Abstract
Acute pancreatitis is most commonly attributed to gallstones, alcohol abuse, and metabolic disorders such as hyperlipidemia and hypercalcemia. Medications are an infrequent yet commonly overlooked etiology of pancreatitis. Although several drugs have been implicated, antidiabetic agents are a rare cause for drug-induced pancreatitis. Canagliflozin is a new drug in the class of SGLT-2 inhibitors used for the treatment of type 2 diabetes mellitus. Serious reported side effects include renal impairment, hyperkalemia, and hypotension. Pancreatitis as a result of canagliflozin, however, is exceedingly rare. Here we describe a case of a 33-year old female who presented with severe acute pancreatitis in the setting of recent initiation of canagliflozin. Given the timing of her presentation and after excluding all other possible etiologies, it was determined that canagliflozin was the likely source of her illness. This case highlights the importance of identifying drug-induced pancreatitis, especially in novel drugs, as it is commonly neglected in patients with multiple medical comorbidities and those taking numerous medications. Prompt identification of drug-induced pancreatitis can improve management as well as decrease morbidity and mortality in these individuals.
Introduction
Acute pancreatitis (AP) is an inflammatory condition of the pancreas characterized clinically by intense epigastric pain radiating to the back along with elevated levels of pancreatic enzymes in the blood. Although pancreatitis is a leading cause of hospitalization in the United States,Citation1 the pathogenesis for this condition is not fully understood. Nevertheless, various etiologies have been shown to increase the risk for and to cause pancreatitis.
Gallstones are the most common cause for AP, accounting for 35%–40% of cases worldwide, and together with alcohol, and metabolic disorders such as hyperlipidemia and hypercalcemia make up around 90% of all cases.Citation2 Medications are infrequently associated with pancreatitis with a reported incidence of only 0.1%–2%Citation3,Citation4 although several drugs have been implicated including diuretics, didanosine, tetracycline, sulfonamides, and steroids, among others.
Diabetic medications have rarely been associated with pancreatitis with the majority of reported cases involving DPP-4 inhibitors (sitagliptin, saxagliptin) and GLP-1 receptor antagonists (exenatide, liraglutide).Citation5 Canagliflozin is a new drug in the class of sodium-glucose cotransporter-2 (SGLT-2) inhibitors used for the treatment of type 2 diabetes mellitus (T2DM). Here we describe a patient who developed a severe manifestation of AP soon after initiation of canagliflozin therapy.
Case presentation
A 33-year old African–American female presented to the emergency room with a 2-day history of progressively worsening nausea, vomiting, and severe abdominal pain. The patient denied any cardiovascular, respiratory, or urinary symptoms nor had she been around any sick contacts. The patient denied any abdominal surgeries or prior history of gallstones, dyslipidemia or pancreatitis. She denied any alcohol, cigarette use, or illicit drug use. She had a history of diabetes mellitus, hypertension and hypothyroidism; however, there was no family history of autoimmune conditions or AP. Her home medications included long-term metformin and levothyroxine. In addition, the patient recently began canagliflozin therapy 2 weeks prior to her admission.
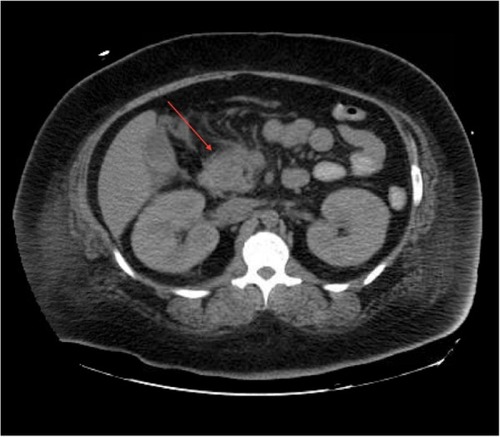
In the emergency department, she was found to be hypotensive with a blood pressure of 79/36, heart rate of 118 bpm, and a respiratory rate of 27. Her temperature was 40.3 degrees Celsius. Serum chemistries revealed leukocytosis with a white blood cell count of 23.6×103/mm3, creatinine of 3.19 mg/dL, calcium of 9.3 mg/dL, amylase 535 IU/L, and lipase 373 IU/L. HbA1c was recorded as 13.5% and her triglyceride level was within normal limits. Additionally, she displayed an elevated anion gap of 19 and acidosis with a pH of 6.89 with blood glucose level of 563 mg/dL and positive urine ketones (beta-hydroxybutyrate: 2.90). Subsequent arterial blood gas revealed PCO2 of 48.8 and FiO2 of 100. Maximum blood alcohol was negative. She was initially diagnosed with diabetic ketoacidosis (DKA) and given several liters of normal saline followed by a bicarbonate and insulin drip. Urine and blood cultures were drawn and she was started on broad-spectrum antibiotics for her fever and leukocytosis. A computerized tomography (CT) scan of the abdomen was obtained due to the elevated pancreatic enzymes and it showed evidence of AP with peripancreatic inflammation and ascites (). Chest X-ray additionally revealed bilateral pleural effusions. Echocardiogram though was unremarkable.
Despite aggressive fluid resuscitation, the patient became progressively more hypotensive and became unresponsive. She was started on a norepinephrine drip, intubated and admitted to the intensive care unit. Her renal function deteriorated with decreased urine output and increasing serum creatinine reaching 4.26 (pre-morbid creatinine: 1.0) with persistent acidemia requiring continuous renal replacement therapy. In the intensive care unit, an APACHE (Acute Physiology and Chronic Health Evaluation) II score was calculated for the patient and revealed an estimated mortality rate of 97.2%.
Despite the poor prognosis the patient’s condition remarkably improved and vasopressors were discontinued and she was successfully extubated after 4 days. The source of leukocytosis was indeterminate as cultures of blood, urine, and mini bronchoalveolar lavage were all negative. After excluding all other causes for her symptoms and given the chronology of her presentation, it was determined that canagliflozin was likely the source of her illness. Canagliflozin was discontinued and the patient has remained stable since discharge.
Discussion
While metformin has been widely accepted as the initial drug of choice for T2DMCitation6 numerous second-line classes of diabetic medications exist for clinicians to choose from, with individual choice determined largely by factors including cost and risk for adverse effects. Canagliflozin is the first SGLT-2 inhibitor approved by the FDA (US Food and Drug Administration) for the treatment of T2DM.Citation7
Normally, plasma glucose is freely filtered by the glomerulus and reabsorbed in the proximal renal tubule, with a small amount excreted directly in the urine. Ninety percent of renal glucose reabsorption occurs in the proximal tubule as a result of SGLT-2.Citation8 Canagliflozin works by inhibiting SGLT-2, causing a dramatic reduction in the kidneys’ capacity to reabsorb glucose, thus leading to a decrease in plasma glucose levels.Citation9 Clinical studies have shown remarkable efficacy with this drug. The Canagliflozin Treatment and Trial Analysis-Monotherapy Trial demonstrated a baseline HbA1c reduction by 0.91%–1.16%, a reduction in systolic blood pressure and body weight, and an increased high-density lipoprotein level.Citation10 Additionally, studies have also shown a 0.81%–0.93% decrease in HbA1c when canagliflozin was given in conjunction with metformin.Citation11
The most common side effects of canagliflozin are urinary tract infections and genital mycotic infections, which occur more frequently in women. Additional potential side effects include nausea, renal impairment, hyperkalemia, and hypoglycemia. Pancreatitis as a result of canagliflozin is exceedingly rare, with an incidence rate of only 2.7 per 1,000 patient-years.Citation12
The exact mechanism of canagliflozin-induced pancreatitis is currently unclear; however, the underlying pathogenesis is unlikely to differ from other causes of AP. In fact, the majority of drug-induced pancreatitis (DIP) appears to be idiosyncratic in nature.Citation13 Idiosyncratic drug reactions are adverse effects not directly related to pharmacodynamic drug mechanisms. These spontaneous and unpredictable adverse complications are a result of abnormal interactions between specific drugs and the patient themselves, and are typically mediated by immunologic or cytotoxic effects triggered by the drug or its metabolites on a specific organ system.Citation14
Nevertheless, DIP is a serious complication and very easily overlooked in patients with multiple comorbidities, those on multiple medications, or those with potential underlying risk factors such as gallstones or alcohol abuse.
We believe this is the second reported incidence of canagliflozin-induced pancreatitis as a PubMed literature search for several variations of “canagliflozin” and “pancreatitis” revealed only one additional case.Citation15 Our case is further unique in that pancreatitis was also associated with acute kidney injury. It remains to be seen whether kidney injury was a sequela of the pancreatitis or a simultaneous additional side effect of canagliflozin. It is important to note that the patient had been on long-term metformin and levothyroxine when this occurred, albeit without a history of pancreatitis or other side effects. Several case reports have suggested a possible role of metformin in causing AP, specifically during periods of renal insufficiency.Citation16,Citation17 Therefore, it is possible that metformin also played a role in precipitating this episode of AP.
Special care must also be given to patients who present with DKA and concurrent abdominal pain. Nair et alCitation18 reported an association between DKA and AP, though the pathogenesis primarily involved transient hyperlipidemia rather than DIP. Regardless, DKA may mask a coexisting AP in 10%–15%.Citation18 The key treatment for DIP is early recognition, exclusion of all other etiologies, and discontinuation of the possible causative medication followed by supportive therapy.
In our case, the adverse event occurred within a reasonable period of drug administration. Laboratory data and radiological imaging confirmed the diagnosis of AP. Additionally, there were no other changes to the patient’s drug regimen in the weeks preceding pancreatitis aside from the addition of canagliflozin. Furthermore, the event was not attributable to any other etiology, as our patient did not have a history of recent infection, gallstones, hypertriglyceridemia, or alcohol intake. Finally, there was no recurrence of pancreatitis following discontinuation of canagliflozin. Therefore, according to the Naranjo probability scale, the adverse event was most likely related to canagliflozin.Citation19
Conclusion
As the prevalence of diabetes in the United States increases, the amount of new medications designed to combat it will increase as well. Our case illustrates the importance of being aware of new antidiabetic drugs such as canagliflozin as potential treatment options for T2DM as well as remaining up to date on potential side effects and drug interactions of these newer agents. DIP is an uncommon yet potentially serious complication of canagliflozin. It remains to be seen whether this is as a result of canagliflozin monotherapy or due to an interaction with metformin in the setting of acute kidney injury. Regardless, DIP may easily be overlooked in patients due to its low incidence, and confounded by the presence of comorbidities, and potential of multiple medication use. Thus it is imperative for clinicians to keep a high index of suspicion in order to prevent morbidity and mortality from disease progression.
Acknowledgments
No outside funding was utilized in this study.
Disclosure
The authors declare that there is no conflict of interests in this work.
References
- PeeryAFDellonESLundJBurden of gastrointestinal disease in the United States: 2012 updateGastroenterology2012143511791187e1322885331
- ForsmarkCEBaillieJAGA Institute Clinical Practice and Economics Committee; AGA Institute Governing BoardAGA Institute technical review on acute pancreatitisGastroenterology200713252022204417484894
- SpanierBWTuynmanHAvan der HulstRWDijkgraafMGBrunoMJAcute Pancreatitis and Concomitant Use of Pancreatitis-Associated DrugsAm J Gastroenterol2011106122183218821912439
- BalaniARGrendellJHDrug-induced pancreatitis: incidence, management and preventionDrug Saf2008311082383718759507
- CohenDReports of pancreatitis are 20–30 times more likely with GLP-1 drugs, analysis findsBMJ2013346f260723613543
- American Diabetes AssociationStandards of medical care in diabetes – 2014Diabetes Care201437Suppl 1S14S8024357209
- US Food and Drug AdministrationFDA approves Invokana to treat type 2 diabetes [press release]US Food and Drug Administration2013 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htmAccessed February 22, 2015
- ChaoECHenryRRSGLT2 inhibition-a novel strategy for diabetes treatmentNat Rev Drug Discov20109755155920508640
- ShaSDevineniDGhoshACanagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjectsDiabetes Obes Metab201113766967221457428
- StenlofKCefaluWTKimKAEfficacy and safety of Canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exerciseDiabetes Obes Metab201315437238223279307
- ValentineVHinnenDClinical implications of canagliflozin treatment in patients with type 2 diabetesClin Diabetes201533151325653467
- Invokana® (canagliflozin) [prescribing information]Titusville, NJJanssen Pharmaceuticals, Inc2014
- HungWYAbreu LanfrancoOContemporary review of drug-induced pancreatitis: A different perspectiveWorld J Gastrointest Pathophyiol201454405415
- BadalovNBaradarianRIswaraKLiJSteinbergWTennerSDrug-induced acute pancreatitis: an evidence-based reviewClin Gastroenterol Hepatol20075664865117395548
- VermaRCanagliflozin-Associated Acute PancreatitisAm J Ther Epub 2014 Sep 2
- FimognariFLCorsonelloAPastorellRAntonelli-IncalziRMetformin-induced pancreatitisDiabetes Care2006295118316644670
- AlsubaieSAlmalkiMHMetformin induced acute pancreatitisDermatoendocrinol20135231731824194972
- NairSYadavDPitchumoniCSAssociation of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKAAm J Gastroenterol200095102795280011051350
- NaranjoCABustoUSellersEMA method for estimating the probability of adverse drug reactionsClin Pharmacol Ther19813022392457249508