Abstract
Objective
Smoking has been associated with tuberculosis (TB); however, the effects of smoking on the effectiveness of TB treatment remain unclear.
Materials and methods
Data were retrieved from case notes and interviews of subjects registered in the TB-reporting system from 2010 to 2012. Study cases were defined as subjects with TB-positive sputum cultures, whereas the controls were defined as subjects with non-TB-related pulmonary diseases. Statistical analyses included logistic regression and multivariate Cox proportional hazard regression models.
Results
A total of 245 cases with cultures positive for TB and 114 controls with non-TB-related pulmonary diseases and negative sputum cultures were recruited. Current smokers had the highest failure rate (33%) for TB treatment, and they had the most severe pulmonary lesions based on chest X-ray grading. Current smokers had a 1.36-fold (95% confidence interval 1.03–2.36, P<0.05) higher odds ratio for cultures positive for TB compared with nonsmokers. In subjects with TB-positive cultures, current smoking was associated with an increase in treatment days required for cultures to convert from positive to negative (hazard ratio 1.12, 95% confidence interval 1.03–1.39; P<0.05).
Conclusion
Longer periods of treatment may be required for TB patients who are current smokers.
Introduction
Tuberculosis (TB) is a communicable disease, widespread throughout the world. The World Health Organization (WHO) estimated that there were 8.6 million TB cases, 1.3 million TB deaths, and an estimated 450,000 people who had developed multidrug-resistant TB in 2013.Citation1 With regard to treatment outcomes, the WHO reported that the success rate of treating TB was 87% among all new TB cases in 2011. In Taiwan, TB has accounted for the highest incidence and mortality rate among all communicable diseases for many years. In 2012, for example, there were 12,338 TB cases and 626 TB-related deaths in Taiwan. Although the annual case numbers and incidence rate of TB in Taiwan have been decreasing, the success rate of treating TB is still below the world average reported by the WHO.Citation1
The numbers of smokers have been continuously increasing in both developing and developed countries during the past several decades, and smoking has been associated with numerous diseases. For example, increasing evidence shows an association between smoking and TB. The correlation suggests that smoking is an important risk factor for the development of TB. Additionally, the risk of mortality from TB is higher in smokers. The possible mechanisms underlying these effects on TB infection in response to cigarette smoke include 1) dysfunctional mucociliary clearance, 2) decreased alveolar macrophage activity, 3) immunosuppression of the pulmonary lymphocytes, 4) inactive natural killer cells, and 5) dysfunctional pulmonary dendritic cells. Previous studies have shown that the severity of pulmonary lesions in TBCitation2,Citation3 and the TB-recurrence rateCitation2 are related to smoking. Therefore, smoking cessation represents an important factor in controlling TB epidemics.Citation4 The objective of the present study was to investigate possible differences between TB-culture conversion (≥180 days) in current smokers compared to nonsmokers and former smokers.
Materials and methods
Study population
This investigation was a retrospective case-control study of cases reported between January 1, 2010 and December 31, 2012. The data were taken from 511 cases in the registry of the TB-reporting system in Taipei Medical University Shuang Ho Hospital in New Taipei (Taiwan). We included patients with TB-positive sputum cultures, of both sexes, aged ≥16 years, whose treatment began during the period of study. An age-matched clinically selected control population was recruited from patients who had visited the chest clinic but who had been diagnosed with conditions other than TB. Subjects for whom the mandatory report was incomplete and for whom the missing data could not be retrieved were excluded. The diagnosis of TB was conducted using sputum cultures according to diagnostic standards.Citation5 Sputum samples from the control subjects were also collected and cultured with negative results. The diagnosis of TB was reviewed at the beginning and end of the TB treatment, and cases for which TB was ruled out by the attending physician were excluded. The duration of sputum-culture conversion from positive to negative was calculated in days. The treatment procedure was the same for all the TB-positive subjects. Personal, clinical, and epidemiological data were obtained from the patients’ clinical progress records. The microbiological laboratory data and medical records of the patients were reviewed.
Patients who smoked at the time of TB diagnosis were considered smokers (≥3 months’ current smoking). Subjects who had quit smoking at least 3 months prior to diagnosis were classified as former smokers, and those who had never smoked were classified as nonsmokers. This study was approved by the Taipei Medical University Joint Institutional Review Board. All patient records were anonymized and deidentified prior to analysis. The methods were carried out in accordance with the approved guidelines. All subjects received written and oral information prior to inclusion and provided informed consent.
Grading of chest X-rays
To quantify the severity of lesions observed in chest X-ray (CXR) images of the TB patients, a scoring method was applied to the present study.Citation6 Standard full-size posteroanterior CXRs were obtained at the time of TB diagnosis. Briefly, the presence of nodules (1–2 mm or >2 mm), patchy or confluent consolidation, cavitation (mm), bronchial lesions or fibrosis, effusion, and lymphadenopathy were recorded in each lung (upper, mid-, or lower zones). Additionally, pulmonary effusion, lymphadenopathy, and the total percentage of each lung affected by any pathology was estimated. To grade the percentage of the lungs that were affected, a visual estimation of the extent of opacification, cavitation, or other pathology as a percentage of the visible lung was conducted. Dense and patchy opacification of a zone was graded. Dense opacification of a zone was graded as 100%, whereas patchy opacification within a zone resulted in scores <100% depending on the extent of opacification. The severity of TB CXRs was blindly scored by two chest physicians (KYL and PHF).
Statistical analysis
A logistic regression model was used to assess the associated risk of smoking in subjects with TB-positive cultures compared with that in subjects with TB-negative cultures. This model produced estimates of the odds ratios and the corresponding 95% confidence intervals (CIs). Multivariate Cox proportional hazard regression was used to assess the hazard ratios and the corresponding 95% CIs for those with positive sputum cultures relative to those with negative cultures. For comparisons among multiple values, one-way analysis of variance with Dunnett’s post hoc test was used. All statistical analyses for this study were performed using SPSS 15.0 software (SPSS Inc, Chicago, IL, USA). The significance criterion was set at P<0.05.
Results
There were 511 subjects included in the registry during the period of study from 2010 to 2012; 245 TB cases eligible for inclusion in the present study were recorded. In this study, we enrolled a ratio of control-group (other pulmonary disease; culture-negative) to TB-group (culture-positive) individuals of 1:2. The demographic characteristics of subjects with TB-negative (n=114) and TB-positive cultures (n=245) are presented in . The percentage of smokers was similar (27%) between the case and control groups, whereas the nonsmokers accounted for 58% of the group with TB-negative cultures and 60% of the group with TB-positive cultures. Clinical and diagnostic characteristics indicated that no HIV coinfections were present at the time of diagnosis, and 26% presented with pulmonary cavitary lesions on CXR. The CXR grade of the group with TB-positive cultures was 33. The number of days for positive cultures to become negative in the TB subjects was recorded: the average was 242 days (median 225 days) and the range was 32–773 days.
Table 1 Demographic characteristics of subjects with negative (n=114) and positive (n=245) cultures from 2010 to 2012
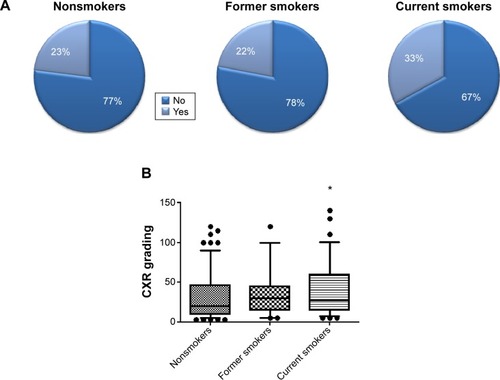
shows the treatment outcomes of patients with TB-positive cultures. The success rates of nonsmokers, former smokers, and current smokers were 77%, 78%, and 67%, respectively. The current smokers had the highest failure rate (33%) of TB treatment. We then further investigated the median days of culture conversion in the nonsmokers, former smokers, and current smokers, which were 239, 275, and 272 days, respectively. illustrates the CXR grades of the nonsmokers, former smokers, and current smokers among the TB subjects. Within this group, current smokers had significantly higher CXR grades than nonsmokers (P<0.05). No difference was observed between nonsmokers and former smokers.
Figure 1 Smoking and the success rate of treating tuberculosis and CXR grading.
shows that the subjects with TB-positive cultures who were current smokers had an odds ratio of 1.36 (95% CI 1.03–2.36, P<0.05) relative to nonsmokers, after adjusting for age, sex, and body mass index. TB subjects who were former smokers had an odds ratio of 1.00 (95% CI 0.71–1.89, P=0.343) relative to never-smokers. We further investigated the association between smoking and days for the sputum cultures to convert from positive to negative, as shown in . In subjects with TB-positive cultures, we observed an association between being a current smoker and an increased risk of a longer duration of culture conversion (hazard ratio 1.12, 95% CI 1.03–1.39; P<0.05). There was no association observed between nonsmokers and former smokers.
Table 2 Estimated ORs (95% CI) of the risk for tuberculosis patients with positive cultures among former smokers and current smokers
Table 3 Hazard ratios of sputum-culture conversion duration (days) in subjects with tuberculosis-positive cultures
Discussion
In this study, an association between current smoking and TB was revealed. These results were consistent with other studies.Citation2,Citation7–Citation9 TB patients who were current smokers had a higher proportion of treatment failure (33%) and higher severity of pulmonary lesions based on scores of the CXR-grading process. These findings may be related to a smoking-related alteration in the immunity of the TB patients, suggesting the possibility that smoking is a risk factor for adverse TB outcomes. Importantly, we showed that TB patients who were current smokers had a longer period of sputum-culture conversion.
In accordance with the WHO DOTS (Directly Observed Treatment, Short-course) program,Citation10 Taiwanese policies target an 85% success rate of TB treatment.Citation2 In the Centers for Disease Control report for Taiwan in 2012,Citation3 new cases with TB-positive sputum cultures accounted for 36% of all new cases. Testing the mucus from the lungs in TB patients provides the best and most accurate diagnosis for active TB. To confirm our hypothesis that cigarette smoke influences the number of treatment days required for sputum cultures to convert from positive to negative, subjects with TB-positive sputum cultures were therefore recruited for the study group. Within this group, the success rates of TB treatment were 77% in never-smokers and 78% in former smokers, whereas the rate was only 67% in current smokers. These treatment-success rates of TB cases with positive sputum cultures were similar to the 66% recorded in 2010 in Taiwan after 12 months of follow-up.Citation3 Both our results and the 2010 Taiwan report demonstrated average outcomes below the WHO target rate. The difference could be partially explained by TB-related risk factors, such as smoking. In the present study, there was a strong association between having TB-positive culture and being a current smoker. This association has also been previously reported.Citation2,Citation7–Citation9 Furthermore, smoking has been related to an increased risk of recurrence after successful anti-TB treatment.Citation2 Yen et al showed that subjects with a current smoking rate of more than ten cigarettes/day had twice the risk of never- and former smokers.Citation2 There was no difference between nonsmokers and former smokers among subjects with TB-positive cultures, which is consistent with the previous report by Leung et al.Citation8 However, the effects of smoking on TB-culture conversion remain unclear.
Smoking has been reported to be harmful to human lungs, and to increase mortality and morbidity.Citation11 We observed that the pulmonary lesions in current-smoker TB subjects were more severe, as measured by such criteria as treatment outcome and CXR grading. Cigarette consumption is likely to increase the risk of mortality and morbidity in both high- and low-income countries. For example, the risk of TB death is approximately 4.5 times higher in smokers than nonsmokers,Citation12 and smokers account for 61% of TB mortalities.Citation12 Additionally, smoking was a factor associated with treatment failure and was a predictive factor of death.Citation13 Notably, we observed an approximately 1.5-fold higher failure rate of TB treatment in current smokers compared to nonsmokers and former smokers. Treatment failure was mainly due to death during the treatment period. If survival is associated with smoking in TB patients, the pulmonary pathology may be more severe in smokers. In the present study, CXR grades in current smokers were significantly higher than in nonsmokers, suggesting that smoking caused the increased severity of TB lesions in the lungs. This concept is supported by previous work.Citation7,Citation11 Together, these results indicate that TB treatment-success rates, survival, and lung lesions are influenced by cigarette smoking. All the evidence implies that smoking may be associated with the efficacy of TB treatment.
If smoking causes faster development of TB lesions, then smoking may alter disease progress in TB patients. No difference has been observed in the time of diagnosis between smokers and nonsmokers with TB.Citation7 A previous report indicated that in 287 new subjects with symptomatic TB, smoking was not identified as a predictive factor in any of the components of diagnostic delay.Citation14 The time between the appearance of TB symptoms and the initiation of anti-TB treatment was similar in smokers and nonsmokers, but smokers presented with more severe lesions. Strikingly, we observed an association between current smoking and the number of days required for sputum-culture conversion. This finding suggests that smoking may prolong the required days in TB patients with positive cultures. The effects of smoking on disease progress have also been investigated in other diseases. For example, some of the chemical compounds in cigarette smoke, such as polycyclic aromatic hydrocarbons, can induce key drug-metabolizing enzymes, including cytochrome P450 and isoforms of the glucuronyltransferase families,Citation15 which can modulate drug pharmacokinetics and efficacy in lung cancer treatment. Laki et al suggested that the identification of patients’ smoking history is important for medical treatments.Citation16 Based on our findings, we suggest that smoking-cessation activities should be included in TB care.
Immunopathological associations between smoking and TB have been revealed.Citation17,Citation18 Smoking decreases the defenses of the respiratory system, alters the mucociliary apparatus through cell destruction and dysfunction, exerts ciliostatic and ciliotoxic effects, and reduces lysozyme A activity.Citation7 Cigarette smoke has been shown to cause structural changes in experimental mycobacterial strains.Citation19 The fluid-producing functions of the lungs in both normal and TB patients have been shown to be increased by smoking.Citation20 Smoking also induces alterations in both natural and acquired cell immunity that affect macrophages and leukocytes.
The limitations of the present study were that the recorded smoking history did not contain sufficient details to allow calculation of the number of cigarette pack-years, and that other recognized risk factors, such as indoor air pollution, were not included. Side effects and multidrug-resistant TB, which were not investigated in this study, may have affected the study outcomes. Additionally, in future studies, the TB drug used should be recorded to permit assessment of the possible interactions between the therapeutic regimens and cigarette smoking.
Conclusion
In conclusion, smoking was significantly associated with the period required for the sputum cultures to convert from TB-positive to TB-negative. This finding has important implications for TB-treatment strategies and control policy, and may be associated with the TB treatment-success rate in Taiwan, which is lower than the DOTS-targeted strategy. Smoking may increase the difficulty of treating TB, as well as prolong the days for each drug cycle. Public awareness needs to be encouraged to counter the continued prevalence of smoking and of TB.
Acknowledgments
The authors wish to thank Mrs Chiao-Ju Fu and Ching-Ling Li for technical assistance in this research. This study was founded by the Ministry of Science and Technology of Taiwan (MOST103-2314-B-038-018 and 104-2621-M-038-002-MY3) and the Shuang Ho Hospital (104-SHH-HCP-015).
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationGlobal Tuberculosis Report 2013GenevaWHO2013
- YenYFYenMYLinYSSmoking increases risk of recurrence after successful anti-tuberculosis treatment: a population-based studyInt J Tuberc Lung Dis20141849249824670708
- Centers for Disease Control TaiwanTaiwan Tuberculosis Control Report 2012TaipeiCDC2012
- UndernerMPerriotJSmoking and tuberculosisPresse Med20124111711180 French22465718
- No authors listedDiagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999Am J Respir Crit Care Med20001611376139510764337
- RalphAPArdianMWigunaAA simple, valid, numerical score for grading chest X-ray severity in adult smear-positive pulmonary tuberculosisThorax20106586386920861290
- Altet-GômezMNAlcaideJGodoyPRomeroMAHernández del ReyIClinical and epidemiological aspects of smoking and tuberculosis: a study of 13,038 casesInt J Tuberc Lung Dis2005943043615830749
- LeungCCYewWWChanCKSmoking and tuberculosis in Hong KongInt J Tuberc Lung Dis2003798098614552569
- LeungCCLiTLamTHSmoking and tuberculosis among the elderly in Hong KongAm J Respir Crit Care Med20041701027103315282201
- World Health OrganizationGlobal Tuberculosis ControlGenevaWHO2011
- MieczkowskiBEzzieMEUpdate on obstructive sleep apnea and its relation to COPDInt J Chron Obstruct Pulmon Dis2014934936224748786
- GajalakshmiVPetoRKanakaTSJhaPSmoking and mortality from tuberculosis and other diseases in India: retrospective study of 43,000 adult male deaths and 35,000 controlsLancet200336250751512932381
- SanthaTGargRFriedenTRRisk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, South India, 2000Int J Tuberc Lung Dis2002678078812234133
- Casal RománMStudy of delays in diagnosing symptomatic pulmonary tuberculosisArch Bronconeumol20044050 Spanish14718123
- O’MalleyMKingANConteMEllingrodVLRamnathNEffects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancerJ Thorac Oncol2014991792624926542
- LakiSKalapos-KovácsBAntalIKlebovichIImportance of drug interactions with smoking in modern drug researchActa Pharm Hung201383107120 Hungarian24575657
- YachDPartnering for better lung health: improving tobacco and tuberculosis controlInt J Tuberc Lung Dis2000469369710949319
- MauryaVVijayanVKShahASmoking and tuberculosis: an association overlookedInt J Tuberc Lung Dis2002694295112475139
- ShprykovASZhdanovVZLazovskaiaALLevchenkoTNTobacco smoke condensate-induced structural changes in mycobacteria tuberculosisProbl Tuberk20023941 Russian11899805
- PikasOBEffect of smoking and alcohol drinking on the moisture-excreting lung functionLik Sprava20006567 Ukrainian11452925
