTThe prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasiaCancer Res200565114496449915930264
- MazharDAngRWaxmanJCOX inhibitors and breast cancerBr J Cancer200694334635016421592
- IshizakiTKatsumataKTsuchidaAEtodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of MMP-9 activityInt J Mol Med200617235736216391837
- IwataCKanoMRKomuroAInhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesisCancer Res20076721101811018917974958
- KarnezisTShayanRCaesarCVEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endotheliumCancer Cell201221218119522340592
- DongXLiRXiuPMeloxicam executes its antitumor effects against hepatocellular carcinoma in COX-2-dependent and -independent pathwaysPLoS One201493e9286424675684
- NaruseTNishidaYHosonoKIshiguroNMeloxicam inhibits osteosarcoma growth, invasiveness and metastasis by COX-2-dependent and independent routesCarcinogenesis200627358459216219634
- GuptaRADuboisRNColorectal cancer prevention and treatment by inhibition of cyclooxygenase-2Nat Rev Cancer200111112111900248
- HarrisREBeebe-DonkJSchullerHMChemoprevention of lung cancer by non-steroidal anti-inflammatory drugs among cigarette smokersOncol Rep20029469369512066194
- MuranushiCOlsenCMPandeyaNGreenACAspirin and non-steroidal anti-inflammatory drugs can prevent cutaneous squamous cell carcinoma: a systematic review and meta-analysisJ Invest Dermatol2014135497598325521453
- WongCCChengKWRigasBPreclinical predictors of anticancer drug efficacy: critical assessment with emphasis on whether nanomolar potency should be required of candidate agentsJ Pharmacol Exp Ther2012341357257822448039
- MatosPKotelevetsLGonçalvesVIbuprofen inhibits colitis-induced overexpression of tumor-related Rac1bNeoplasia201315110211123359345
- SuBXuBWanJCorrelation between long-term aspirin use and F-fluorodeoxyglucose uptake in colorectal cancer measured by PET/CTPLoS One2014910e10945925290692
- LiggettJLChoiCKDonnellRLNonsteroidal anti-inflammatory drug sulindac sulfide suppresses structural protein nesprin-2 expression in colorectal cancer cellsBiochim Biophys Acta20141840132233124080406
- LiggettJLMinKWSmolenskyDBaekSJA novel COX-independent mechanism of sulindac sulfide involves cleavage of epithelial cell adhesion molecule proteinExp Cell Res201432611924859349
- LiggettJLZhangXElingTEBaekSJAnti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targetsCancer Lett2014346221722424486220
- VidalACHowardLEMoreiraDMCastro-SantamariaRAndrioleGLFreedlandSJAspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE studyClin Cancer Res201521475676225520389
- ChanATAspirin and familial adenomatous polyposis: coming full circleCancer Prev Res (Phila)20114562362721543340
- KimKYJeonSWParkJGRegression of colonic adenomas after treatment with sulindac in familial adenomatous polyposis: a case with a 2-year follow-up without a prophylactic colectomyAnn Coloproctol201430420120425210691
- XieGSunYNieTPhospho-ibuprofen (MDC-917) is a novel agent against colon cancer: efficacy, metabolism, and pharmacokinetics in mouse modelsJ Pharmacol Exp Ther2011337387688621422165
- ChengKWWongCCAlstonNAerosol administration of phospho-sulindac inhibits lung tumorigenesisMol Cancer Ther20131281417142823645590
- HuangLWongCCChengKWRigasBPhospho-aspirin-2 (MDC-22) inhibits estrogen receptor positive breast cancer growth both in vitro and in vivo by a redox-dependent effectPLoS One2014911e11172025369051
- MukherjeeDNissenSETopolEJRisk of cardiovascular events associated with selective COX-2 inhibitorsJAMA2001286895495911509060
- BresalierRSSandlerRSQuanHCardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trialN Engl J Med2005352111092110215713943
- BaronJASandlerRSBresalierRSA randomized trial of rofecoxib for the chemoprevention of colorectal adenomasGastroenterology200613161674168217087947
- FujimuraTOhtaTOyamaKMiyashitaTMiwaKCyclooxygenase-2 (COX-2) in carcinogenesis and selective COX-2 inhibitors for chemoprevention in gastrointestinal cancersJ Gastrointest Cancer2007382–4788219031117
- LanasABaronJASandlerRSPeptic ulcer and bleeding events associated with rofecoxib in a 3-year colorectal adenoma chemoprevention trialGastroenterology2007132249049717258718
- PattersonSLColbert MaressoKHawkECancer chemoprevention: successes and failuresClin Chem20135919410123150056
- CooperDLMurrellDEConderCMExacerbation of celecoxib-induced renal injury by concomitant administration of misoprostol in ratsPLoS One201492e8908724586517
- KawasakiASuzukiKTakekawaHCo-occurrence of multiple cerebral infarctions due to hypercoagulability associated with malignancy and meningeal carcinomatosis as the initial manifestation of gastric cancerBMC Neurol20141416025103421
- TianSHirshfieldKMJabbourSKSerum biomarkers for the detection of cardiac toxicity after chemotherapy and radiation therapy in breast cancer patientsFront Oncol2014427725346912
- AurielERegevKKorczynADNonsteroidal anti-inflammatory drugs exposure and the central nervous systemHandb Clin Neurol201411957758424365321
- SaffRRBanerjiAManagement of patients with nonaspirin-exacerbated respiratory disease aspirin hypersensitivity reactionsAllergy Asthma Proc2015361343925562554
- SiokaCKyritsisAPCentral and peripheral nervous system toxicity of common chemotherapeutic agentsCancer Chemother Pharmacol200963576176719034447
- BruchimIGoldbergAFishmanAConfino-CohenRCarboplatin hypersensitivity: evaluation and successful desensitization protocolImmunotherapy20146890591225313569
- BakSAndersenMTsiropoulosIRisk of stroke associated with non steroidal anti-inflammatory drugs: a nested case-control studyStroke200334237938612574546
- CruzRJJrVincenziRKetzerBMCecilioALCepedaLASpontaneous intratumoral bleeding and rupture of giant gastric stromal tumor (>30 cm) in a young patientWorld J Surg Oncol200867618627622
- KwonYAhnJSJeonSRIntratumoral bleeding in meningioma after gamma knife radiosurgeryJ Neurosurg2002975 SupplS657S662
- Lakshmi PrasadGRamdurgSRSuriAMahapatraAKA rare association of meningioma with intratumoral bleed and acute subdural hematomaNeurol India201058697797821150084
- ChenWCLiYDChiangPHComparison of proton pump inhibitor and histamine-2 receptor antagonist in the prevention of recurrent peptic ulcers/erosions in long-term low-dose aspirin users: a retrospective cohort studyBiomed Res Int2014201469356725295267
- BonelliPTuccilloFMFedericoAIbuprofen delivered by poly(lactic-co-glycolic acid) (PLGA) nanoparticles to human gastric cancer cells exerts antiproliferative activity at very low concentrationsInt J Nanomedicine201275683569123180963
- MouradJJThe evolution of systolic blood pressure as a strong predictor of cardiovascular risk and the effectiveness of fixed-dose ARB/CCB combinations in lowering levels of this preferential targetVasc Health Risk Manag2008461315132519337545
- KalaitzidisRGBakrisGLThe current state of RAAS blockade in the treatment of hypertension and proteinuriaCurr Cardiol Rep200911643644219863868
- VajapeyRRiniDWalstonJAbadirPThe impact of age-related dysregulation of the angiotensin system on mitochondrial redox balanceFront Physiol2014543925505418
- SchwedaFSalt feedback on the renin-angiotensin-aldosterone systemPflugers Arch2014467356557625502115
- TamaratRSilvestreJSDurieMLevyBIAngiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathwaysLab Invest200282674775612065685
- AgerEINeoJChristophiCThe renin-angiotensin system and malignancyCarcinogenesis20082991675168418632755
- ArrietaOGuevaraPEscobarEGarcía-NavarreteRPinedaBSoteloJBlockage of angiotensin II type I receptor decreases the synthesis of growth factors and induces apoptosis in C6 cultured cells and C6 rat gliomaBr J Cancer20059271247125215785746
- SuganumaTInoKShibataKFunctional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal disseminationClin Cancer Res20051172686269415814650
- MiyajimaAKikuchiEKosakaTOyaMAngiotensin II type 1 receptor antagonist as an angiogenic inhibitor in urogenital cancerRev Recent Clin Trials200942757819463103
- WasaJSugiuraHKozawaEKohyamaKYamadaKTaguchiOThe tumor suppressive effect of angiotensin II type 1 receptor antagonist in a murine osteosarcoma modelAnticancer Res201131112312721273589
- ShirotakeSMiyajimaAKosakaTRegulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancerAm J Pathol201218031008101622226738
- UemuraHNakaigawaNIshiguroHKubotaYAntiproliferative efficacy of angiotensin II receptor blockers in prostate cancerCurr Cancer Drug Targets20055530732316101380
- UemuraHIshiguroHKubotaYPharmacology and new perspectives of angiotensin II receptor blocker in prostate cancer treatmentInt J Urol2008151192618184167
- IzzedineHEderhySGoldwasserFManagement of hypertension in angiogenesis inhibitor-treated patientsAnn Oncol200920580781519150949
- MasamuneAHamadaSKikutaKThe angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in miceScand J Gastroenterol201348560260923477656
- WilsonJSPirolaRCApteMVStars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progressionFront Physiol201455224592240
- KosakaTMiyajimaATakayamaEAngiotensin II type 1 receptor antagonist as an angiogenic inhibitor in prostate cancerProstate2007671414917044086
- KosugiMMiyajimaAKikuchiEHoriguchiYMuraiMAngiotensin II type 1 receptor antagonist candesartan as an angiogenic inhibitor in a xenograft model of bladder cancerClin Cancer Res20061292888289316675585
- AlhusbanAAl-AzayzihAGocAGaoFFaganSCSomanathPRClinically relevant doses of candesartan inhibit growth of prostate tumor xenografts in vivo through modulation of tumor angiogenesisJ Pharmacol Exp Ther2014350363564524990940
- OkazakiMFushidaSHaradaSThe angiotensin II type 1 receptor blocker candesartan suppresses proliferation and fibrosis in gastric cancerCancer Lett20143551465325224569
- RiveraEArrietaOGuevaraPDuarte-RojoASoteloJAT1 receptor is present in glioma cells; its blockage reduces the growth of rat gliomaBr J Cancer20018591396139911720480
- ParkYAChoiCHDoIGDual targeting of angiotensin receptors (AGTR1 and AGTR2) in epithelial ovarian carcinomaGynecol Oncol2014135110811725014541
- ChoiCHParkYAChoiJJAngiotensin II type I receptor and miR-155 in endometrial cancers: synergistic antiproliferative effects of anti-miR-155 and losartan on endometrial cancer cellsGynecol Oncol2012126112413122525818
- Diop-FrimpongBChauhanVPKraneSBoucherYJainRKLosartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumorsProc Natl Acad Sci U S A201110872909291421282607
- FunaoKMatsuyamaMKawahitoYTelmisartan is a potent target for prevention and treatment in human prostate cancerOncol Rep200820229530018636189
- OzekiKTanidaSMorimotoCTelmisartan inhibits cell proliferation by blocking nuclear translocation of ProHB-EGF C-terminal fragment in colon cancer cellsPLoS One201382e5677023451083
- NakagawaTKubotaTKabutoMKoderaTCaptopril inhibits glioma cell invasion in vitro: involvement of matrix metalloproteinasesAnticancer Res1995155B198199
- WilliamsRNParsonsSLMorrisTMRowlandsBJWatsonSAInhibition of matrix metalloproteinase activity and growth of gastric adenocarcinoma cells by an angiotensin converting enzyme inhibitor in in vitro and murine modelsEur J Surg Oncol20053191042105015993560
- AttoubSGabenAMAl-SalamSCaptopril as a potential inhibitor of lung tumor growth and metastasisAnn N Y Acad Sci20081138657218837885
- VolpertOVWardWFLingenMWCaptopril inhibits angiogenesis and slows the growth of experimental tumors in ratsJ Clin Invest19969836716798698858
- de Groot-BesselingRRRuersTJvan KraatsAAAnti-tumor activity of a combination of plasminogen activator and captopril in a human melanoma xenograft modelInt J Cancer2004112232933415352048
- MiyajimaAAsanoTHayakawaMCaptopril restores transforming growth factor-beta type II receptor and sensitivity to transforming growth factor-beta in murine renal cell cancer cellsJ Urol2001165261662011176447
- HiiSINicolDLGotleyDCThompsonLCGreenMKJonssonJRCaptopril inhibits tumour growth in a xenograft model of human renal cell carcinomaBr J Cancer19987768808839528828
- SmallWJrMolteniAKimYTTaylorJMChenZWardWFCaptopril modulates hormone receptor concentration and inhibits proliferation of human mammary ductal carcinoma cells in cultureBreast Cancer Res Treat19974432172249266101
- KohSLAgerEICostaPLMalcontenti-WilsonCMuralidharanVChristophiCBlockade of the renin-angiotensin system inhibits growth of colorectal cancer liver metastases in the regenerating liverClin Exp Metastasis2014314395405
- KastREKarpel-MasslerGHalatschMECUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomideOncotarget20145188052808225211298
- ReddyMKBaskaranKMolteniAInhibitors of angiotensin-converting enzyme modulate mitosis and gene expression in pancreatic cancer cellsProc Soc Exp Biol Med199521032212268539259
- YoshijiHKuriyamaSKawataMThe angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factorClin Cancer Res2001741073107811309359
- YoshijiHKuriyamaSFukuiHPerindopril: possible use in cancer therapyAnticancer Drugs200213322122811984065
- OgilvieRIAnandSRoyPDe SouzaSPerindopril for control of blood pressure in patients with hypertension and other cardiovascular risk factors: an open-label, observational, multicentre, general practice-based studyClin Drug Investig20082811673686
- NoguchiRYoshijiHKuriyamaSCombination of interferon-beta and the angiotensin-converting enzyme inhibitor, perindopril, attenuates murine hepatocellular carcinoma development and angiogenesisClin Cancer Res2003916 Pt 16038604514676130
- YoshijiHNoguchiRKuriyamaSYoshiiJIkenakaYCombination of interferon and angiotensin-converting enzyme inhibitor, perindopril, suppresses liver carcinogenesis and angiogenesis in miceOncol Rep200513349149515706423
- YoshijiHKuriyamaSNoguchiRCombination of vitamin K2 and the angiotensin-converting enzyme inhibitor, perindopril, attenuates the liver enzyme-altered preneoplastic lesions in rats via angiogenesis suppressionJ Hepatol200542568769315826718
- YasumatsuRNakashimaTMasudaMEffects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cellsJ Cancer Res Clin Oncol20041301056757315449186
- UemuraHHasumiHKawaharaTPilot study of angiotensin II receptor blocker in advanced hormone-refractory prostate cancerInt J Clin Oncol200510640541016369744
- WilopSvon HobeSCrysandtMEsserAOsiekaRJostEImpact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapyCancer Res Clin Oncol20091351014291435
- TatokoroMFujiiYKawakamiSPhase-II trial of combination treatment of interferon-α, cimetidine, cyclooxygenase-2 inhibitor and renin-angiotensin-system inhibitor (I-CCA therapy) for advanced renal cell carcinomaCancer Sci2011102113714320973869
- NakaiYIsayamaHIjichiHPhase I trial of gemcitabine and candesartan combination therapy in normotensive patients with advanced pancreatic cancer: GECA1Cancer Sci201210381489149222515232
- NakaiYIsayamaHIjichiHA multicenter phase II trial of gemcitabine and candesartan combination therapy in patients with advanced pancreatic cancer: GECA2Invest New Drugs20133151294129923690239
- van der KnaapRSiemesCCoeberghJWvan DuijnCMHofmanAStrickerBHRenin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: the Rotterdam StudyCancer2008112474875718181094
- SiragyHMA current evaluation of the safety of angiotensin receptor blockers and direct renin inhibitorsVasc Health Risk Manag2011729731321633727
- LundeHHednerTSamuelssonODyspnoea, asthma, and bronchospasm in relation to treatment with angiotensin converting enzyme inhibitorsBMJ1994308692018218298346
- TschöpeCSchultheissHPWaltherTMultiple interactions between the renin-angiotensin and the kallikrein-kinin systems: role of ACE inhibition and AT1 receptor blockadeJ Cardiovasc Pharmacol200239447848711904521
- DicpinigaitisPVAngiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelinesChest20061291 SupplS169S173
- StoneCAKennyRANolanBLawlorPGAutonomic dysfunction in patients with advanced cancer; prevalence, clinical correlates and challenges in assessmentBMC Palliat Care201211322379978
- LissoniPPittalisSArdizzoiaAPrevention of cytokine-induced hypotension in cancer patients by the pineal hormone melatoninSupport Care Cancer1996443133168829312
- SelcukbiricikFTuralDSenelTESarıcaASoylukOSerdengectiSBilateral ischemic optic neuropathy developed under interferon therapyCase Rep Ophthalmol Med2012201210273923119208
- GuillemPPapachristosIPeillonCTribouletJPEtilefrine use in the management of post-operative chyle leaks in thoracic surgeryInteract Cardiovasc Thorac Surg20043115616017670203
- KranzfelderMGertlerRHapfelmeierAFriessHFeithMChylothorax after esophagectomy for cancer: impact of the surgical approach and neoadjuvant treatment: systematic review and institutional analysisSurg Endosc201327103530353823708712
- PokornáJMachalaLRezáčováPKonvalinkaJCurrent and novel inhibitors of HIV proteaseViruses2009131209123921994591
- BrikAWongCHHIV-1 protease: mechanism and drug discoveryOrg Biomol Chem20031151412929379
- PerryCMFramptonJEMcCormackPLSiddiquiMACvetkovićRSNelfinavir: a review of its use in the management of HIV infectionDrugs200565152209224416225378
- MoniniPSgadariCBarillariGEnsoliBHIV protease inhibitors: antiretroviral agents with anti-inflammatory, anti-angiogenic and anti-tumour activityJ Antimicrob Chemother200351220721112562682
- SgadariCBarillariGToschiEHIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcomaNat Med20028322523211875492
- ChowWAJiangCGuanMAnti-HIV drugs for cancer therapeutics: back to the future?Lancet Oncol2009101617119111246
- GillsJJLopiccoloJTsurutaniJNelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivoClin Cancer Res200713175183519417785575
- YangYIkezoeTTakeuchiTHIV-1 protease inhibitor induces growth arrest and apoptosis of human prostate cancer LNCaP cells in vitro and in vivo in conjunction with blockade of androgen receptor STAT3 and AKT signalingCancer Sci200596742543316053514
- YangYIkezoeTNishiokaCNFV, an HIV-1 protease inhibitor, induces growth arrest, reduced Akt signalling, apoptosis and docetaxel sensitisation in NSCLC cell linesBr J Cancer200695121653166217133272
- GuanMFousekKChowWANelfinavir inhibits regulated intramembrane proteolysis of sterol regulatory element binding protein-1 and activating transcription factor 6 in castration-resistant prostate cancerFEBS J2012279132399241122540830
- GuanMFousekKJiangCNelfinavir induces liposarcoma apoptosis through inhibition of regulated intramembrane proteolysis of SREBP-1 and ATF6Clin Cancer Res20111771796180621355074
- BonoCKarlinLHarelSThe human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivoHaematologica20129771101110922271897
- KastREHalatschMEMatrix metalloproteinase-2 and -9 in glioblastoma: a trio of old drugs – captopril, disulfiram and nelfinavir – are inhibitors with potential as adjunctive treatments in glioblastomaArch Med Res201243324324722564423
- JiangWMikochikPJRaJHHIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrestCancer Res20076731221122717283158
- BrüningABurgerPVogelMNelfinavir induces the unfolded protein response in ovarian cancer cells, resulting in ER vacuolization, cell cycle retardation and apoptosisCancer Biol Ther20098322623219106637
- PoreNGuptaAKCernigliaGJNelfinavir down-regulates hypoxia-inducible factor 1 alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapyCancer Res200666189252925916982770
- SrirangamAMitraRWangMEffects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancerClin Cancer Res20061261883189616551874
- KumarSBryantCSChamalaSRitonavir blocks AKT signaling, activates apoptosis and inhibits migration and invasion in ovarian cancer cellsMol Cancer200982619386116
- McLeanKVanDeVenNASorensonDRDaudiSLiuJRThe HIV protease inhibitor saquinavir induces endoplasmic reticulum stress, autophagy, and apoptosis in ovarian cancer cellsGynecol Oncol2009112362363019147209
- ViciPMarianiLPizzutiLEmerging biological treatments for uterine cervical carcinomaJ Cancer201452869724494026
- GanttSCasperCAmbinderRFInsights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and preventionCurr Opin Oncol201325549550223872785
- ZhangRLuoDMiaoRHsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosisOncogene200524243954396315782121
- PyrkoPKardoshAWangWXiongWSchönthalAHChenTCHIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stressCancer Res20076722109201092818006837
- ZhangKKaufmanRJSignaling the unfolded protein response from the endoplasmic reticulumJ Biol Chem200427925259352593815070890
- PajonkFHimmelsbachJRiessKSommerAMcBrideWHThe human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cellsCancer Res200262185230523512234989
- BrunnerTBGeigerMGrabenbauerGGPhase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancerJ Clin Oncol200826162699270618509182
- Alonso-BasantaMFangPMaityAHahnSMLustigRADorseyJFA phase I study of nelfinavir concurrent with temozolomide and radiotherapy in patients with glioblastoma multiformeJ Neurooncol2014116236537224194293
- BlumenthalGMGillsJJBallasMSA phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumorsOncotarget20145188161817225327558
- BuijsenJLammeringGJansenRLPhase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancerRadiother Oncol2013107218418823647753
- RenganRMickRPrymaDA phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical responseJ Thorac Oncol20127470971522425919
- HooverACMilhemMMAndersonCMEfficacy of nelfinavir as monotherapy in refractory adenoid cystic carcinoma: results of a phase II clinical trialHead Neck Epub201435
- PenzakSRChuckSKManagement of protease inhibitor-associated hyperlipidemiaAm J Cardiovasc Drugs2002229110614727985
- CalzaLManfrediRChiodoFUse of fibrates in the management of hyperlipidemia in HIV-infected patients receiving HAARTInfection2002301263111876511
- KalraSKalraBAgrawalNUnnikrishnanAUnderstanding diabetes in patients with HIV/AIDSDiabetol Metab Syndr201131221232158
- GillsJJLopiccoloJDennisPANelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagyAutophagy20084110710918000394
- YogasundaramHPutkoBNTienJHydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatmentCan J Cardiol201430121706171525475472
- RahimRStroblJSHydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cellsAnticancer Drugs200920873674519584707
- BrüningAGingelmaierAFrieseKMylonasINew prospects for nelfinavir in non-HIV-related diseasesCurr Mol Pharmacol201032919720359290
- PadhyBMGuptaYKDrug repositioning: re-investigating existing drugs for new therapeutic indicationsJ Postgrad Med201157215316021654146
- SchneiderBPLiLShenFGenetic variant predicts bevacizumab-induced hypertension in ECOG-5103 and ECOG-2100Br J Cancer201411161241124825117820
