Abstract
Background
Patients with ischemic cardiomyopathy (ICM) may continue to experience persistent chest pain and/or dyspnea despite pharmacologic therapy and revascularization. We hypothesized that ranolazine would reduce anginal symptoms or dyspnea in optimally treated ICM patients.
Methods
In this randomized, double-blind, crossover-design pilot study, 28 patients with ICM (ejection fraction less or equal 40%) were included after providing informed consent. A total of 24 patients completed both placebo and ranolazine treatments and were analyzed. All patients were on treatment with a beta blocker, an angiotensin-converting enzyme inhibitor (or angiotensin receptor blocker), and at least one additional antianginal drug. After randomization, patients received up to 1,000 mg ranolazine orally twice a day, as tolerated, versus placebo. The primary end point was change in angina as assessed by the Seattle Angina Questionnaire (SAQ), or in dyspnea as assessed by the Rose Dyspnea Scale (RDS). Change in the RDS and SAQ score from baseline was compared, for ranolazine and placebo, using the Wilcoxon signed rank test or paired t-test.
Results
Patients had the following demographic and clinical variables: mean age of 71.5 years; male (82.1%); prior coronary bypass surgery (67.9%); prior coronary percutaneous intervention (85.7%); prior myocardial infarction (82.1%); diabetes (67.9%); and mean ejection fraction of 33.1%. No statistical difference was seen between baseline RDS score and that after placebo or ranolazine (n=20) (P≥0.05). There was however, an improvement in anginal frequency (8/10 patients) (P=0.058), quality of life (8/10 patients) (P=0.048), and mean score of all components of the SAQ questionnaire (n=10) (P=0.047) with ranolazine compared with placebo.
Conclusion
In optimally treated ICM patients with continued chest pain or dyspnea, ranolazine possibly had a positive impact on quality of life, a reduction in anginal frequency, and an overall improvement in the mean SAQ component score compared with baseline. Ranolazine did not change the dyspnea score compared with baseline.
Introduction
Patients with ischemic cardiomyopathy (ICM) and reduced ejection fraction (EF) may continue to experience persistent chest pain and dyspnea despite optimal medical therapy and/or revascularization. In a recent study of a large database from Duke University Medical Center, 59% of patients with ICM and reduced EF were found to continue to have angina pectoris despite medical therapy, and this was associated with an increased risk of cardiovascular death and cardiovascular hospitalization.Citation1 Angina in patients with ICM does respond favorably to afterload reduction and nitrate therapy.Citation2 However, in some patients, this may persist, likely due to microvascular disease, diastolic dysfunction, and diffuse nontreatable coronary artery disease. Even in patients with nonischemic cardiomyopathy, angina is prevalent and is likely due to reduction in myocardial perfusion at rest and stress, and, subsequently, subendocardial ischemia.Citation3
Ranolazine has been shown to reduce anginal frequency and to increase exercise time without angina or electrocardiographic ischemic changes when compared with placebo, in patients on anti-ischemic drugs.Citation4,Citation5 Its safety has been validated by the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes (MERLIN)-TIMI 36 trial.Citation6 MERLIN demonstrated no increase in arrhythmia or mortality in patients with acute coronary syndromes treated with ranolazine compared with placebo.
Patients with reduced EF and known diabetes were included in the ranolazine trials, and there was no evidence that they responded less to treatment. No safety issues were raised in these subgroups. However, there is no randomized double blind study that has evaluated ranolazine in patients with continued angina and ICM with an EF of less than 40%.
The purpose of this randomized pilot study was to determine the efficacy and safety of ranolazine compared with placebo, in symptomatic patients with ICM and reduced EF despite optimal medical therapy and revascularization.
Methods
This was a prospective, double-blind, crossover, randomized pilot trial (ClinicalTrials.gov identifier: NCT01345188) in which a total of 28 patients with ICM nontreatable by further revascularization were enrolled and randomly assigned to an initial treatment with ranolazine or placebo, in addition to conventional medical treatment for their cardiomyopathy. Patients were on angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), a beta blocker, and at least one additional anti-ischemic drug (amlodipine or long-acting nitrate).
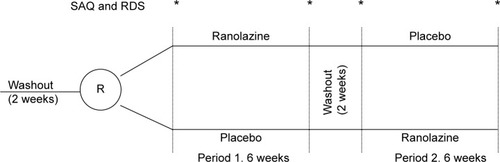
Following a 2-week washout period, patients were randomized to either placebo or ranolazine for 6 weeks followed by a 2-week washout period before crossing over to the alternative treatment for 6 additional weeks (). A total of 24 patients were able to complete the study and were treated randomly with both placebo and ranolazine. During the study, patients were kept on the same antianginal drugs in addition to the study drug. The primary end point of the study was the improvement in angina as assessed by the Seattle Angina Questionnaire (SAQ),Citation7 or in dyspnea as assessed by the Rose Dyspnea Scale (RDS).Citation8 The SAQ and RDS were administered at baseline immediately prior to randomization, after 6 weeks of each treatment with either placebo or ranolazine, and at the end of the 2-week washout period immediately prior to crossover treatment. The investigators and the patient remained blinded to the treatment until the completion of the trial.
Figure 1 Crossover randomized design of ranolazine versus placebo in patients with ischemic cardiomyopathy and persistent angina or dyspnea.
The SAQCitation7 score was determined by a core laboratory (CV Outcomes, Inc. www.cvoutcomes.org) that was blinded to patient treatment. SAQ is a self-administered questionnaire that objectively scores five components of coronary artery disease presentation, including anginal stability, anginal frequency, physical limitation, treatment satisfaction, and disease perception. Scoring is done by assigning each response an ordinal value. A low score indicates a low level of functioning. Typically, scores for each component are analyzed and compared independently. Although a mean score of SAQ components was calculated in this report, its value has not been validated.
The RDSCitation8 is a four-item questionnaire that evaluates a patient’s dyspnea with regular activities. Each question answered positively is given a score of 1, and the total score is 4. A higher score indicates dyspnea leading to more physical limitation.
Patients were included in the study if they: 1) had ICM with continued symptoms on guideline-directed medical treatment, where optimal medical treatment was defined as treatment with two anti-ischemic agents (amlodipine or long-acting nitrates on top of beta blockers) as well as an ACEI/ARB unless contraindicated, and continued symptoms defined as significant exertional angina or dyspnea, interfering with the patient’s daily activity; 2) had angiographic documentation of coronary artery disease that was not amenable to treatment by coronary intervention (already treated or nontreatable); 3) had a recent EF of less than or equal to 40% within 6 months of enrollment, as assessed by echocardiography or isotope ventriculography; and 4) were able to sign an informed consent before enrollment. There was no prespecified exclusion based on QTc or renal function. Dialysis patients were excluded. The protocol was conducted using Good Clinical Practice (http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/) and was approved by the Institutional Review Board at UnityPoint Health System, Trinity Medical Center.
There was a high prevalence of patients with prior diabetes mellitus (67.9%) and prior myocardial infarction (82.1%). The mean EF was 33.1%. All patients received ranolazine 500 mg tablets or a matching placebo to take twice daily for 1 week. If tolerated, both ranolazine and the matching placebo were increased to 1,000 mg orally twice a day. If the patient experienced side effects from the high dose of ranolazine or placebo, the drug was resumed at 500 mg orally twice daily (). All patients had an electrocardiogram, a physical exam, vital signs, and chest pain and/or dyspnea assessment with the SAQ and RDS, respectively, at each visit. Compliance with medications was assessed at each visit (baseline and end of each treatment).
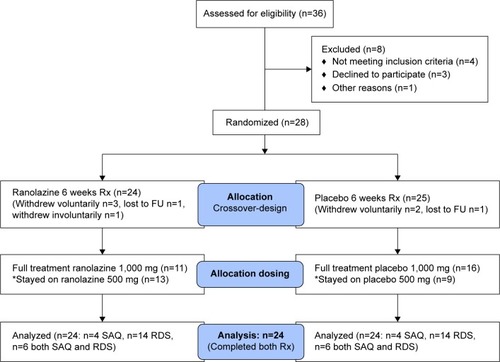
Figure 2 CONSORT flow diagram of the Ranexa® trial in ischemic cardiomyopathy patients with reduced ejection fraction.
Statistical analysis
RDS scores at baseline and after treatment with ranolazine or placebo were compared using the Wilcoxon signed rank test. Continuous data from the SAQ was compared using paired t-test. In this exploratory randomized pilot study, no statistical power or sample size was prespecified.
Results
A total of 28 patients were included in this study. In all, 24 patients received the allocated ranolazine treatment, and 25 patients received the placebo treatment. A total 24 patients completed both treatment arms and were the subject of this analysis (). Of the 24 patients analyzed, ten patients had angina and 20 patients had dyspnea (six patients had both). Demographic and clinical variables are presented in . The majority of the patients had advanced heart failure symptoms based on dyspnea with minimal activity, indicating the extent of limitation on their daily activity. The mean QTc was 429 msec (range 320–534 msec). Also, three patients had a creatinine over 2 mg/dL, but no patients were on dialysis.
Table 1 Descriptive and clinical characteristics
The pretreatment baseline SAQ and RDS scores prior to randomization (after the first washout period) and before crossover treatment (after the second washout period) were not statistically different. Among the patients with dyspnea (n=20), no statistically significant change in mean RDS score was seen following treatment with either placebo or ranolazine (−0.34 versus −0.45, respectively) (P≥0.05), although a type II error cannot be excluded because of the small number of patients in the study. There was, however, an apparent improvement in anginal frequency (8/10 patients) (P=0.058), quality of life (8/10 patients) (P=0.048), and average score of all components of the SAQ questionnaire (n=10) (P=0.047) between baseline and after treatment with ranolazine ( and ).
Figure 3 Line graph showing the change in scores of each component and the mean score of the Seattle Angina Questionnaire, with ranolazine (A) and placebo (B), when compared at baseline and after 6 weeks of treatment.
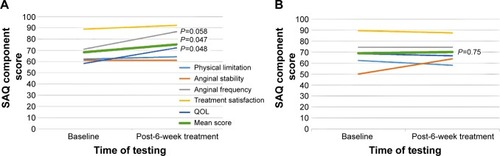
Table 2 Seattle Angina Questionnaire variables
The most common side effects reported in the ranolazine arm included nausea, dizziness, constipation, headache, hypotension, and dyspnea; all have been previously reported with ranolazine.Citation4,Citation5 No sustained ventricular arrhythmia was noted. In the placebo patients, dizziness was reported. One patient had to withdraw after transferring to a nursing home. Three patients withdrew voluntarily shortly after randomization, and one person was lost to follow up. In all, 11/24 (45.8%) patients on ranolazine were able to be increased to 1,000 mg twice a day. The remaining 13/24 (54.2%) patients remained at 500 mg twice a day because of: 1) intolerance to high dose related to side effects (6/24); 2) potential drug interactions with metformin, simvastatin, or digoxin (as per investigator’s preference) (4/24); or 3) for other, undetermined reasons (3/24). On the other hand, 16/24 (66.7%) patients on placebo had their dose increased to 1,000 mg twice a day.
Two serious adverse events (SAEs) occurred: one was a stroke in a patient who was on ranolazine but who had a history of atrial fibrillation; another patient was admitted to the hospital with exacerbation of his heart failure, but this patient also had pneumonia and sepsis. Both SAEs were considered unrelated to the study drug.
Discussion
Ranolazine is a selective inhibitor of the late Na+ current, reducing excess Na+ entry into the cells and, therefore, reducing Na+/Ca2+ exchanger-mediated Ca2+ overload. Ca2+ overload is associated with mechanical and electrical instability and mitochondrial dysfunction, all leading to diastolic dysfunction, arrhythmia, and cell injury.Citation9 In the Monotherapy Assessment of Ranolazine in Stable Angina (MARISA) trial, ranolazine increased exercise duration, time to 1 mm ST depression, and time to angina. Ranolazine minimally affected heart rate and systolic blood pressure.Citation4 Exercise duration was dose-dependent, as were side effects. More adverse events were noted at the 1,500 mg twice daily dosing, which is not an approved dose. In MARISA, 16.8% of patients had class I/II heart failure, and the treatment effect in this subgroup was not different from the entire cohort.Citation4
Similarly, in the Combination Assessment of Ranolazine in Stable Angina (CARISA) trial,Citation5 exercise duration was also significantly increased following ranolazine compared with placebo. Also time to angina was increased, and the number of anginal attacks and use of nitroglycerin were reduced with ranolazine compared with placebo. In the CARISA study, 28% to 31% of patients had congestive heart failure. Ranolazine effectiveness was not affected by the presence of heart failure.
Intracellular Na+ is increased in patients with heart failure, probably related, in part, to increase in CaMKII-dependent phosphorylation of Na+ channels, with subsequent increase in the inward Na+ current.Citation10,Citation11 In the present study, anginal frequency and the mean of SAQ component scores was improved with ranolazine in patients with ICM and reduced EF, possibly related to reduction of intracellular Na+ and Ca2+, and improvement in diastolic dysfunction and coronary perfusion. Dyspnea score, however, was not changed with ranolazine. The etiology of dyspnea can be multifactorial, including fluid overload, deconditioning, obesity, ischemia, and pulmonary conditions. Therefore, dyspnea may have not been a reliable symptom to measure the effectiveness of ranolazine in patients with ICM and reduced EF. Although ranolazine may positively influence ischemia-induced dyspnea, it is likely that it has no effect on other causes of dyspnea. Also, it should be noted that most of the study patients had very advanced New York Heart Association functional class III symptoms despite medical treatment and, therefore, may represent a cohort of patients with advanced disease that may not be responsive to ranolazine.
Guideline-based therapy considers beta blockers and ACEI as ACC/AHA Class I treatment in patients with ischemic heart disease and reduced left-ventricular function.Citation12 The addition of a Ca2+ channel blocker, nitrates, or ranolazine is also considered class I therapy in ischemic heart disease patients with continued symptoms and already receiving beta blockers. In our study, patients were on a beta blocker and an ACEI/ARB. Also, all of them received one additional anti-ischemic drug in addition to beta blockers, meeting guideline-based therapy for ischemic heart disease. They were included in the study only after failure of two anti-ischemic drugs and, therefore, they represent a challenging subgroup of patients to treat. Despite this, ranolazine demonstrated a possible benefit in this subgroup, as demonstrated by the SAQ.
Ranolazine at a 500 mg twice daily dose was well-tolerated in this study. However, 6/24 (25%) patients could not tolerate the higher dose of 1,000 mg twice daily. Also, 44.8% in the ranolazine arm were advanced to the higher dose versus 66.7% in the placebo arm, which may reflect on some increase in side effects with ranolazine at higher dosing in ICM. There were no fatal or sustained ventricular arrhythmias noted, consistent with the drug safety profile.
Conclusion
In conclusion, ranolazine possibly exerted a positive effect in reducing angina and improving the composite SAQ score when compared with placebo, in patients with ICM and reduced EF. Quality of life also improved significantly. Ranolazine, however, had no impact on the dyspnea score. A large randomized trial is needed to validate these findings.
Limitation of the study
In any crossover randomized trial, patients may become aware of the active drug because of either its effectiveness or side effects, which could bias their reporting of symptoms. Also, the impact of the initial treatment may have influenced the response to the alternative subsequent treatment, although this is less likely given the 2-week washout period and the short half-life of ranolazine (7 hours). In fact, the baseline SAQ scores at onset of randomization and after the 2-week washout period were statistically not different. Furthermore, the use of spironolactone was not a prerequisite to enroll patients in this study. Whether the use of aldosterone inhibitors would have affected the findings of this study is unclear. It is, however, unlikely, as the same patients were crossed over randomly to both placebo and ranolazine on the background of unchanged baseline medical therapy. Finally, this study was an exploratory pilot trial with no prespecified statistical power or sample size calculations. The positive outcomes on angina, however, should encourage a larger trial to validate these findings.
Acknowledgments
The authors wish to express their sincere thanks to Janelle Goins for her very valuable help, advice, and support in conducting this study.
Disclosure
This study was supported by a research grant from Gilead. Dr Shammas is a speaker for and is on the advisory board of Gilead.
The authors report no other conflicts of interest in this work.
References
- MentzRJFiuzatMShawLKComparison of clinical characteristics and long-term outcomes of patients with ischemic cardiomyopathy with versus without angina pectoris (from the Duke Databank for Cardiovascular Disease)Am J Cardiol201210991272127722325975
- LevineTBLevineABVincenziAKeteyianSJBillingsJLeschMResponse of symptomatic myocardial ischemia in ischemic cardiomyopathy to intensive vasodilator therapyAm J Cardiol19978022122149230164
- PasternacANobleJStreulensYElieRHenschkeCBourassaMGPathophysiology of chest pain in patients with cardiomyopathies and normal coronary arteriesCirculation19826547787897199403
- ChaitmanBRSkettinoSLParkerJOMARISA InvestigatorsAnti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe anginaJ Am Coll Cardiol20044381375138215093870
- ChaitmanBRPepineCJParkerJOCombination Assessment of Ranolazine In Stable Angina (CARISA) InvestigatorsEffects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trialJAMA2004291330931614734593
- MorrowDASciricaBMKarwatowska-ProkopczukESkeneAMcCabeCHBraunwaldEMERLIN-TIMI 36 InvestigatorsEvaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trialAm Heart J200615161186.e11186.e916781216
- SpertusJAJonesPMcDonellMFanVFihnSDHealth status predicts long-term outcome in outpatients with coronary diseaseCirculation20021061434912093768
- RoseGABlackburnHCardiovascular survey methodsMonogr Ser World Health Organ1968561188
- BelardinelliLAntzelevitchCFraserHInhibition of late (sustained/persistent) sodium current: a potential drug target to reduce intracellular sodium-dependent calcium overload and its detrimental effects on car-diomyocyte functionEur Heart J Suppl20046Suppl IS13S17
- PieskeBMaierLSPiacentinoVWeisserJHasenfussGHouserSRate dependence of [Na+]i and contractility in nonfailing and failing human myocardiumCirculation2002106444745312135944
- WagnerSDybkovaNRasenackECCa2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channelsJ Clin Invest2006116123127313817124532
- FihnSDGardinJMAbramsJAmerican College of Cardiology Foundation/American Heart Association Task Force2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsCirculation201212625e354e47123166211
