Abstract
It has been recognized that perioperative hemostasis management after joint-replacement surgery for hemophilia patients is complicated and cumbersome, due to the necessity of rigorous monitoring for clotting-factor levels throughout the infusion. Between 2005 and 2014, we examined seven patients with hemophilia A (ten joints: six hips and four knees) receiving total hip or knee arthroplasty (THA or TKA) for hemophilic arthropathy. One male patient (31 years old) showed an intra-articular hematoma formation after THA (case 1). In another male patient (46 years old) receiving TKA, the postoperative trough factor VIII level became lower significantly than reference levels (80%–100% for the 5–10 postoperative days) recommended by the guidelines from the Japanese Society on Thrombosis and Hemostasis, despite sufficient coagulant based on the guidelines being administered (case 2). In the latter patient, deep infection and hematoma formation were observed postoperatively. In this article, we provide a detailed clinical report regarding these two complication cases at the early postoperative periods, and the management of bleeding control for hemophilia patients is discussed.
Introduction
Hemophilia is an X-linked congenital hemorrhagic disorder due to deficiency in clotting factors, either factor VIII (in hemophilia A) or factor IX (in hemophilia B). Some hemophilia patients can experience severe impairment in their daily activities, due to the joint destruction induced by the repeated intra-articular hemorrhaging (hemophilic arthropathy).Citation1 Therefore, they may require receipt of total hip and knee arthroplasty (THA and TKA) potentially at a younger age than nonhemophilic patients with such joint diseases as osteoarthritis or rheumatoid arthritis. A rigorous hemostatic control as well as careful rehabilitation is essential for hemophilia patients after joint replacement by monitoring clotting-factor levels through continuous infusion. Nevertheless, even though comprehensive care was provided by a medical team of experts (doctors/nurses) in the field of hemophilia, it was previously reported that postoperative complication rates were significantly higher in hemophilic patients than nonhemophilic patients.Citation2–Citation5 Only a few reports have been published on the replacement of clotting factors and their activity levels in hemophilia patients after total joint arthroplasty.Citation6–Citation10 In these contexts, we report in detail on the postoperative transition of factor VIII-activity levels in hemophilia A patients receiving THA and TKA, and also on the observed two complications among seven hemophilia patients.
Patients and methods
This study was preliminarily approved by the institutional review board of Tokyo Medical University and all patients gave thier informed consent before participation in this study. We examined a total of seven hemophilic patients (ten joints: six hips and four knees) receiving THA or TKA for treatment of hemophilic arthropathy between 2005 and 2014 at our institution. THA and TKA were performed on six hips of four patients and on four knees of four patients, respectively. All patients were diagnosed with hemophilia A, of which six patients (nine joints: six hips and three knees) were categorized as a severe hemophilia with clotting factor-activity level <1%, and the remaining patient (one knee) as moderate hemophilia with an activity level of 1%–5%. Six patients (nine joints: five hips and four knees) were hepatitis C-positive, and three patients (four joints: three hips and one knee) were HIV-positive (of which one patient exhibited low preoperative CD4 counts of 196 cells/μL). The mean age of the patients was 46.0 years (range: 26–61 years). The development of inhibitors to factor VIII was not detected in any case.
Cementless THA was performed by a posterolateral approach using a Mallory–Head acetabular shell (Biomet Inc, Warsaw, IN, USA) and a Bi-Metric™ femoral stem (Biomet Inc). The acetabular liners were 33 kGy γ-irradiated conventional ultrahigh-molecular-weight polyethylene (ArCom®; Biomet Inc), coupled with a 28 mm cobalt chrome alloy. Controlled hypotensive anesthesia was performed by anesthesiologists in order to reduce perioperative blood loss. Cemented cruciate retaining-type TKA was performed by parapatellar or mid-vastus approach, and a tourniquet was used in all cases to restrain hemorrhage. Prior to implant installation, a tourniquet was deflated once in order to examine bleeding status and if necessary to achieve hemostasis. The knee-joint components were Duracon and Scorpio NRG (Stryker Corporation, Kalamazoo, MI, USA), which were implanted in three and four patients, respectively. The tibial components were 30 kGy γ-irradiated conventional ultrahigh-molecular-weight polyethylene (N2-Vac; Stryker Corporation) coupled with cobalt chrome femoral components.
For patients prior to 2008 (four joints: two hips and two knees), perioperative hemostatic control was performed by physicians specializing in hemophilia according to the guidelines defined by the World Federation of Hemophilia.Citation1 On the other hand, for patients after 2008 (six joints: four hips and two knees), the arthroplasty procedures were performed in accordance with the guidelines defined by the Japanese Society on Thrombosis and Hemostasis.Citation11 The latter guidelines recommend that trough clotting-factor levels should be maintained between 80% and 100% by conducting continuous infusion for 5–10 postoperative days (PODs), and subsequently be controlled between 20% and 80% depending on the postoperative course of patients.
The median (25th–75th percentile) operation time, intraoperative bleeding amount, and postoperative bleeding amount were 92.0 (72.5–107.0) minutes (THA 80.5 [58.8–89.3] minutes, TKA 107.0 [101.0–108.5] minutes), 322.0 (204.5–605.0) mL (THA 427.5 [242.0–885.5] mL, TKA 263.5 [165.5–342.8] mL), and 592.0 (390.8–991.0) mL (THA 497.5 [253.5–1,282.5] mL, TKA 671.0 [500.8–857.0] mL), respectively. Drain-tube removal, getting out of bed, and ambulation were done on a median of POD 3.0 (2.0–3.0) (THA 2.5 [2.0–3.0], TKA 3.0 [3.0–4.5]), 3.0 (2.8–4.3) (THA 3.0 [2.0–4.3], TKA 3.5 [3.0–4.8]), and 6.0 (4.0–8.3) (THA 5.5 [4.0–9.3], TKA 7.0 [4.5–8.8]), respectively.
Perioperatively administered clotting factor VIII was Advate® (Baxter International Inc, Deerfield, IL, USA) for six joints (four hips, two knees), Kogenate® FS (Bayer AG, Leverkusen, Germany) for one joint (one hip), Recombinate® (Baxter) for two joints (one hip, one knee), and Cross-Eight M® (Japanese Red Cross Society, Tokyo, Japan) for one joint (one knee). A single intravenous injection of factor VIII by bolus administration was conducted preoperatively for one patient (one knee) with moderate hemophilia. For six patients (six hips, three knees) with severe hemophilia, preoperative single intravenous injection by continuous infusion was conducted initially and then switched to bolus administration.
We confirmed that one male patient receiving THA showed an intra-articular hematoma (henceforth referred to as case 1). In another male patient receiving TKA, we observed that the trough factor VIII level was significantly lower than the reference value defined in the aforementioned Japanese guidelines.Citation11 The latter patient experienced deep infection and hematoma formation postoperatively (henceforth referred to as case 2). In the next section, we provide a detailed clinical report regarding these two patients with early postoperative complications.
Results
Case 1
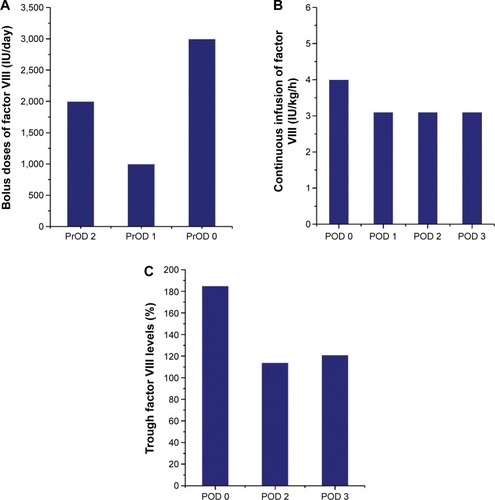
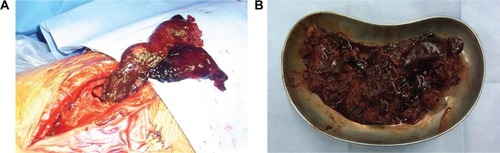
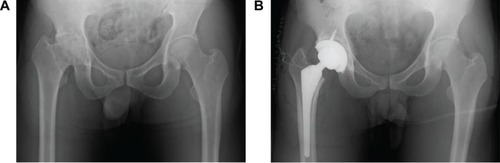
The patient was a 31-year-old male (height 180 cm, weight 95 kg, body mass index [BMI] 29.3 kg/m2) with severe hemophilia A. He was HIV-positive, and had undergone insulin therapy for diabetes mellitus. Preoperative CD4 count, blood glucose level, and HbA1c level were 196 cells/μL, 198 mg/dL, and 7.1%, respectively. The severity of the affected hip joint was stage V according to the Arnold–Hilgartner classification ().Citation12 Cementless THA was performed using the posterolateral approach. The postoperative radiograph was shown in . The operation time was 102 minutes. The amount of intraoperative and postoperative bleeding was 908 mL and 570 mL, respectively. On POD 2, the patient started using a wheelchair after the removal of the drainage tube. Ambulation exercises started from POD 6, and the patient was discharged on POD 16. Clotting factor VIII (Kogenate FS) was administered preoperatively (3,000 IU/day just prior to THA), and subsequently continuous infusion was conducted at 4 IU/kg/h (). The trough factor VIII level was 185% immediately after THA. From POD 1, the dosage was reduced to 3.1 IU/kg/h, and then the factor level decreased to 114% and 121% on PODs 1 and 3, respectively (). After being discharged from the hospital, the patient was rehospitalized due to hematoma formation. On POD 71, surgical removal of the hematoma was performed ().
Figure 1 Anteroposterior hip radiographs of case 1 (31-year-old male with severe hemophilia).
Case 2
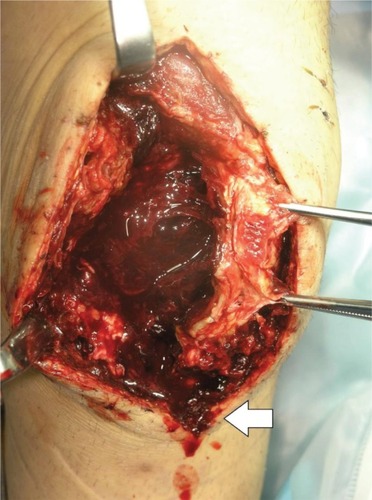
The patient was a 46-year-old male (height 152 cm, weight 50 kg, BMI 21.6 kg/m2) with severe hemophilia A and hepatitis C/HIV-positive. The preoperative CD4 count was 892 cells/μL. The severity of the affected knee joint was stage IV according to the Arnold–Hilgartner classification ().Citation12 Cemented cruciate retaining-type TKA was performed by the mid-vastus approach. The operation time was 99 minutes, and the amount of intraoperative bleeding was 224 mL. Postoperative radiographs are shown in . Although a drainage tube was kept inserted after TKA, frequent dressing replacement was needed, due to a large amount of subcutaneous bleeding. The amount of postoperative bleeding was 614 mL. The patient started using a wheelchair after the removal of drainage tube on POD 3. Ambulation and range-of-motion exercises started from POD 4. However, the exercises were discontinued, due to the low trough factor VIII level (70%) on POD 6. Pain and swelling of the left knee joint increased from POD 10. A severe fever (39°C) was observed on POD 12, and coagulase-negative Staphylococcus was detected from blood culture and aspirate samples. The patient was thus diagnosed with early deep infection following TKA. Surgical debridement was performed, and closed continuous irrigation was initiated. According to our observation during the debridement, a hematoma filled in the intra-articular cavity and connected to the distal subcutaneous area (), which implies a possible route of infection.
Figure 4 Anteroposterior and lateral knee radiographs of case 2 (46-year-old male).
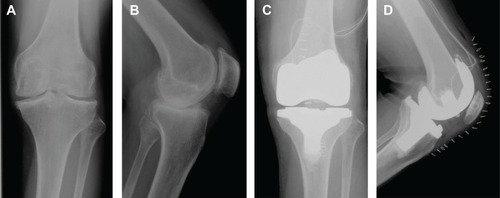
Figure 5 Intraoperative view of hematoma connecting to the distal subcutaneous area (white arrow) in case 2 (46-year-old male).
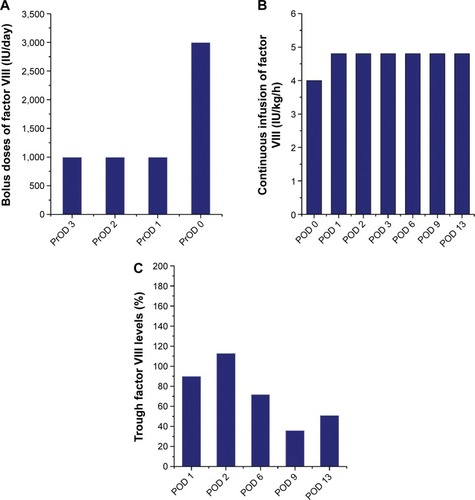
Clotting factor VIII (Advate) was administered preoperatively (3,000 IU/day just prior to TKA), and subsequently continuous infusion was conducted at 4 IU/kg/h (). The trough factor VIII level was 90% on POD 1, and the dosage of Advate was then increased to 4.8 IU/kg/h (). The trough level increased to 113% on POD 2, but subsequently decreased to 72% and 36% on PODs 6 and 9, respectively. Although sufficient clotting factor was administered according to the Japanese guidelines,Citation11 trough factor VIII activity remained at much lower levels than the reference (ie, 80%–100%). Note that there was no development of factor VIII inhibitors in this case.
Discussion
Postoperative complications, including hematoma and infection, have previously been reported for hemophilia patients,Citation2,Citation3,Citation13,Citation14 and thus it has been considered that rigorous hemostatic control should be performed for hemophilia patients through clotting-factor replacement after arthroplasty. According to recent publications on hemophilia patients receiving TKA, postoperative complication rates were 2.8%–12.5% for hematoma and 1.4%–15.5% for infection, and revision rates were 4.6%–20.0% at a mean follow-up of 5.1–11.8 years.Citation2–Citation5,Citation15 Although hemophilia patients receiving THA showed a lower risk of hematoma and infection, higher revision rates (7.4%–13.3%) were reported at a mean follow-up of 6.3–11.0 years compared to hemophilia patients receiving TKA. Therefore, both THA and TKA are likely to entail greater risks of failure for hemophilic patients than nonhemophilic patients. Furthermore, it is considered that HIV-positive patients with CD4 counts of ≤200 cells/μL can also be exposed to a higher risk of infection,Citation16 and repeated self-administration of clotting factors may become a route of infection.Citation17–Citation19 In general, hemophilia patients are often HIV-positive, and there has been a lot of research done regarding a correlation between postoperative infection and HIV.4,15,219–22 Nevertheless, this correlation remains to be fully elucidated for hemophilia patients.
In the current report, one male patient infected at an early POD (case 2) required frequent dressing replacement due to continuous bleeding from the surgical wound. According to the intraoperative findings after the infection, a hematoma filled in the intra-articular cavity and connected to the distal subcutaneous area, which was considered a possible route of the infection. Poor postoperative hemostatic control can delay the initiation of range-of-motion or ambulation exercise, due to the necessity of bed rest. In addition, the formation of an intra-articular hematoma can lead to prosthetic loosening and consequently to an increased rate of revision arthroplasty. Therefore, in the perioperative and postoperative periods, clotting-factor replacement is regarded as one of the most important treatment strategies for preventing complications. This treatment commonly starts during the operation, since clotting-factor levels are more easily controlled by continuous infusion than bolus administration.
Negrier et alCitation23 recommended using Advate at an initial dose of 3.2–3.7 IU/kg/h, while TakedaniCitation9 reported initial mean doses of Kogenate FS, Advate, and Cross-Eight M for continuous infusion during arthroplasty of 2.9±0.3, 3.5±0.9, and 3.2±0.5 IU/kg/h, respectively. In our present case of early postoperative infection (case 2), the initial mean dose for continuous infusion of Advate was 4.0 IU/kg/h and then increased to 4.8 IU/kg/h. Nevertheless, we found clotting-factor levels decreased ().
Since only a small fraction of factor VIII circulates outside the vascular system, several authors have reported that underweight patients are subject to being underdosed and obese patients to being overdosed by applying the following formula: dose = (body weight [kg] × desired factor VIII increase [%])/2.Citation24–Citation27 Henrard et alCitation26 proposed that factor VIII dosing can be adjusted for patients with a BMI <20 kg/m2 or ≥30 kg/m2. In this study, the BMI of cases 1 and 2 was 29.3 and 21.6 kg/m2, respectively, and thus the currently applied doses based on the guidelinesCitation1,Citation11 seem to be acceptable for those patients.
According to a report by Wong et alCitation13 on 556 knees following TKA, the postoperative infection rate was 9.2% in the patient group receiving clotting-factor replacement based on the World Federation of Hemophilia guidelines.Citation1 On the other hand, the other patient group, maintaining higher clotting-factor levels than the guidelines’ recommendation, exhibited a significantly lower infection rate (2.5%). Note that we experienced some patients with significantly reduced activity levels regardless of the proper administration based on the guidelines. This fact can suggest great importance in maintaining a higher level of each factor than the guidelines’ recommendation through careful daily monitoring.
The hematoma formation observed in case 1 can be associated with the discontinued self-administration of the clotting factor after his discharge from the hospital. Therefore, adequate instruction for continuous self-administration should be given to a hemophilia patient who undergoes joint-replacement surgery. Moreover, it should be carefully considered whether or not a compromised host with HIV or diabetes is a proper candidate for arthroplasty. Lastly, in order to control bleeding, it is essential to conduct daily monitoring of clotting factor-activity levels through close cooperation among orthopedists, hemophilia experts, and physiotherapists.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Federation of HemophiliaGuidelines for the Management of HemophiliaMontrealWFH2005
- Rodriguez-MerchanECGomez-CarderoPJimenez-YusteVInfection after total knee arthroplasty in haemophilic arthropathy with special emphasis on late infectionHaemophilia2011175e831e83221507150
- WangKStreetADowrickALiewSClinical outcomes and patient satisfaction following total joint replacement in haemophilia – 23-year experience in knees, hips and elbowsHaemophilia2012181869321649799
- SolimenoLPMancusoMEPastaGSantagostinoEPerfettoSMannucciPMFactors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institutionBr J Haematol2009145222723419236610
- SilvaMLuckJVLong-term results of primary total knee replacement in patients with hemophiliaJ Bone Joint Surg Am2005871859115634817
- ZinggPOFucenteseSFLutzWBrandBMamischNKochPPHaemophilic knee arthropathy: long-term outcome after total knee replacementKnee Surg Sports Traumatol Arthrosc201220122465247022293897
- YooMCChoYJKimKIRamtekeAChunYSThe outcome of cementless total hip arthroplasty in haemophilic hip arthropathyHaemophilia200915376677319444974
- BaeDKYoonKHKimHSSongSJTotal knee arthroplasty in hemophilic arthropathy of the kneeJ Arthroplasty200520566466816310005
- TakedaniHContinuous infusion during total joint arthroplasty in Japanese haemophilia A patients: comparison study among two recombinants and one plasma-derived factor VIIIHaemophilia201016574074620398072
- ShethDSOldfieldDAmbroseCClyburnTTotal knee arthroplasty in hemophilic arthropathyJ Arthroplasty2004191566014716652
- Japanese Society on Thrombosis and HemostasisAcute hemorrhage in hemophilia patients without inhibitors: guidelines for clotting factor replacement therapy in the treatment and surgery2008 Available from: http://www.jsth.org/committee/pdf/03_inhibitor_H1_B.pdfAccessed August 24, 2015 Japanese
- ArnoldWDHilgartnerMWHemophilic arthropathy. Current concepts of pathogenesis and managementJ Bone Joint Surg Am1977593287305849938
- WongJMMannHAGoddardNJPerioperative clotting factor replacement and infection in total knee arthroplastyHaemophilia201118416
- SikkemaTBoerboomALMeijerKA comparison between the complications and long-term outcome of hip and knee replacement therapy in patients with and without haemophilia; a controlled retrospective cohort studyHaemophilia201117230030321070490
- GoddardNJMannHALeeCATotal knee replacement in patients with end-stage haemophilic arthropathy: 25-year resultsJ Bone Joint Surg Br20109281085108920675751
- RagniMVCrossettLSHerndonJHPostoperative infection following orthopaedic surgery in human immunodeficiency virus-infected hemophiliacs with CD4 counts <, or =200/mm3J Arthroplasty19951067167218749751
- ChiangCCChenPQShenMCTsaiWTotal knee arthroplasty for severe haemophilic arthropathy: long-term experience in TaiwanHaemophilia200814482883418510565
- LuckJVSilvaMRodriguez-MerchanECGhalamborNZahiriCAFinnRSHemophilic arthropathyJ Am Acad Orthop Surg200412423424515473675
- NorianJMRiesMDKarpSHambletonJTotal knee arthroplasty in hemophilic arthropathyJ Bone Joint Surg Am20028471138114112107312
- Rodriguez-MerchanECTotal knee replacement in haemophilic arthropathyJ Bone Joint Surg Br201089218618817322432
- GoddardNJRodriguez-MerchanECWiedelJDTotal knee replacement in haemophiliaHaemophilia20028338238612010438
- HicksJLRibbansWJBuzzardBInfected joint replacements in HIV-positive patients with haemophiliaJ Bone Joint Surg Br20018371050105411603522
- NegrierCShapiroABerntorpESurgical evaluation of a recombinant factor VIII prepared using a plasma/albumin-free method: efficacy and safety of Advate in previously treated patientsThromb Haemostasis2008100221722318690340
- BjörkmanSBerntorpEPharmacokinetics of coagulation factors: clinical relevance for patients with haemophiliaClin Pharmacokinet2001401181583211735604
- BjörkmanSBlanchetteVSFischerKComparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age-related differences and implications for dose tailoringJ Thromb Haemost20108473073620398185
- HenrardSSpeybroeckNHermansCImpact of being underweight or overweight on factor VIII dosing in hemophilia A patientsHaematologica20139891481148623645693
- GrahamAJaworskiKPharmacokinetic analysis of anti-hemophilic factor in the obese patientHaemophilia201420222622924252161