Abstract
Respiratory diseases are a very common source of morbidity and mortality among children. Health care providers often face a dilemma when encountering a febrile infant or child with respiratory tract infection. The reason expressed by many clinicians is the trouble to confirm whether the fever is caused by a virus or a bacterium. The aim of this review is to update the current evidence on the virus-induced bacterial infection. We present several clinical as well in vitro studies that support the correlation between virus and secondary bacterial infections. In addition, we discuss the pathophysiology and prevention modes of the virus–bacterium coexistence. A search of the PubMed and MEDLINE databases was carried out for published articles covering bacterial infections associated with respiratory viruses. This review should provide clinicians with a comprehensive idea of the range of bacterial and viral coinfections or secondary infections that could present with viral respiratory illness.
Introduction
Viral respiratory tract infections (VRTIs) are very common in children and their presentations vary from simple colds to life-threatening infections.Citation1–Citation5 The detection of a respiratory virus does not necessarily infer that the child has only a viral infection,Citation6 since outbreaks of VRTIs are being linked to increased incidence of bacterial coinfections.Citation7 The human body is usually capable of eliminating respiratory viral infections with no sequelae; however, in some cases, viruses bypass the immune response of the airways, causing conceivable severe respiratory diseases.Citation8 Robust mechanical and immunosuppressive processes protect the lungs against external infections, but a single respiratory tract infection might change immunity and pathology.Citation9
Health care providers often face a dilemma when encountering a febrile infant or child with respiratory tract infection. The reason expressed by many clinicians is the challenge to confirm whether the fever is caused by a virus or bacterium.Citation10 Acute otitis media (AOM) is a usual bacterial coinfection that occurs in 20%–60% of cases of VRTIs.Citation11–Citation14 In addition, almost 60% of children with VRTI have changes in the maxillary, ethmoidal, and frontal sinuses.Citation11,Citation12 Moreover, in the year 1918, it was estimated that 40–50 million individuals died from the influenza pandemic, many of which were due to secondary bacterial pneumonia with Streptococcus pneumoniae.Citation15
Search strategy and selection criteria
A search of the PubMed database and Google was carried out, using different combinations of the following terms: virus, induced, bacteria, pathogenesis, prevention, vaccine, and children. In addition, we searched the references of the identified articles for additional articles. We then reviewed abstracts and titles and included studies that were relevant to the topic of interest. Finally, the search was limited to studies of disease in humans that were published in English and Spanish from 1918 to the end of 2014 ().
Airway epithelium
The epithelium () is usually covered by a layer of mucus that functions as a boundary.Citation16 Mucins, which are charged glycoproteins, are the main components of mucus.Citation17,Citation18 MUC5AC and MUC5B are the most common mucins in the human sputum, and they assist the innate immune system through their anti-inflammatory and antiviral properties.Citation19,Citation20 In addition, they facilitate trapping and clearance of viruses; however, overproduction of those mucins might have a paradoxical effect.Citation18,Citation19
Figure 2 Airway epithelium.
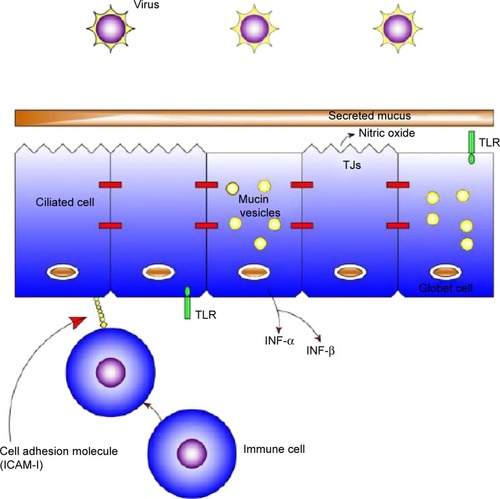
The airway epithelium not only functions as a physical barrier but also recognizes microorganisms through pattern recognition receptors such as Toll-like receptors (TLRs),Citation18 nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and retinoic acid-inducible gene (RIG)-like helicases.Citation21,Citation22
TLRs are single, noncatalytic, membrane-spanning receptor proteins used by the innate immune system.Citation23 Respiratory viruses collaborate with TLR lanes, leading to extended bacterial load in the lungs.Citation21,Citation24
In comparison, NLRs and RIG-like helicases activate innate immune responses through cytosolic sensing of viral and bacterial components.Citation22,Citation25 Nod1 and Nod2, which are family members of NLRs, are induced by molecules synthesized during the production and/or degradation of bacterial peptidoglycan.Citation26–Citation29 In addition, many epithelial cells express the classical antiviral interferons (INFs), especially IFN-α and IFN-β.Citation30,Citation31 Moreover, the respiratory virus-infected epithelia facilitates the attraction of inflammatory cells, including natural killer cells, neutrophils, macrophages, and eosinophils from the bloodstream into the infected site.Citation32 Finally, the airway epithelium consists of many molecules including intercellular adhesion molecule 1 (ICAM-1), carcinoembryonic antigen-related cellular adhesion 1 (CEACAM-1), and platelet-activating factor receptor (PAF-r).Citation33 Viruses have an effect in modulating these receptors, leading to an increase risk of bacterial adherence; for example, rhinovirus upregulates the expression of PAF-r, leading to the binding of S. pneumoniae to bronchial epithelial cells.Citation34
Pathogenesis of superimposed secondary bacterial infection
Different mechanisms might contribute to the debilitation in host defense of the respiratory tract against bacteria following viral infection. Some of the mechanisms have been extrapolated from studies conducted in animal models of sequential infections by respiratory viruses and several bacterial pathogens.
Virus inflicts impairment on host epithelial cells
Mammalian cells are prone to bacterial attachment during a viral illness.Citation8,Citation35 Viruses can debilitate the mucociliary clearance structure, leading to the increased attachment of bacteria to mucins and colonization; moreover, the condensed mucus will impede the penetration of antibacterial material and immune cells.Citation36 Viruses like the respiratory syncytial virus (RSV) can damage ciliated cells, resulting in ciliostasis and, therefore, deterioration of mucociliary clearance.Citation37 The same concept applies to an influenza virus infection, leading to decreased tracheal mucociliary velocity and clearance of S. pneumoniae.Citation35,Citation38 Moreover, virus-induced cell death debilitates the mechanical elimination of the attached pathogens and displays novice receptors for bacterial adherence.Citation39 Studies have shown that the RSV virus induces the adherence of S. pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenza to airway epithelial cells.Citation40–Citation43 In addition, adenovirus and rhinovirus play the same role in the adherence of S. pneumoniae to the airway epithelial cells;Citation8,Citation44 however, the measles virus decreases the risk of adherence of streptococcal bacteria, implying that every virus has a specific mode of changing the host cell membrane.Citation44 Moreover, bacterial adhesion might also be a result of the upregulation of surface receptors including PAF-r, which is involved in pneumococcal invasion.Citation45,Citation46
In patients with cystic fibrosis, bacterial adherence forms a biofilm, creating permanent airway colonization with P. aeruginosa.Citation47 Viruses such as RSV, rhinovirus, and influenza virus also lead to pneumococcal biofilm formation on the airway lining.Citation48 Furthermore, RSV increases the risk of adherence of Staphylococcus aureus and Bordetella pertussis to Hep-2 (human epidermoid cancer) epithelial cells.Citation49,Citation50
Virus effect on the immune system
Post-viral sustained desensitization of lung sentinel cells to TLR signals may be one possible contributor to the common secondary bacterial pneumonia associated with viral infection. For instance, TLR4 and TLR5 pathways are altered after influenza virus infection, resulting in decreased neutrophil attraction, thereby leading to increased attachment of S. pneumonia and P. aeruginosa to the airway epithelial cells.Citation25
The interrelation between host cells and microorganisms during an infection induces immune responses that include the generation of proinflammatory molecules. Despite their crucial role as a bactericidal, proinflammatory cytokines such as TNF-α produced in response to infection could be detrimental to the host cells.Citation51 During a viral infection, TLR and RIG-I-like receptor activation induces production of type I IFNs, which can augment the inflammatory response to TLR ligands including lipopolysaccharide (LPS).Citation52,Citation53 In addition, certain bacteria such as S. aureus integrate into the A549 respiratory epithelial cells (adeno-carcinomic human-alveolar basal-epithelial cells) during a respiratory viral infection by increasing the expression of ICAM-1.Citation54 RSV differs from influenza virus in that the former upregulates cellular receptors including CEACAM-1 and ICAM-1, which eventually leads to bacterial infection.Citation45 Finally, interaction between type I IFNs and Nod1/Nod2 signaling leads to bacterial recognition, but indicts harmful effects in the virally infected host.Citation55
Clinical presentation
Corollary and secondary bacterial infections in patients with viral diseases are known to coexist. A study conducted by O’Brien et alCitation56 showed that the influenza virus (H1N1) was the culprit of the severe pneumococcal pneumonia outbreak among children that occurred in Iowa in the mid-1990s. Other studies have shown that almost one-third of children with community acquired pneumonia (CAP) had mixed (viral and bacterial) infection.Citation57,Citation58 Moreover, a study in France showed that influenza virus infection was the direct cause of CAP in 12% of children.Citation59 Syrjanen et alCitation60 found in their study that the isolation of S. pneumonia from the nasopharyngeal area was higher during respiratory infection without concomitant AOM. Viral respiratory infection due to RSV, influenza virus (type A or B), and adenovirus increase the incidence of otitis media (OM) and recurrent OM in children.Citation61,Citation62 Ruuskanen et alCitation63 found that there is a concrete association between AOM and 57% of children with RSV, 33% with parainfluenza type 3 virus, 30% with adenovirus, 35% with influenza A virus, 28% with parainfluenza type 1 virus, 18% with influenza B virus, and 10% with parainfluenza type 2 virus infections; the most common bacteria isolated from tympano-centesis were H. influenzae, S. pneumoniae, Branhamella catarrhalis, and Mycoplasma pneumonia. Another study showed that the rates of bacteremia and OM were 18% and 44%, respectively, in children with viral-induced bronchiolitis.Citation11 The highest incidence of AOM is usually 2–5 days after an upper respiratory infection.Citation64,Citation65 Isolation of viruses alone from sinus aspirates or in concomitance with bacteria proposes the role of viruses in the induction of bacterial sinusitis,Citation62 with rhinovirus and parainfluenza viruses being the culprits.Citation66 The rate of bacteremia in children with acute bronchiolitis ranges from 0.2% to 1.4%.Citation67–Citation75 In addition, the rate of bacterial urinary tract infection (UTI) in children with bronchiolitis can be as high as 11.4%.Citation67 In a recent study, Hendaus et alCitation76 assessed the prevalence of UTI in infants and children with bronchiolitis. The study included 835 hospitalized children with acute bron-chiolitis. The results disclosed that UTI was found in 13.4% with bronchiolitis triggered by a respiratory viruses such as rhinovirus (31%), adenovirus (14%), parainfluenza virus type 4 (14%), bocavirus (10%), human metapneumovirus (10%), coronavirus (7%), parainfluenza virus type 3 (3.4%), parainfluenza virus type 2 (3.4%), parainfluenza virus type 2 (3.4%), and H1N1 (3.4%). Rittichier et alCitation77 have researched the effect of respiratory viruses on the risk of acquiring serious bacterial infection, including UTI. The study concluded that febrile infants with enterovirus had a coexisting rate of serious bacterial infection of 6.6%.
Role of myxovirus resistance protein 1 (MxA) in differentiating between viral and bacterial infections
The human myxovirus resistance protein 1 (MxA) is an important intermediary of the IFN-induced antiviral response against a variety of viruses. MxA expression is firmly modified by type I and type III IFNs, which also requires signal transducer and activator of transcription 1 signaling. Additionally, MxA has many characteristics similar to the superfamily of large guanosine triphosphatases.Citation78 MxA analysis could be beneficial to differentiate between bacterial and viral infections. Engelmann et alCitation79 conducted a prospective, multicenter cohort study in different pediatric emergency departments in France on the role of MxA in the diagnosis of viral infections. MxA blood values were calculated in infants and children with verified bacterial or viral infections, uninfected controls, and infections of unknown origin. A receiver operating characteristic analysis was used to verify the diagnostic performance of MxA. The study, which included 553 children, showed that MxA was significantly higher in children with viral versus bacterial infections and uninfected controls (P<0.0001). Additionally, MxA levels were significantly higher in children with clinically diagnosed viral infections than in those with clinically diagnosed bacterial infections (P<0.001).Citation79 Other authors have also reported the usefulness of blood MxA testing in patients with viral infections.Citation80,Citation81 The use MxA in diagnosing viral infection is very promising, especially in patients who are at risk of infectious complications. Two separate studies have shown that blood MxA is beneficial in differentiating between viral illness and acute graft-versus-host disease after allogenic stem cell transplantation.Citation82,Citation83
Prevention of secondary bacterial infection
It has been recommended that treatment or prevention of a viral disease may be a superior method for diminishing of complications from influenza.Citation84,Citation85 Since viral infections might lead to secondary bacterial infection, it is prudent to vaccinate patients with the influenza vaccine to diminish the risk of OM in children and pneumonia in adults.Citation62
It has also been published that live attenuated influenza vaccine is effective in reducing the incidence of all-cause AOMCitation86–Citation88 and pneumoniaCitation89 compared to placebo in children. In addition, the intranasal influenza vaccine can reduce OM by 44%.Citation90 Moreover, studies have shown that a combined influenza/pneumococcal vaccine is efficient in the prevention of OM in children and pneumonia.Citation91,Citation92 However, the credit of protection was awarded to the influenza vaccine since studies have shown that pneumococcal vaccine has no benefit in the reduction of AOM.Citation93,Citation94 In addition, the pneumococcal polysaccharide vaccine showed no efficacy in the prevention of pneumonia in adults.Citation95
Treatment of viral infection is anticipated to prevent bacterial superinfections. Currently, the only respiratory virus that is pharmacologically treatable is the influenza viruses (Type A and B).Citation62 Neuraminidase inhibitors can potentially diminish the morbidity related to influenza.Citation96 Oseltamivir can reduce the incidence of AOM in preschool children,Citation97 and the reduction rate can be up to 44%.Citation98 A meta-analysis review showed that oral oseltamivir reduces the rate of hospitalization by 25% and morbidity by 75%.Citation99 In addition, its use can reduce the use of antibiotics by up to 50%,Citation100,Citation101 The same concept of protection applies to vaccines that prevent against RSV infections.Citation62 The vaccine available for RSV is palivizumab (MedImmune, Gaithersburg, MD, USA), a humanized monoclonal antibody that perceives the fusion protein of RSV. The other monoclonal antibody that is under clinical trials is motavizumab (MedImmune), which has a higher affinity for RSV fusion protein than palivizumab and can prevent against medically attended lower respiratory tract infection.Citation102
Conclusion
The rate of concurrent serious bacterial infections with viral illness is appreciable. Similar emphasis must be given to the prevention and treatment of viral illnesses, especially in young children. Furthermore, health care providers should emphasize to parents on the importance of clinical follow-up of infants and young children diagnosed with VRTI. Moreover, the introduction of MxA in the diagnosis of viral illnesses in children is promising.
Disclosure
The authors declare no conflicts of interest in this work.
References
- TregoningJSSchwarzeJRespiratory viral infections in infants: causes, clinical symptoms, virology, and immunologyClin Microbiol Rev2010231749820065326
- RegameyNKaiserLRoihaHLPaediatric Respiratory Research GroupViral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort studyPediatr Infect Dis J200827210010518174876
- GreenbergSBRespiratory viral infections in adultsCurr Opin Pulm Med20028320120811981309
- SlootsTPWhileyDMLambertSBNissenMDEmerging respiratory agents: new viruses for old diseases?J Clin Virol200842323324318406664
- Van der ZalmMMvan EwijkBEWilbrinkBUiterwaalCSWolfsTFvan der EntCKRespiratory pathogens in children with and without respiratory symptomsJ Pediatr20091543396400400.e118823911
- RawlinsonWDWaliuzzamanZCarterIWBelessisYCGilbertKMMortonJRAsthma exacerbations in children associated with rhinovirus but not human metapneumovirus infectionJ Infect Dis200318781314131812696012
- BeadlingCSlifkaMKHow do viral infections predispose patients to bacterial infections?Curr Opin Infect Dis200417318519115166819
- VareilleMKieningerEEdwardsMRRegameyNThe airway epithelium: soldier in the fight against respiratory virusesClin Microbiol Rev201124121022921233513
- WalzlGTafuroSMossPOpenshawPJHussellTInfluenza virus lung infection protects from respiratory syncytial virus-induced immunopathologyJ Exp Med200019291317132611067880
- RalstonSHillVWatersAOccult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic reviewArch Pediatr Adolesc Med20111651095195621969396
- LehtinenPJarttiTVirkkiRBacterial coinfections in children with viral wheezingEur J Clin Microbiol Infect Dis200625746346916819619
- VesaSKleemolaMBlomqvistSTakalaAKilpiTHoviTEpidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of agePediatr Infect Dis J200120657458111419498
- BulutYGuvenMOtluBAcute otitis media and respiratory virusesEur J Pediatr200716622322816967296
- YanoHOkitsuNHoriTDetection of respiratory viruses in nasopharyngeal secretions and middle ear fluid from children with acute otitis mediaActa Otolaryngol200812916
- MalliaPJohnstonSLInfluenza infection and COPDInt J Chron Obstruct Pulmon Dis200721556418044066
- VoynowJARubinBKMucins, mucus, and sputumChest2009135250551219201713
- ThorntonDJRousseauKMcGuckinMAStructure and function of the polymeric mucins in airways mucusAnnu Rev Physiol20087045948617850213
- RoseMCVoynowJARespiratory tract mucin genes and mucin glycoproteins in health and diseasePhysiol Rev200686124527816371599
- VoynowJAGendlerSJRoseMCRegulation of mucin genes in chronic inflammatory airway diseasesAm J Respir Cell Mol Biol200634666166516456183
- RoseMCNickolaTJVoynowJAAirway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasiaAm J Respir Cell Mol Biol200125553353711713093
- AkiraSPathogen recognition by innate immunity and its signalingProc Jpn Acad Ser B Phys Biol Sci2009854143156
- AkiraSUematsuSTakeuchiOPathogen recognition and innate immunityCell200612478380116497588
- KannegantiTDLamkanfiMNunezGIntracellular NOD-like receptors in host defense and diseaseImmunity20072754955917967410
- HanssonGKEdfeldtKToll to be paid at the gateway to the vessel wallArterioscler Thromb Vasc Biol20052561085108715923538
- DidierlaurentAGouldingJPatelSSustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infectionJ Exp Med2008205232332918227219
- TingJPDavisBKCATERPILLER: a novel gene family important in immunity, cell death, and diseasesAnnu Rev Immunol20052338741415771576
- ChamaillardMHashimotoMHorieYAn essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acidNat Immunol2003470270712796777
- GirardinSEBonecaIGCarneiroLANod1 detects a unique muropeptide from gram-negative bacterial peptidoglycanScience20033001584158712791997
- GirardinSEBonecaIGVialaJNod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detectionJ Biol Chem20032788869887212527755
- InoharaNOguraYFontalbaAHost recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s diseaseJ Biol Chem20032785509551212514169
- BaslerCFGarcía-SastreAViruses and the type I interferon antiviral system: induction and evasionInt Rev Immunol2002214–530533712486817
- KatzeMGHeYGaleMJrViruses and interferon: a fight for supremacyNat Rev Immunol20022967568712209136
- MessageSDJohnstonSLHost defense function of the airway epithelium in health and disease: clinical backgroundJ Leukoc Biol200475151712972516
- IshizukaSYamayaMSuzukiTEffects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cellsJ Infect Dis2003188121928193914673774
- PittetLAHall-StoodleyLRutkowskiMRHarmsenAGInfluenza virus infection decrease stracheal mucociliary velocity and clearance of Streptococcus pneumoniaeAm J Respir Cell Mol Biol201042445046019520922
- WilsonRDowlingRBJacksonADThe biology of bacterial-colonization and invasion of the respiratory mucosaEur Respir J199697152315308836669
- TristramDAHicksWJrHardRRespiratory syncytial virus and human bronchial epitheliumArch Otolaryngol Head Neck Surg19981247777839677113
- McCullersJAIversonARMcKeonRMurrayPJThe platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenzaScand J Infect Dis2008401111717852951
- BragonziACopreniEde BentzmannSUlrichMConeseMAirway epithelial cell-pathogen interactionsJ Cyst Fibros20043suppl 219720115463958
- AvadhanulaVWangYPortnerAAddersonENon typeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoproteinJ Med Microbiol2007561133113717761473
- FukasawaCIshiwadaNOgitaJHishikiHKohnoYThe effects of disodium cromoglycate on enhanced adherence of Haemophilus influ-enzae to A549 cells infected with respiratory syncytial virusPediatr Res20096616817319390482
- HamentJMAertsPCFleerADirect binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine modelPediatr Res2005581198120316306193
- Van EwijkBEWolfsTFAertsPCRSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cellsPediatr Res20076139840317515861
- WangJHKwonHJJangYJRhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneouslyLaryngoscope20091191406141119434681
- AvadhanulaVRodriguezCADevincenzoJPRespiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent mannerJ Virol20068041629163616439519
- McCullersJARehgJELethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptorJ Infect Dis2002186334135012134230
- HassettDJKorfhagenTRIrvinRTPseudomonas aeruginosa bio-film infections in cystic fibrosis: insights into pathogenic processes and treatment strategiesExpert Opin Ther Targets20101411713020055712
- MorrisDPBacterial biofilm in upper respiratory tract infectionsCurr Infect Dis Rep2007918619217430699
- SaadiATBlackwellCCEsserySDDevelopmental and environmental factors that enhance binding of Bordetella pertussis to human epithelial cells in relation to sudden infant death syndrome (SIDS)FEMS Immunol Med Microbiol19961651598954353
- SaadiATBlackwellCCRazaMWFactors enhancing adherence of toxigenic Staphylococcus aureus to epithelial cells and their possible role in sudden infant death syndromeEpidemiol Infect19931105075178519316
- CookDNPisetskyDSSchwartzDAToll-like receptors in the pathogenesis of human diseaseNat Immunol2004597597915454920
- NansenARandrup ThomsenAViral infection causes rapid sensitization to lipopolysaccharide: central role of IFN-alpha betaJ Immunol200116698298811145676
- DoughtyLNguyenKDurbinJBironCA role for IFN-alpha beta in virus infection-induced sensitization to endotoxinJ Immunol20011662658266411160329
- PassarielloCSchippaSContiCRhinoviruses promote internalisation of Staphylococcus aureus into non-fully permissive cultured pneumocytesMicrobes Infect2006875876616513395
- KimYGParkJHReimerTViral infection augments Nod1/2 signaling to potentiate lethalityCell Host Microbe20119649650721669398
- O’BrienKLWaltersMISellmanJSevere pneumococcal pneumonia in previously healthy children: the role of preceding influenza infectionClin Infect Dis20003078478910816149
- Nascimento-CarvalhoCMRibeiroCTCardosoMRThe role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing countryPediatr Infect Dis J2008271093994118756190
- JuvénTMertsolaJWarisMEtiology of community-acquired pneumonia in 254 hospitalized childrenPediatr Infect Dis J200019429329810783017
- Pons-CatalanoCValletCLorrotMCommunity acquired pneumonia and influenza in childrenArch Pediatr200310121056106014643533
- SyrjanenRKKilpiTMKaijalainenTHHervaEETakalaAKNasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years oldJ Infect Dis200118445145911471103
- HendersonFWCollierAMSanyalMAA longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusionN Engl J Med1982306137713836281639
- PeltolaVTMcCullersJARespiratory viruses predisposing to bacterial infections: role of neuraminidasePediatr Infect Dis J2004231 supplS87S9714730275
- RuuskanenOArolaMPutto-LaurilaAMertsolaJMeurmanOViljanenMKAcute otitis media and respiratory virus infectionsPediatr Infect Dis J1989894992495520
- KoivunenPKontiokariTNiemelaMPokkaTUhariMTime to development of acute otitis media during an upper respiratory tract infection in childrenPediatr Infect Dis J19991830330510093961
- HeikkinenTTemporal development of acute otitis media during upper respiratory tract infectionPediatr Infect Dis J1994136596617970958
- GwaltneyJMSinusitisMandellGLBennettJEDolinRMandell, Douglas and Bennett’s Principles and Practices of Infectious Diseases4th edNew YorkChurchill Livingstone1995585590
- PurcellKFergieJConcurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infectionsArch Pediatr Adolesc Med2002156432232411929363
- GreenesDSHarperMBLow risk of bacteremia in febrile children with recognizable viral syndromesPediatr Infect Dis J19991825826110093948
- BilavskyEShouvalDSYarden-BilavskyHFischNAshkenaziSAmirJA prospective study of the risk for serious bacterial infections in hospitalized febrile infants with or without bronchiolitisPediatr Infect Dis J200827326927018277919
- LuginbuhlLMNewmanTBPantellRHFinchSAWassermanRCOffice-based treatment and outcomes for febrile infants with clinically diagnosed bronchiolitisPediatrics2008122594795418977972
- LiebeltELQiKHarveyKDiagnostic testing for serious bacterial infections in infants aged 90 days or younger with bronchiolitisArch Pediatr Adolesc Med1999153552553010323635
- AntonowJAHansenKMcKinstryCAByingtonCLSepsis evaluations in hospitalized infants with bronchiolitisPediatr Infect Dis J19981732312369535251
- LevineDAPlattSLDayanPSMulticenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infectionsPediatrics200411361728173415173498
- Oray-SchromPPhoenixCSt MartinDAmoateng-AdjepongYSepsis workup in febrile infants 0–90 days of age with respiratory syncytial virus infectionPediatr Emerg Care200319531431914578830
- KuppermannNBankDEWaltonEASenacMOJrMcCaslinIRisks for bacteremia and urinary tract infections in young febrile children with bronchiolitisArch Pediatr Adolesc Med199715112120712149412595
- HendausMAAlhammadiAHKhalifaMSMuneerEChandraPRisk of urinary tract infection in infants and children with acute bronchiolitisPaediatr Child Health2015205e25e2926175566
- RittichierKRBryanPABassettKEDiagnosis and outcomes of enterovirus infections in young infantsPediatr Infect Dis J200524654655015933567
- HallerOKochsGHuman MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activityJ Interferon Cytokine Res2011311798721166595
- EngelmannIDubosFLobertPEDiagnosis of viral infections using myxovirus resistance protein A (MxA)Pediatrics20151354e985e99325802344
- NakabayashiMAdachiYItazawaTMxA-based recognition of viral illness in febrile children by a whole blood assayPediatr Res200660677077417065575
- KawamuraMKusanoAFuruyaANew sandwich-type enzyme-linked immunosorbent assay for human MxA protein in a whole blood using monoclonal antibodies against GTP-binding domain for recognition of viral infectionJ Clin Lab Anal201226317418322628233
- YoshimasuTManabeAEbiharaYMxA expression in patients with viral infection after allogeneic stem cell transplantationBone Marrow Transplant200332331331612858204
- GhidiniCZanottiCBoccacciSLanfranchiACaimiLImbertiLMxA RNA quantification in febrile patients who underwent hematopoietic cell transplantation for primary immunodeficiencyDiagn Mol Pathol201120211111621532489
- GravensteinSDavidsonHECurrent strategies for management of influenza in the elderly populationClin Infect Dis20023572973712203171
- McCullersJABartmessKCRole of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniaeJ Infect Dis20031871000100912660947
- HeikkinenTBlockSLTobackSLWuXAmbroseCSEffectiveness of intranasal live attenuated influenza vaccine against all-cause acute otitis media in childrenPediatr Infect Dis J201332666967423271441
- FelixFGomesGACabralGACordeiroJRTomitaSThe role of new vaccines in the prevention of otitis mediaBraz J Otorhinolaryngol200874461361618852991
- RussellFMulhollandKPrevention of otitis media by vaccinationDrugs200262101441144512093313
- NicholKLWuorenmaJvon SternbergTBenefits of influenza vaccination for low-, intermediate-, and high-risk senior citizensArch Intern Med1998158176917769738606
- MarchisioPCavagnaRMaspesBEfficacy of intranasal virosomal influenza vaccine in the prevention of recurrent acute otitis media in childrenClin Infect Dis200235216817412087523
- ChristensonBHedlundJLundberghPOrtqvistAAdditive preventive effect of influenza and pneumococcal vaccines in elderly personsEur Respir J200423336336815065822
- HedlundJChristensonBLundberghPOrtqvistAEffects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-upVaccine2003213906391112922125
- VeenhovenRBogaertDUiterwaalCBrouwerCKiezebrinkHBruinJEffect of conjugate pneumococcal vaccine followed by poly-saccharide pneumococcal vaccine on recurrent acute otitis media: a randomised studyLancet200336193762189219512842372
- FortanierACVenekampRPBoonackerCWPneumococcal conjugate vaccines for preventing otitis mediaCochrane Database Syst Rev20144CD00148024696098
- JacksonLANeuzilKMYuOVaccine Safety DatalinkEffectiveness of pneumococcal polysaccharide vaccine in older adultsN Engl J Med20033481747175512724480
- McNichollIRMcNichollJJNeuraminidase inhibitors: zanamivir and oseltamivirAnn Pharmacother2001351577011197587
- WangKShun-ShinMGillPPereraRHarndenANeuraminidase inhibitors for preventing and treating influenza in children (published trials only)Cochrane Database Syst Rev20124CD00274422513907
- WhitleyRJHaydenFGReisingerKSOral oseltamivir treatment of influenza in childrenPediatr Infect Dis J20012012713311224828
- MuthuriSGMylesPRVenkatesanSLeonardi-BeeJNguyen-Van-TamJSImpact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patientsJ Infect Dis2013207455356323204175
- KaiserLWatCMillsTMahoneyPWardPHaydenFImpact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizationsArch Intern Med20031631667167212885681
- TreanorJJHaydenFGVroomanPSEfficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial: US Oral Neuraminidase Study GroupJAMA20002831016102410697061
- Carbonell-EstranyXSimõesEADaganRMotavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trialPediatrics20101251e35e5120008423