Abstract
Bronchodilators are the most important drugs used for the treatment of chronic obstructive pulmonary disease (COPD). In particular, these therapeutic agents are mostly long-acting compounds utilized via inhalation, and include LAMA (long-acting muscarinic receptor antagonists) and LABA (long-acting β2-adrenoceptor agonists). Because LAMA and LABA induce bronchodilation by distinct mechanisms of action, LABA/LAMA combinations provide a reciprocal potentiation of the pharmacological effects caused by each component. Hence, many COPD patients who do not achieve a satisfactory control of their symptoms using a single, either LAMA or LABA bronchodilator, can experience relevant benefits with the use of LAMA/LABA fixed combinations. Many different LAMA/LABA combinations have been recently developed and evaluated in randomized clinical trials. In this context, our review focuses on the pharmacological mechanisms underpinning the bronchodilation elicited by the LAMA tiotropium bromide and the LABA olodaterol. We also discuss the results of the most important clinical studies carried out in COPD patients to assess the efficacy and safety of tiotropium/olodaterol combinations.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous respiratory disorder affecting more than 200 million patients worldwide.Citation1 Current knowledge indicates that both prevalence and incidence of this disease are continuously increasing, thus leading COPD to predictably become by 2020 the third leading cause of death in the world. Arising from complex interactions between genetic factors and environmental agents, mainly including tobacco smoke and airborne pollutants, COPD is prominently featured by a scarcely reversible and progressively worsening airflow limitation.
Bronchodilators are the most important drugs used for COPD treatment, and they are usually utilized as inhaled long-acting compounds, including LAMA (long-acting muscarinic receptor antagonists) and LABA (long-acting β2-adrenoceptor agonists).Citation2 The excellent therapeutic profile of both LAMA and LABA depends on their effective ability to counteract the bronchoconstrictive cholinergic tone, which in COPD patients represents the predominant functional cause of airflow limitation. This bronchomotor tone is largely sustained by an excessive amount of acetylcholine (ACh) within the airways of subjects with COPD. High levels of ACh are indeed released by vagal nerve reflexes triggered by stimulation of airway sensory nerve endings.Citation3,Citation4 Hence, the increase in baseline cholinergic bronchomotor tone of smokers with COPD is closely related to disease severity.Citation5 Once released into the airways from postganglionic parasympathetic nerve terminals, ACh stimulates postjunctional cholinergic muscarinic receptors. In particular, ACh-induced contraction of airway smooth muscle (ASM) cells is mainly due to activation of the M3 subtype of muscarinic receptors. Coupling of activated muscarinic M3 receptors to cell membrane Gq protein leads to stimulation of the catalytic activity of phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate, thereby generating the two intracellular second messengers inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG).Citation6 DAG activates protein kinase C (PKC), which increases the sensitivity of ASM contractile apparatus to calcium ions (Ca2+), whereas IP3 elicits Ca2+–dependent bronchoconstriction via a rapid mobilization of Ca2+ from intracellular stores such as the sarcoplasmic reticulum.Citation7 The subsequent remarkable increase in cytosolic Ca2+ levels is responsible for the sequential activation of Ca2+–calmodulin complex, myosin light chain kinase, and actin–myosin contractile apparatus.Citation7–Citation9 Furthermore, the enhanced bronchoconstrictive cholinergic tone occurring in COPD also results from the overexpression of muscarinic M3 receptors, associated with an upregulation of M3 receptor-coupled signaling pathways.Citation10,Citation11 In this regard, it is noteworthy that key proinflammatory stimuli involved in COPD pathobiology, including cigarette smoke and tumor necrosis factor-α, are able to stimulate Gq protein expression.Citation12,Citation13
These molecular mechanisms can be effectively neutralized by currently used inhaled bronchodilators. In fact, LAMA act as potent competitive antagonists of airway muscarinic receptors, while LABA are powerful functional antagonists of bronchoconstriction.Citation14 In both cases, these drugs are able to markedly counteract the exaggerated cholinergic bronchomotor tone affecting the airways of COPD patients. Within this evolving therapeutic scenario, tiotropium bromide represents the first, most studied, and most used LAMA for COPD treatment.Citation2,Citation15,Citation16 Olodaterol is a new LABA recently introduced in clinical practice, characterized by very interesting therapeutic properties.Citation17 Therefore, acting by different pharmacological mechanisms, tiotropium and olodaterol can reciprocally potentiate their broncodilating actions. Such a dual bronchodilation has been successfully tested in several clinical trials evaluating in COPD patients the effects of fixed-dose combinations (FDC) of tiotropium and olodaterol, assembled in the same inhaler that simultaneously delivers the two drugs.Citation18 On the basis of the above considerations, the aim of this concise review article is to outline the mechanisms of action of tiotropium and olodaterol, as well as to discuss the efficacy and safety of tiotropium/olodaterol co-formulations in COPD treatment.
Mechanism of action and therapeutic profile of tiotropium
Tiotropium bromide is a cornerstone of inhaled bronchodilator therapy, and its introduction in clinical practice provided a prominent evolution within the context of pharmacological competitive antagonism of muscarinic receptors. High-affinity binding of tiotropium to transmembrane Gq-coupled airway muscarinic M3 receptors inhibits ACh-dependent and IP3-mediated release of Ca2+ from intracellular stores, thus completely preventing cholinergic bronchoconstriction (). The effective and long-lasting blockade of muscarinic M3 receptors, operated by tiotropium, is due to its very slow dissociation rate from these receptors.Citation19 The molecular basis of such a highly durable residence time of tiotropium at a level of M3 receptors has been recently elucidated in terms of chemical drug–receptor interactions. In particular, the aromatic portion of the drug deeply penetrates into a hydrophobic region of the M3 receptor, where the hydroxyl groups of tiotropium form strong double hydrogen bonds with a specific asparagine residue (N508) of the receptor protein.Citation19 Therefore, this molecular interaction is essential for the slowly dissociating properties of tiotropium, regarding its binding pattern to the M3 receptor. Among currently available LAMA, tiotropium occupies for the longest time the muscarinic M3 receptor, whose blockade lasts for more than 24 hours thus allowing a once-daily administration.Citation20,Citation21 On the contrary, tiotropium rapidly dissociates from M2 muscarinic receptors, that in human airways are predominantly located on postganglionic vagal nerve terminals, where they act as inhibitory autoreceptors which repress ACh release.Citation22,Citation23 This kinetic receptor selectivity is the key mechanism underpinning the persistent bronchodilation induced by tiotropium.
Figure 1 Tiotropium: mechanism of bronchodilating action.
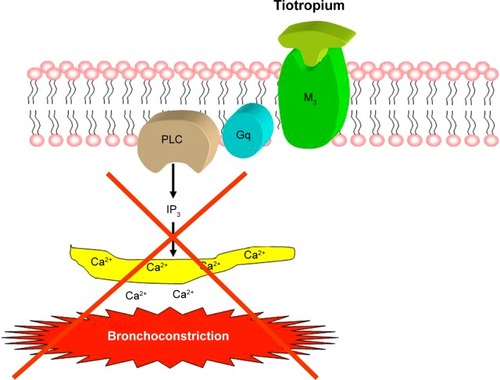
With regard to lung function, the potent bronchodilating effect of tiotropium is responsible for a significant and prolonged increase in forced expiratory volume in 1 second (FEV1).Citation24 It has also been shown that the bronchodilatory action of tiotropium does not decrease during long-term therapy, and this observation rules out the development of pharmacological tolerance. Indeed, the large multicentric trial UPLIFT (Understanding Potential Long-Term Impacts on Function with Tiotropium) involving almost 6,000 COPD patients worldwide, demonstrated that tiotropium elicited a constant FEV1 improvement throughout the overall 4-year study period, with no detectable losses of bronchodilating effectiveness during such a long time span.Citation25 The excellent bronchodilatory activity of tiotropium promotes air egression from enlarged alveolar spaces, thereby decreasing lung hyperinflation. This deflating effect of tiotropium is clinically very relevant, because air trapping is the predominant cause of the main COPD symptoms, namely dyspnea and exercise intolerance. In fact, tiotropium affects static lung volumes thus reducing residual volume and functional residual capacity, as well as increasing inspiratory capacity.Citation26 These changes result in significant improvements in both exertional dyspnea and exercise endurance.Citation27
The therapeutic impact on lung hyperinflation also contributes to the remarkable tiotropium capability of lowering the annual rate and severity of COPD exacerbations. Indeed, lung deflation correlates better than FEV1 increase with reduction in exacerbation frequency.Citation28 In particular, by persistently decreasing airway resistance and facilitating lung emptying, tiotropium can prevent COPD exacerbations and attenuate dyspnea during an exacerbation. On the other hand, the degree of baseline bronchial obstruction is predictive of exacerbation severity.Citation29 Therefore, it is reasonable that severe airflow limitation fosters the development of COPD exacerbations, which in turn promote a further worsening of airway narrowing, thus triggering a deleterious and self-perpetuating vicious circle which can be interrupted by an effective and persistent bronchodilation. Tiotropium could contribute to decrease COPD exacerbations also by reducing sputum production.Citation30 In fact, mucus hypersecretion may favor airflow obstruction and pulmonary infections, thereby enhancing the risk of COPD exacerbations. The inhibitory effect of tiotropium on sputum production is likely mediated by blockade of muscarinic M3 receptors expressed on airway mucous glands.Citation31 Another putative mechanism potentially contributing to the positive effects of tiotropium on COPD exacerbations can be attributable to the reported anti-inflammatory activity of this drug. COPD exacerbations are indeed associated with a relevant amplification of airway inflammation.Citation32 In this regard, it is notable that a relevant anti-inflammatory action can be exerted on lung alveolar macrophages by tiotropium via suppression of ACh-induced release of leukotriene B4,Citation33 a powerful neutrophil chemoattractant. Moreover, tiotropium is able to inhibit the pro-inflammatory actions stimulated by ACh in neutrophils isolated from COPD patients.Citation10,Citation34 These anti-inflammatory effects of tiotropium appear to be also due to blockade of muscarinic M3 receptors located on inflammatory cells.Citation21 Taken together, the above considerations suggest that several mechanisms are responsible for the potent preventive action on COPD exacerbations provided by tiotropium, which in this regard resulted to be equivalent to the fluticasone propionate/salmeterol combination, and superior to either salmeterol alone or indacaterol, as shown by INSPIRE (Investigating New Standards for Prophylaxis in Reducing Exacerbations), POET (Prevention of Exacerbations with Tiotropium), and INVIGORATE (Indacaterol: Providing Opportunity to Re-engage Patients with Life) trials, respectively.Citation35–Citation37
Mechanism of action and therapeutic profile of olodaterol
All β2-adrenergic receptor (β2-AR) agonists, including olodaterol, induce bronchodilation by relaxing ASM regardless of the nature and multitude of constricting stimuli, thus acting as functional antagonists of bronchoconstriction. These drugs occupy and activate β2-ARs, which are coupled to the stimulatory G protein (Gs) that is in turn responsible for stimulation of adenylyl cyclase and the subsequent increase in the intracellular concentration of the second messenger cyclic AMP (cAMP) ().Citation14 The latter activates cAMP-dependent protein kinase A (PKA), which phosphorylates several targets within the cell,Citation17 thereby leading to inhibition of myosin light chain kinase and activation of MLC phosphatase which result in ASM relaxation. In addition, β2-adrenergic agonists promote Ca2+ sequestration inside the intracellular stores and, by facilitating the opening of large conductance Ca2+–activated K+ channels, these drugs induce the repolarization of ASM cell membrane.Citation17
Figure 2 Olodaterol: mechanism of bronchodilating action.
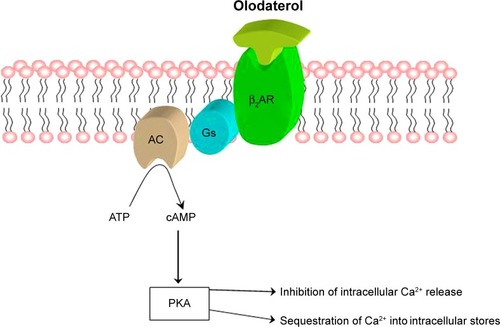
Olodaterol is a new LABA, recently developed for the treatment of COPD, characterized by a very long, 24-hour-lasting bronchodilatory action.Citation38 Olodaterol tightly binds to β2-AR, from which this drug dissociates very slowly.Citation39 Such a pharmacological profile is due to the formation of a highly stable ternary complex consisting of olodaterol, β2-AR, and Gs protein ().Citation39 The long-lasting persistence on β2-AR of olodaterol, with its high-affinity binding modality, results in a durable activation of the cAMP-mediated signaling pathway. On the contrary, olodaterol dissociates quite rapidly from β1-AR,Citation39 thereby exhibiting a very high β2-AR selectivity. Another positive feature of olodaterol is the fast onset of its bronchodilatory action. Moreover, the pronounced β2-AR selectivity of olodaterol is associated with a remarkable intrinsic activity in regard to β2-AR stimulation.Citation38 Indeed, olodaterol behaves as a nearly full β2-AR agonist, whereas this drug acts as a partial β1-AR agonist.Citation38 Hence, this pharmacological profile can be therapeutically very useful, in that it provides a very effective bronchodilation, potentially paralleled by a minimization of cardiovascular side effects, mainly caused by activation of cardiac β1-ARs.
Olodaterol exerts an efficacious, 24-hour-lasting protection against experimental ACh-induced bronchoconstriction in both guinea pigs and dogs.Citation38 Used by COPD patients once daily at dosages ranging from 2 to 20 µg, olodaterol elicits dose-dependent, significant increases in FEV1Citation40,Citation41 Two replicate, randomized, double-blind, placebo-controlled, and parallel-group phase III studies, carried out in patients with moderate to very severe COPD, have shown that olodaterol 5 and 10 µg significantly enhanced trough FEV1, and this bronchodilation was associated with an incidence of adverse events (AEs) comparable to that caused by placebo.Citation42 Such findings were further corroborated by other two replicate, multicenter studies, which also demonstrated that the improvements in lung function produced by olodaterol translated into symptomatic benefits for COPD patients.Citation43 The bronchodilating action of olodaterol was also confirmed by another randomized controlled trial, which did not find any difference in efficacy and tolerability between 5 and 10 µg dosages, thus suggesting the selection of the 5 µg dose in clinical practice.Citation44 Furthermore, utilized by COPD patients at the once-daily dosage of 5 µg, olodaterol induced a similar 24-hour bronchodilation pattern when compared with either once-daily 10 µg or twice-daily 5 µg.Citation45 When comparatively evaluated in COPD patients under similar trial conditions, olodaterol and indacaterol, another ultralong-acting β2-AR agonist, exhibited an overlapping bronchodilatory efficacy in terms of 24-hour increases in trough FEV1.Citation46 Overall, the safety profile of olodaterol used at both dosages of once-daily 5 and 10 µg, especially with regard to cardiovascular side effects, resulted to be comparable to placebo and to twice-daily administered formoterol.Citation47
Dual bronchodilation with tiotropium/olodaterol
In patients with COPD characterized by a marked airflow limitation, in whom treatment with a single long-acting bronchodilator (LABA or LAMA) does not provide an adequate control of respiratory symptoms, a LABA/LAMA combination therapy can be usefully considered. Indeed, several LABA/LAMA combinations have been recently developed (). In particular, a potent LABA such as olodaterol induces a direct bronchodilating effect, mediated by a β2-AR-dependent rapid and sustained increase in the intracellular concentration of cAMP, which is responsible for the very effective functional antagonism of ASM contraction. Therefore, the integration of this mechanism of action with the postjunctional competitive antagonism of M3 muscarinic receptors, namely at the level of ASM cell membrane, makes it possible for olodaterol and tiotropium, when used together, to maximize the resultant bronchodilation.Citation18 Moreover, β2-adrenergic agonists inactivate the signaling cascade underlying ACh-induced bronchoconstriction, thus enhancing the bronchodilating action of a LAMA-like tiotropium. This mechanism is mediated by cAMP-dependent transcriptional stimulation of the expression of regulator of G-protein signaling 2, which specifically inhibits the activation of the Gq protein coupled to M3 muscarinic receptors.Citation48 Furthermore, by blocking M3 muscarinic receptors, tiotropium inhibits receptor coupling to the signal transduction pathway leading to the sequential activation of Gq and PLC, which is in turn responsible for the synthesis of DAG, the intracellular second messenger that activates PKC.Citation7 PKC phosphorylates both β2-AR and Gs protein, thereby uncoupling two key components of the signaling pathway responsible for the bronchodilating action of β2-adrenergic agonists.Citation49 Moreover, PKC can also phosphorylate and activate G-protein-coupled receptor kinase 2, an enzyme which in turn promotes the phosphorylation of specific threonine and serine residues located within the C-terminal cytoplasmatic tail of β2-AR, thus further enhancing β2-AR/Gs uncoupling as well as facilitating binding of β-arrestins to the C-terminus of β2-AR, with subsequent intracellular receptor sequestration.Citation50,Citation51 Therefore, by abrogating these effects of ACh, tiotropium can prevent β2-AR desensitization, thus maintaining and potentiating the bronchodilating action of LABA such as olodaterol. The cooperation between LABA and LAMA also extends to the prejunctional site, namely at the level of vagal postganglionic nerve endings. Prejunctional inhibition of ACh release is indeed mediated by β2-AR activation, as well as by stimulation of M2 muscarinic autoreceptors.Citation52 The function of prejunctional M2 autoreceptors is largely preserved by tiotropium, which rapidly dissociates from these receptors, whereas this drug blocks for a long time the postjunctional M3 muscarinic receptors, mainly responsible for bronchoconstriction and mucus hypersecretion. These positive pharmacological interactions are further amplified by the different distribution patterns of receptor targets along the bronchial tree. Indeed, the density of bronchodilating β2-ARs progressively increases from proximal to distal airways, while vagal cholinergic innervation is mostly distributed within the central airways, where bronchoconstrictive muscarinic M3 receptors are present in higher numbers.Citation53 LAMA/LABA combinations can thus provide an extensive bronchodilation across the entire respiratory system.
Table 1 LABA/LAMA fixed dose combinations
On the basis of the pharmacological concepts as discussed in the previous section of this paper, several controlled trials have been performed to evaluate the potential benefits of tiotropium/olodaterol in COPD treatment.Citation18 Preclinical studies, carried out in anesthetized dogs, demonstrated that the endotracheal administration of tiotropium/olodaterol combination provided, with respect to either monotherapy (tiotropium or olodaterol), a better protection against ACh-induced bronchoconstriction.Citation18 More importantly, many clinical trials conducted in COPD patients with regard to evaluation of lung function, dyspnea, and quality of life have shown that greater improvements can be achieved with tiotropium/olodaterol combination, when compared to each drug used alone. In particular, two replicate, double-blind, randomized, 12-week studies named ANHELTO-1 and ANHELTO-2, tested in COPD patients the efficacy of tiotropium 18 µg once daily, administered through the HandiHaler device, plus olodaterol 5 µg once daily, delivered via the Respimat inhaler, versus tiotropium 18 µg once daily (via HandiHaler) plus placebo (via Respimat).Citation54 Acting together, tiotropium and olodaterol induced, when compared to tiotropium plus placebo, higher increases in trough (pre-dose) FEV1 as well as in FEV1 area under the curve from 0 to 3 hours response (FEV1AUC0–3), and better improvements in health status evaluated by St George’s Respiratory Questionnaire. The efficacy and safety profile of tiotropium/olodaterol combinations administered via a Respimat Soft Mist inhaler is currently being investigated by the so-called TOviTO program.Citation18 This is a network of ten phase 3 studies, involving more than 8,000 COPD patients with moderate to very severe disease. Within the context of the TOviTO program, TOnado-1 and TOnado-2 trials globally evaluated the effects of FDC of tiotropium (2.5 or 5 µg) and olodaterol (5 µg), delivered once-daily through the same Respimat device for 52 weeks, which were compared with the mono-components (tiotropium or olodaterol alone).Citation55,Citation56 At both dosages, tiotropium/olodaterol enhanced trough FEV1 and FEV1AUC0–3, producing significantly greater effects than each single drug. Moreover, the FDC of tiotropium 5 µg/olodaterol 5 µg improved St George’s Respiratory Questionnaire total score at 24 weeks more than either tiotropium or olodaterol. Furthermore, when compared to each monotherapy component throughout the 52-week treatment period, both FDC of tiotropium + olodaterol reduced the daily use of rescue medications, and also induced a trend for decrease in the annual rate of COPD exacerbations. Recently, further valuable information has been extrapolated from a post hoc analysis of these two TOnado studies.Citation57 Hence, FDC tiotropium + olodaterol resulted to be more effective than either mono-therapy in improving lung function across all subgroups of enrolled subjects, including those who had or had not previously received a maintenance treatment with a LAMA or a LABA, as well as patients with different degrees of COPD severity. However, tiotropium/olodaterol evoked higher increases in trough FEV1 and FEV1AUC0–3 among patients with less severe disease. This observation is consistent with a post hoc analysis of the UPLIFT trial, which showed that tiotropium was more efficacious in patients with moderate COPD (GOLD stage 2), who achieved a slower disease progression during such a long, 4-year study.Citation58 Taken together, these considerations about the timing of bronchodilator therapy are very important in light of the well-known concept implying that in COPD a lung function decline proceeds at faster rates during earlier, rather than later disease stages.Citation59,Citation60 This suggests that a maximal, dual-action maintenance bronchodilator treatment should probably be implemented quite precociously throughout the natural history of COPD. Consistently with such a speculation, the post hoc analysis of TOnado trials also indicates that younger patients are more responsive than older ones to the positive effects of FDC tiotropium/olodaterol on FEV1AUC0–3.Citation57 This finding is likely attributable to the better respiratory functional response detectable in less severe patients, rather than to a primary age effect. A timely and optimal bronchodilation could also be useful in those subjects who, though not being susceptible to a rapid worsening of lung function, however, are characterized by a very high risk of developing a long-course COPD because of the occurrence of a FEV1 <80% of predicted value already in their early adulthood.Citation61 In addition to eliciting higher incremental benefits with regard to changes in both trough FEV1 and FEV1AUC from 0 to 24 hours response (FEV1AUC0–24) when compared to monotherapies with either tiotropium or olodaterol, according to an investigational study (VIVACITO) evaluating 24-hour dynamic respiratory function and static pulmonary volumes, FDC of tiotropium (2.5 or 5 µg) and olodaterol (5 µg), delivered once-daily via Respimat inhaler for 6 weeks, also reduced lung hyperinflation more efficiently than each drug used alone.Citation62 Indeed, tiotropium/olodaterol decreased lung residual volume and functional residual capacity to a greater extent than either tiotropium or olodaterol at both peak and trough measurements. VIVACITO is another trial of the TOviTO program. The results of VIVACITO study thus suggest that dual bronchodilation is more effective than each monotherapy in promoting lung emptying, thus implying that tiotropium/olodaterol lowers air trapping better than either single drug. Such findings are very important with regard to the positive actions of bronchodilator treatment on the main COPD symptoms, namely dyspnea and exercise intolerance, which are strongly dependent on lung hyperinflation, rather than on FEV1 decrease. In fact, the reported changes in static lung volumes translate into greater clinical effects, with respect to placebo, achievable by treatment with FDC tiotropium/olodaterol in terms of reduced intensity of breathing discomfort during exercise, assessed by the Borg scale.Citation63 Other ongoing studies are included within the TOviTO program.Citation18 In particular, OTEMTO 1 and 2 are aimed to further assess the clinical and functional outcomes of two different doses of tiotropium/olodaterol, and ENERGITO is a comparative trial which is evaluating tiotropium/olodaterol versus fluticasone propionate/salmeterol. Moreover, TORRACTO, PHYSACTO, and MORACTO 1 and 2 have been designed to assess the effects of tiotropium/olodaterol on exercise tolerance, whereas the aim of DYNAGITO is to evaluate the potential impact of the treatment with tiotropium/olodaterol on COPD exacerbations and survival. In consideration of the current, insufficient available data regarding these key issues for COPD management,Citation64 the results of this latter study will be very important to better elucidate the effects of tiotropium/olodaterol on crucial parameters such as exacerbations and mortality.
A further advantage of the combined use of tiotropium and olodaterol is represented by the device used for this dual bronchodilator therapy. Indeed, Respimat is a soft mist inhaler which delivers these drugs regardless of inspiratory effort and patient coordination.Citation65 Such valuable features allow one to overcome some critical limitations related to the need of hand-breathing coordination, required by pressurized metered dose inhalers, as well as to the necessity of deep inspiration, essential for activation of dry powder inhalers (DPIs). Respimat is portable, propellant-free, does not need a spacer/holding chamber, and it uses mechanical energy for actuation.Citation65 The key technical component of Respimat is “Uniblock,” a combination of filters and nozzles made of silicon and glass, which allows two converging jets of solution to collide at a controlled angle, thus generating a fine aerosol of inhalable droplets.Citation66 The fine mist generated by Respimat makes it possible to deliver small-sized drug particles capable of also reaching peripheral airways, ie, the main site of COPD pathobiology. In fact, it has been shown that Respimat guarantees a high lung deposition of delivered drugs.Citation67 Taken together, these characteristics of Respimat reasonably explain why this device can be satisfactorily used by almost all COPD patients,Citation68 with a consequent increase in their adherence to inhaled treatment.
Safety and tolerability
Overall, the combined bronchodilator treatment with tiotropium/olodaterol is well tolerated and is characterized by a good safety profile in COPD patients. Given together via different devices, tiotropium and olodaterol appeared to be quite safe during the comprehensive evaluation performed within the context of ANHELTO-1 and ANHELTO-2 studies.Citation54 Indeed, the incidence of AEs was considered to be low in both trials. Although in ANHELTO-1 more serious AEs were reported with tiotropium + olodaterol (7.1%) when compared to tiotropium + placebo (4.6%), the incidence of these AEs resulted to be similar in ANHELTO-2 (4.2% and 4.7%, respectively). Also when evaluated as FDC in TOnado-1 and TOnado-2 trials, tiotropium + olodaterol combinations elicited similar patterns of AEs with respect to the individual therapeutic components.Citation55 The majority of reported AEs were mild to moderate in severity. The most common AEs were respiratory events, including upper respiratory infections which were more frequent in tiotropium/olodaterol FDC arms, as well as COPD exacerbations, which instead resulted to be more frequent among patients undergoing treatment with either tiotropium or olodaterol monotherapies. No significant abnormalities in vital signs or laboratory parameters were found in both TOnado-1 and TOnado-2 studies.Citation55 With regard to the subset of patients with cardiovascular comorbidities, similar incidences of major adverse cardiac events were detected across all treatment groups. These observations were also corroborated by the results of VIVACITO trial.Citation62 According to this study, the incidence of AEs was similar among all treatment groups, including tiotropium/olodaterol FDC 2.5/5 µg, tiotropium/olodaterol FDC 5/5 µg, tiotropium monotherapy, olodat-erol monotherapy, and placebo. The most frequent AEs were nasopharyngitis, with incidences ranging from 6.5% to 10.1%, and COPD worsening, with incidences between 5.1% and 12.3%. With regard to each study arm, the incidences of serious AEs were 2.9% for placebo, 5.8% for olodaterol alone, 3.6% for tiotropium 2.5 µg alone, 2.2% for tiotropium 5 µg alone, 2.9% for tiotropium/olodaterol FDC 2.5/5 µg, and 0.7% for tiotropium/olodaterol FDC 5/5 µg. No safety alarms were detected with regard to vital signs such as heart rate and arterial blood pressure.Citation62 With regard to COPD cardiovascular comorbidities and patient survival, it is noteworthy that tiotropium HandiHaler has been shown to exert beneficial effects.Citation25,Citation69 However, tiotropium could increase the risk for tachyarrhythmias and death in subjects with either unstable cardiac disease or moderate to severe chronic kidney disease.Citation70 Of course, these particular problems should be considered with careful attention also in regard to the eventual use of tiotropium/olodaterol combinations.
Conclusion
Tiotropium/olodaterol is a novel LAMA/LABA combination recently developed within the evolving scenario of COPD treatment. This innovative bronchodilator strategy exploits the reciprocal potentiation of the different mechanisms of action activated by LABA and LAMA. In particular, COPD patients could usefully benefit from the recently discussed synergistic interaction between LAMA and LABA,Citation71 which can obviously provide higher advantages with respect to a simple additive cooperation. Indeed, the resulting optimization and maximization of bronchodilation makes it possible to achieve better improvements in subjective symptoms, lung function, and overall quality of life, eventually associated with clinically relevant decreases in the annual rates of COPD exacerbations. Acting together tiotropium and olodaterol provide a powerful and persistent bronchodilation, responsible for an effective lung deflation which guarantees relevant therapeutic benefits with special regard to improvements in dyspnea and exercise tolerance. In particular, the rapidly acting and long-lasting LABA olodaterol represents the ideal partner for tiotropium, the most studied and tested LAMA in both controlled trials and clinical practice. Indeed, the addition of olodaterol to tiotropium allows one to overcome the only limit of such a highly valuable LAMA, ie, its relatively late onset of action. Furthermore, once-daily delivery of tiotropium/olodaterol FDC via a very efficient and simple-to-use inhaler device such as Respimat significantly contributes to enhance the therapeutic efficacy of dual bronchodilation, as well as to increase patient adherence to inhaled treatment.
Disclosure
G Pelaia received lecture fees and consultancy fees from Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi, Dompè, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis. A Vatrella received lecture fees and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Dompè, GlaxoSmithKline, Guidotti-Malesci, Mundipharma, Novartis. R Maselli received lecture fees and consultancy fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Guidotti-Malesci, Mundipharma, Novartis. MT Busceti, L Gallelli, C Calabrese, R Terracciano, and N Lombardo report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- CazzolaMPageCPCalzettaLMateraMGPharmacology and therapeutics of bronchodilatorsPharmacol Rev201264345050422611179
- ColeridgeHMColeridgeJCSchultzHDAfferent pathways involved in reflex regulation of airway smooth musclePharmacol Ther19894211632657805
- GronebergDAQuarcooDFrossardNNeurogenic mechanisms in bronchial inflammatory diseasesAllergy200459111139115215461593
- GrossNJCoESkorodinMSCholinergic bronchomotor tone in COPD: estimates of its amount in comparison with that in normal subjectsChest19899659849872805869
- HallIPSecond messengers, ion channels and pharmacology of airway smooth muscleEur Respir J20001561120112710885434
- PelaiaGRendaTGallelliLMolecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthmaRespir Med200810281173118118579364
- BerridgeMJInositol trisphosphate and calcium signalingNature199336164103153258381210
- SomlyoAPSomlyoAVSignal transduction and regulation in smooth muscleNature199437265032312367969467
- ProfitaMGiorgiRDSalaAMuscarinic receptors, leukotriene B4 production, and neutrophilic inflammation in COPD patientsAllergy200560111361136916197467
- CasarosaPKiechleTSiegerPThe constitutive activity of the human muscarinic M3 receptor unmasks differences in the pharmacology of anticholinergicsJ Pharmacol Exp Ther2010333120120920035022
- ChibaYMurataMUshikuboHEffect of cigarette smoke exposure in vivo on bronchial smooth muscle contraction in vitro in ratsAm J Respir Cell Mol Biol200533615741581
- HottaKEmalaCWHirshmanCATNF-α up-regulates Giα and Gqα protein expression and function in human airway smooth muscle cellsAm J Physiol19992763 Pt 1L405L41110070103
- BarnesPJBiochemical basis of asthma therapyJ Biol Chem201128638328993290521799015
- DisseBSpeckGARomingerKLTiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung diseaseLife Sci1999646–745746410069510
- BarnesPJThe pharmacological properties of tiotropiumChest20001172 Suppl63S66S10673478
- CazzolaMPageCPRoglianiPMateraMGβ2-Agonist therapy in lung diseaseAm J Respir Crit Care Med2013187769069623348973
- MuruganandanSJayaramLProfile of a fixed-dose combination of tiotropium/olodaterol and its potential in the treatment of COPDInt J Chron Obstruct Pulmon Dis2015101179118926124657
- TautermannCSKiechleTSeeligerDMolecular basis for the long duration of action and kinetic selectivity of tiotropium for the muscarinic M3 receptorJ Med Chem201356218746875624088171
- CasarosaPBouyssouTGermeyerSPreclinical evaluation of long-acting muscarinic antagonists: comparison of tiotropium and investigational drugsJ Pharmacol Exp Ther2009330266066819478135
- KistemakerLEMGosensRAcetylcholine beyond bronchoconstriction: roles in inflammation and remodelingTrends Pharmacol Sci201536316417125511176
- ScullionJEThe development of anticholinergics in the management of COPDInt J Chron Obstruct Pulmon Dis200721334018044064
- RestrepoRDUse of inhaled anticholinergic agents in obstructive airway diseaseRespir Care200752783385117594728
- AnzuetoATashkinDMenjogeSOne-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropiumPulm Pharmacol Ther2005182758115649848
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- CelliBZuWallackRWangSImprovement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumesChest200312451743174814605043
- O’DonnellDEFlugeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J200423683284015218994
- WedzichaJADecramerMSeemungalTAThe role of bronchodilator treatment in the prevention of exacerbations of COPDEur Respir J20124061545155422835613
- WilkinsonTMHurstJRPereraWREffect of interactions between lower airway bacterial and rhinoviral infections in exacerbations of COPDChest2006129231732416478847
- BatemanEDRennardSBarnesPJAlternative mechanisms for tiotropiumPulm Pharmacol Ther200922653354219635581
- MeursHDekkersBGJMaarsinghHMuscarinic receptors on airway mesenchymal cells: novel findings for an ancient targetPulm Pharmacol Ther201326114515522842340
- PapiABellettatoCMBraccioniFInfections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med2006173101114112116484677
- BuhlingFLiederNKuhlmannUCTiotropium suppresses acetylcholine-induced release of chemotactic mediators in vitroRespir Med2007101112386239417761412
- SantusPBuccellatiCCentanniSBronchodilators modulate inflammation in chronic obstructive pulmonary diseasePharmacol Res201266434334822659487
- WedzichaJACalverleyPMSeemungalTAThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med20081771192617916806
- VogelmeierCHedererBGlaabTTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med2011364121093110321428765
- DecramerMLChapmanKRDahlROnce-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomized, blinded, parallel-group studyLancet Respir Med20131752453324461613
- BouyssouTCasarosaPNalineEPharmacological characterization of olodaterol, a novel inhaled β2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical modelsJ Pharmacol Exp Ther20103341536220371707
- CasarosaPKollakIKiechleTFunctional and biochemical rationales for the 24-hour-long duration of action of olodaterolJ Pharmacol Exp Ther2011337360060921357659
- van NoordJASmeetsJJDrenthBM24-hour bronchodilation following a single dose of the novel β2-agonist olodaterol in COPDPulm Pharmacol Ther201124666667221839850
- Maleki-YazdiMRBeckEHamiltonAMA randomised, placebo-controlled, Phase II, dose-ranging trial of once-daily treatment with olodaterol, a novel long-acting β2-agonist, for 4 weeks in patients with chronic obstructive pulmonary diseaseRespir Med2015109559660525829298
- FergusonGTFeldmanGJHofbauerPEfficacy and safety of olodaterol once daily delivered by Respimat in patients with GOLD 2–4 COPD: results from two replicate 48-week studiesInt J Chron Obstruct Pulmon Dis2014962964524966672
- KochAPizzichiniEHamiltonALung function efficacy and symptomatic benefit of olodaterol once daily delivered by Respimat in patients with GOLD 2–4 COPD: results from two replicate 48-week studiesInt J Chron Obstruct Pulmon Dis2014969771425045258
- FeldmanGJBernsteinJAHamiltonAThe 24-h FEV1 time profile of olodaterol once daily via Respimat and formoterol twice daily via Aerolizer in patients with GOLD 2–4 COPD: results from two replicate 6-week crossover studiesSpringerPlus2014341925187881
- JoosJFAumannJLCoeckCA randomised, double-blind, four-way, crossover trial comparing the 24-h FEV1 profile for once-daily versus twice-daily treatment with olodaterol, a novel long-acting β2-agonist, in patients with chronic obstructive pulmonary diseaseRespir Med2015109560661525776199
- RoskellNSAnzuetoAHamiltonAOnce-daily long-acting β-agonists for chronic obstructive pulmonary disease: an indirect comparison of olodaterol and indacaterolInt J Chron Obstruct Pulmon Dis2014981382425114521
- McGarveyLNiewoehnerDMagderSOne-year safety of olodaterol once daily via Respimat in patients with GOLD 2–4 chronic obstructive pulmonary disease: results of a pre-specified pooled analysisCOPD201510.3109/15412555.2014.991864
- HoldenNSBellMJRiderCFβ2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoidsProc Natl Acad Sci U S A201110849197131971822080612
- PelaiaGMarsicoSARegulation of β2-adrenergic receptors and the implications for bronchial asthma: an updateMonaldi Arch Chest Dis19944921251308049696
- ChuangTTIacovelliLSalleseMG protein-coupled receptors: heterologous regulation of homologous desensitization and its implicationsTrends Pharmacol Sci199617114164218990958
- PennRBEmbracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthmaNaunyn Schmiedebergs Arch Pharmacol2008378214916918278482
- MateraMGPageCVCazzolaMNovel bronchodilators for the treatment of chronic obstructive pulmonary diseaseTrends Pharmacol Sci201132849550621683458
- BarnesPJDistribution of receptor targets in the lungProc Am Thorac Soc20041134535116113456
- ZuWallackRAllenLHernandezGEfficacy and safety of combining olodaterol Respimat and tiotropium HandiHaler in patients with COPD: results of two randomized, double-blind, active controlled studiesInt J Chron Obstruct Pulmon Dis201491133114425342898
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combinations versus mono-components in COPD (GOLD 2–4)Eur Respir J201545496997925573406
- RabeKFTreatment of COPD and the TOnado trial: a tempest in a teapot?Eur Respir J201545486987125829428
- FergusonGTFlezarMKornSEfficacy of tiotropium + olodaterol in patients with chronic obstructive pulmonary disease by initial severity and treatment intensity: a post hoc analysisAdv Ther201532652353626112656
- DecramerMCelliBKestenSEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomized controlled trialLancet200937496961171117819716598
- BridevauxP-OGerbaseMWProbst-HenschNMLong-term decline in lung function, utilization of care and quality of life in modified GOLD stage 1 COPDThorax200863976877418505800
- VestboJEdwardsLDScanlonPDChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med2011365131184119221991892
- LangePCelliBAgustiALung-function trajectories leading to chronic obstructive pulmonary diseaseN Engl J Med2015373211112226154786
- BeehK-MWestermanJKirstenA-MThe 24-hour lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary diseasePulm Pharmacol Ther201532535925956072
- MaltaisFIturriJGKirstenAEffects of 12 weeks of once-daily tiotropium and olodaterol fixed-dose combination on exercise endurance in patients with COPDThorax201469Suppl 2A186A187
- CazzolaMRoglianiPOraJMateraMGOlodaterol + tiotropium bromide for the treatment of chronic obstructive pulmonary diseaseExpert Rev Clin Pharmacol20158552953926294073
- BrandPHedererBAustenGHigher lung deposition with Respimat soft mist inhaler than HFA-MDI in COPD patients with poor techniqueInt J Chron Obstruct Pulmon Dis20083476377019281091
- DalbyRNEicherJZierenbergBDevelopment of Respimat soft mist inhaler and its clinical utility in respiratory disordersMed Devices20114145155
- PitcairnGReaderSPaviaDDeposition of corticosteroid aerosol in the human lung by Respimat soft mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhalerJ Aerosol Med200518326427216181001
- DekhuijzenPNRVinckenWVirchowJCPrescription of inhalers in asthma and COPD: towards a rational, rapid and effective approachRespir Med2013107121817182124120398
- MathioudakisAGKanavidisPChatzimavridou-GrigoriadouVTiotropium HandiHaler improves the survival of patients with COPD: a systematic review and meta-analysisJ Aerosol Med Pulm Drug Deliv2014271435023521168
- MathioudakisAGMastorisIChatzimavridou-GrigoriadouVThe risk of tachyarrhythmias in patients with moderate-to-severe chronic kidney disease receiving tiotropium bromideInt J Cardiol201519710510626142194
- CalzettaLMateraMGCazzolaMPharmacological interaction between LABAs and LAMAs in the airways: optimizing synergyEur J Pharmacol201576116817325981302
