Abstract
Background
Secondary hyperparathyroidism (SHPT) is a major disorder in patients with chronic renal disease with or without dialysis. Air pollution has been confirmed as being associated with increased incidence of human morbidity and mortality. To our knowledge, investigating air pollution as a dialysis-unrelated factor for SHPT in patients undergoing dialysis is limited. We developed this study to assess the effect of air pollution and other important risk factors on SHPT in patients undergoing peritoneal dialysis (PD).
Materials and methods
We recruited a total of 141 patients who did not have diabetes mellitus, were nonsmokers, and were undergoing PD in this cross-sectional study. We analyzed the difference in air quality based on the patients’ living areas. We estimated demographic, hematological, nutritional, inflammatory, biochemical, air pollutant, and dialysis-related data based on this cross-sectional study. Subgroup analysis of the relationship between air pollutants and the clinical variables and having or not having hyperparathyroidism (HPT) (intact parathyroid hormone level ≥180 pg/dL) was also performed.
Results
A total of 141 patients undergoing PD (30 men and 111 women) were enrolled in the study. Sixty-eight patients had SHPT. In a binary logistic regression, high environmental CO exposure (odds ratio [OR] 3.22, 95% confidence interval [CI] 1.42–7.28; P=0.005), serum phosphate levels (OR 1.66, 95% CI 1.17–2.37; P=0.005), hypoalbuminemia (OR 3.76, 95% CI 1.29–10.94; P=0.015), and use of calcitriol (OR 8.25, 95% CI 3.43–19.85; P<0.001) were positively associated with SHPT.
Conclusion
The findings of this cross-sectional study indicated the presence of an association between environmental CO exposure and SHPT in patients undergoing PD who did not have diabetes mellitus. Therefore, poor environmental air quality may be a risk factor for deterioration of SHPT in patients undergoing PD.
Introduction
Chronic kidney disease (CKD), a global noncommunicable disease, is not only a medical but also a public health problem.Citation1 Secondary hyperparathyroidism (SHPT), a common disorder in CKD and patients undergoing dialysis resulting from progressively impaired metabolism of calcium, phosphate, and vitamin D, leads to abnormal bone and mineral metabolism.Citation2,Citation3 SHPT could be treated with vitamin D sterols, calcimimetics, and parathyroidectomy. In patients undergoing dialysis, with their calcium level corrected by dialysis and calcium supplements, the role of controlling the level of phosphate is more important for SHPT. Age, sex, diabetes mellitus (DM), dialysis duration, and hyperphosphatemia are associated with SHPT in patients undergoing dialysis.Citation4 In 2002, Agarwal et al showed that children living in areas of high atmospheric pollution have high parathyroid hormone (PTH) levels.Citation5 Recently, Hosseinpanah et al reported that the incidence of SHPT among women living in a polluted area was high.Citation6 In these studies, they did not show which air pollutants were associated with SHPT. To our knowledge, investigations are limited regarding air pollution as a dialysis-unrelated factor for SHPT in patients undergoing peritoneal dialysis (PD). The aim of this cross-sectional survey was to assess the role of air pollution and other clinical variables on SHPT in patients undergoing PD.
Materials and methods
This cross-sectional study complied with the guidelines of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Chang Gung Memorial Hospital, a medical center in the northern part of Taiwan. Because this was a retrospective cross-sectional design, no informed consent was needed. In addition, all patients’ information was securely protected (by delinking identifying information from the main data set) and was available to investigators only. Furthermore, all patients’ records or information were anonymized and deidentified before analysis. All the data were analyzed anonymously. Finally, all primary data were collected according to STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
Study population
We recruited 141 patients who had been undergoing continuous ambulatory PD or automated PD for at least 4 months and regularly followed up at a PD center in Chang Gung Memorial Hospital. All patients were recruited between October 1 and November 30, 2009. PD supplies (continuous ambulatory PD and automated PD solutions) were obtained from Baxter Healthcare SA, Singapore. Patients who developed dialysis-related peritonitis or active infection within 3 months before the study were excluded. The 141 patients had no history of DM, primary HPT, parathyroidectomy, or smoking habits. Age, sex, use of vitamin D3 (calcitriol), use of calcium-based phosphate binders, use of aluminum-based phosphate binders, use of calcimimetics, and clinical data were obtained from the patients’ medical records.
Sample collection
Fasting blood, urine, and dialysate samples were collected on the same day between October 1 and November 30, 2009 during each patient’s yearly routine examination. Residual renal function was calculated as follows: (renal normalized urea nitrogen clearance + renal normalized creatinine [Cr] clearance)/2. The plasma, dialysate, and urine concentrations of Cr, serum albumin, and urea nitrogen were measured using routine laboratory methods. Protein nitrogen appearance (PNA) was normalized to body weight (nPNA). High levels of SO2, NO2, CO, ozone O3, and particulate matter (PM) with an aerodynamic diameter of <10 μm and <2.5 μm (PM10 and PM2.5, respectively) were defined as greater than or equal to the median value of SO2 (4.4 ppb), NO2 (20.1 ppb), CO (0.53 ppm), O3 (28.7 ppb), PM10 (49.1 μg/m3), and PM2.5 (29.69 μg/m3). Anuria was defined as a 24-hour urine volume <50 cm3. HPT was defined as an intact PTH (iPTH) level ≥180 pg/dL.Citation7 Hypoalbuminemia was defined as albumin <3.8 g/dL.Citation8
Air-quality status and analysis
To verify our hypothesis that air-pollutant levels are correlated with iPTH values in patients undergoing PD, we analyzed the database from the Taiwan Air Quality Monitoring Network operated by the Environmental Protection Administration.Citation9 We recorded and analyzed the difference in air quality according to the patients’ living areas. The referenced items included the previous 1-year (12 months) average concentrations of PM10, PM2.5, SO2, NO2, CO, and O3. Air-pollution levels were recorded by a network of 24 monitoring stations near or in the patients’ living areas throughout Taiwan. The 141 patients lived in 25 different districts. In this study, generally, data on air pollutants were obtained from the monitoring station in the same district as the patient. If patients’ living districts did not have a monitoring station, the air-pollutant data from the nearest station were used (within 15 km). If the patient lived between two monitoring stations, the air-pollutant data of the nearest station were selected. Terrain was also a factor; therefore, data from the nearest monitoring station and on the same side of the mountain as where patients lived were selected. The level of air pollutants was checked every hour for 1 year. As a result, we calculated the average of approximately 8,760 (24×365) pieces of data for every monitoring station to determine the 1-year average level of air pollutants in this study.
Statistical analysis
The Kolmogorov–Smirnov test was used to test if variables were normally distributed. A P-value >0.05 was required to assume a normal distribution. Data are expressed in terms of medians and interquartile range in nonnormal distribution variables and as means ± standard deviation in normal distribution variables. Comparisons between groups were performed using the Mann–Whitney test and Student’s t-test. The frequency of categorical variables is expressed as numbers of patients. The χ2 or Fisher’s exact tests were used to analyze the correlation between categorical variables. To calculate the relative correlation of HPT (iPTH ≥180 pg/dL), odds ratios (ORs) and 95% confidence intervals (CIs) were obtained using binary logistic regression models. Logarithmic conversion was made for iPTH, nPNA, high-sensitivity (hs)-CRP, and aluminum levels. In logistic regression analysis, univariate and forward logistic regression analysis were used (variables with P<0.1 in the univariate regression were used in the forward method) for the association between HPT and clinical variables. The following factors were investigated: high SO2 level, high NO2 level, high CO level, high O3 level, high PM10 level, high PM2.5 level, age, PD duration, serum Cr level, logarithmic nPNA level, hypoalbuminemia, serum corrected calcium level, serum phosphate level, anuria condition, logarithmic hs-CRP level, total (renal and peritoneal) Cr clearance, use of calcitriol, use of calcium-based phosphate binders, use of aluminum-based phosphate binders, logarithmic blood aluminum level, anuria, coronary artery disease, hypertension, and high education level. All the nominal variables in linear regression were dummy coding transformed. Missing data were dealt with by list-wise deletion. All statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 141 patients without DM from a single PD center were enrolled in this study. lists the characteristics of the study subjects (mean age 50 years). Of the patients, 111 were women, and their mean duration of PD was 67.3 months. Furthermore, the mean total normalized Cr clearance was 59.25 L/week/1.73 m2. The median iPTH level was 166 pg/dL (71.75–525 pg/dL). The median hs-CRP level was 2.7 mg/L (1.14–7.29 mg/L). The median concentration of PM10 was 49.1 μg/m3, SO2 4.4 ppb, NO2 20.1 ppb, CO 0.53 ppm, O3 28.7 ppb, and PM2.5 29.69 μg/m3. None of these patients smoked. There were 51 patients receiving vitamin D3 supplements, 131 receiving calcium-based phosphate binders, and eleven receiving aluminum-based phosphate binders; none of the 141 patients was receiving calcimimetics, lanthanum carbonate, or sevelamer.
Table 1 Characteristics of the PD patients
Patients undergoing PD with or without hyperparathyroidism
We categorized our patients undergoing PD into without (n=73) and with (n=68) HPT (). Compared with patients without HPT, PD duration (75.11±40.72 vs 60.19±39.07 months, P=0.02), blood inorganic phosphate level (5.23±1.29 vs 4.8±1.09 mg/dL, P=0.03), environmental NO2 level (20.1 [16.3–21.0] vs 21.0 [18.02–22.9] ppb, P=0.02), and environmental CO level (0.53 [0.4–0.57] vs 0.57 [0.44–0.66] ppm, P=0.01) were significantly higher in patients with HPT. Residual renal function (0.39 [0–5.18] vs 3.33 [0–10.7], P=0.02) was lower in patients with HPT.
Table 2 Comparison of patients with iPTH ≥180 pg/dL and without iPTH <180 pg/dL hyperparathyroidism
Factors associated with hyperparathyroidism in patients undergoing PD
To investigate the factors associated with HPT in our study patients, we used univariate binary logistic regression and multivariate logistic regression methods for analyses. High SO2 exposure, high NO2 exposure, high CO exposure, high O3 exposure, high PM10 exposure, high PM2.5 exposure, age, serum Cr level, total normalized Cr clearance values, log nPNA, hypoalbuminemia, blood corrected Ca level, blood inorganic phosphate level, log hs-CRP, PD duration, anuria status, coronary artery disease, use of calcitriol, use of calcium-based phosphate binders, use of aluminum-based phosphate binders, hypertension, high education level, and log Al were investigated as clinical variables. shows that in univariate binary logistic regression, high NO2 exposure (OR 2.3, 95% CI 1.16–4.57; P=0.01), high CO exposure (OR 2.437, 95% CI 1.231–4.823; P=0.01), blood inorganic phosphate levels (OR 1.36, 95% CI 1.02–1.82; P=0.03), hypoalbuminemia (OR 2.56, 95% CI 1.06–6.18; P=0.03), PD duration (OR 1.01, 95% CI 1.001–1.018; P=0.03), use of calcitriol (OR 6.83, 95% CI 3.12–14.96; P<0.001), use of aluminum-based phosphate binders (OR 12.41, 95% CI 1.54–99.81; P=0.01), and anuria status (OR 2.15, 95% CI 1.11–4.34; P=0.02) were positively associated with HPT. Furthermore, in the forward model of binary logistic regression, after adjustment for related factors, high CO exposure (OR 3.226, 95% CI 1.42–7.28; P=0.005), blood inorganic phosphate levels (OR 1.66, 95% CI 1.17–2.37; P=0.005), hypoalbuminemia (OR 3.76, 95% CI 1.29–10.94; P=0.01), and use of calcitriol (OR 8.25, 95% CI 3.43–19.85; P<0.001) were positively associated with HPT.
Table 3 Logistic regression analysis between hyperparathyroidism (iPTH $180 pg/dL) and clinical variables (n=141)
Factors associated with high and low CO-level exposure in patients undergoing PD
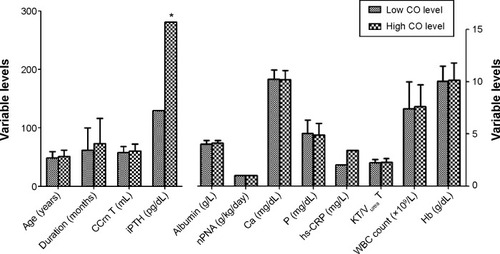
Patients were divided into two subgroups (low, n=80; high, n=61) according to the median environmental CO value (0.53 ppm). Compared with low CO-exposure levels, patients exposed to high CO levels had higher iPTH levels (281 [99–603] vs 130 [67.4–407.25] pg/dL, P=0.04). In addition, age (49.17±10.31 vs 51.18±10.64 years, P=0.26), PD duration (62.4±38.02 vs 73.93±42.82 months, P=0.09), total Cr clearance (58.06±10.3 vs 60.8±12.04 L/week/1.73 m2, P=0.15), nPNA (1.025 [0.88–1.15] vs 1.04 [0.9–1.2] g/kg/day, P=0.7), Ca (10.24±0.87 vs 10.21±0.87 mg/dL, P=0.88), phosphate (5.07±1.27 vs 4.92±1.12 mg/dL, P=0.47), hs-CRP (2.07 [1.12–6.02] vs 3.42 [1.21–9.99] mg/L, P=0.17), KT/Vurea T (2.24±0.3 vs 2.29±0.37, P=0.39), white blood cell count (7.43±2.58 vs 7.6±2.09×109/L, P=0.65), and hemoglobin (10.06±1.41 vs 10.18±1.62 g/dL, P=0.64) were not significantly different in patients living in low and high CO-exposure areas ().
Figure 1 Comparison of patients living in high and low CO-exposure areas.
Abbreviations: iPTH, intact parathyroid hormone; nPNA, normalized protein nitrogen appearance; P, phosphate; hs-CRP, high-sensitivity C-reactive protein; CCrn T, total creatinine clearance; KT/Vurea T, peritoneal KT/Vurea + renal KT/Vurea; WBC, white blood cell; Hb, hemoglobin.
Discussion
In this study, we showed that after adjustment for related risk factors, ambient CO level was significantly positively associated with SHPT in patients undergoing PD who did not have DM.
In patients with CKD, with the decline in renal function and loss of renal 1α-hydroxylase, the concentration of the active form vitamin D also decreases.Citation10 Vitamin D deficiency is a known risk factor for the development of SHPT in patients with chronic renal failure. SHPT has a complex pathogenesis primarily caused by a decrease in calcitriol and anomalies in regulation of serum calcium and phosphorus levels. In patients with chronic renal failure, serum levels of 1,25(OH)2D were positively correlated with estimated Cr clearance.Citation11 However, it is interesting that in 1982, Lambert et al showed that without vitamin D purveyance, a serum level of calcitriol was detected in five adult patients undergoing anephric hemodialysis.Citation12 This phenomenon suggests the existence of extrarenal production of calcitriol. In an in vitro study, calcitriol was synthesized by human decidua and placenta,Citation13 rat placenta tissue,Citation14 human bone cell cultures,Citation15 human macrophages,Citation16 and isolated calvarial cells.Citation17 Additionally, several studies identified another pathway for synthesis of calcitriol in the skin.Citation18,Citation19 Working in concert with these studies, Krause recently pointed out that in long-term patients undergoing hemodialysis, the use of sun-simulating artificial lamps could significantly increase blood calcitriol and calcidiol levels.Citation20 Krause also pointed out that increased skin ultraviolet (UV)-B radiation could improve the residual capacity of the kidneys’ endocrine function in patients with CKD to produce more 1,25(OH)2D3.
There are many exogenous factors affecting the dermal production of cholecalciferol, such as sunlight, air pollution, sunscreen, clothing, latitude, and season.Citation21 The most notable of these factors in urbanized society is air pollution. In many industrialized cities, air pollution can absorb or obstruct UVB, such as absorption by nitrated and aromatic aerosols, nitrated aromatic gases and benzaldehyde, which are coexhausted with CO, were hypothesized to have reduced observed downward global UVA and UVB irradiance,Citation22 thus reducing the dermal photosynthesis of precholecalciferol. This may result in an increased risk of vitamin D deficiency in people who live in polluted cities.Citation21 It has been previously raised that air-pollution exposure can lead to a variety of complications such as increased risk of cardiovascular events,Citation23–Citation25 cerebrovascular accidents,Citation26,Citation27 specific cancers,Citation28 and respiratory systemic diseases.Citation23,Citation24 Agarwal et al and Hosseinpanah et al showed that even healthy women or children living in areas of high atmospheric pollution had higher PTH levels than those living in low-pollution areas.Citation5,Citation6
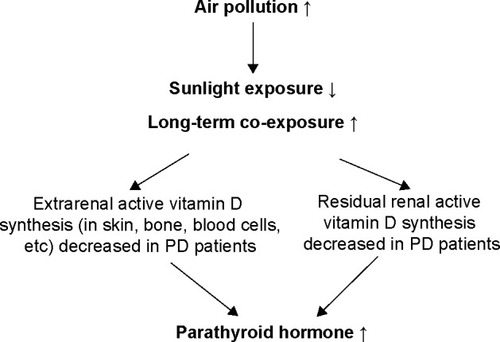
The most likely explanation is that UVB radiation is absorbed or obstructed by air pollution from industrial or vehicular sources and reduces dermal vitamin D synthesis.Citation21,Citation29 Neither Agarwal et al nor Hosseinpanah et al determined what the main chemical particles are that affect vitamin D synthesis. However, in a recent animal study, Feger et al showed that by decreasing transcript levels of Cyp27b1, CO plays a role in decreasing 1,25(OH)2D3 synthesis.Citation30 To the best of our knowledge, direct evidence on the correlation between air pollution and parathyroid dysfunction is limited. Based on the cited studies, except for the relationship between the kidneys and active vitamin D synthesis, we could make an inference that via the pathway of decreasing residual renal and extrarenal active vitamin D synthesis, high ambient CO levels could be associated with increasing PTH synthesis in our patients ().
Figure 2 Inference of the correlation between air pollution, CO, and parathyroid hormone.
We also found that hypoalbuminemia was correlated with high PTH levels when it was selected as an adjustment factor in the stepwise logistic regression analysis (). However, in patients with chronic renal failure, Ishimura et alCitation11 showed that levels of 1,25(OH)2D3, 1,25(OH)2D3, and 25(OH)2D3 were positively correlated with serum albumin levels. In an animal study, dogs with hypoalbuminemia had high PTH levels and low 25(OH)2D3 concentrations.Citation31 The positive correlation between serum vitamin D-binding protein and serum albumin levelCitation32 and the previously cited studies could support our observations.
This study had some limitations. First, this was a small-population study to investigate such a large number of variables. Second, we did not check 25(OH)D3 or 1,25(OH)2 D3 serum levels because of the cross-sectional study design. The positive correlation between air pollution and vitamin D levels in patients undergoing PD is worth further evaluation. Third, we had little information on the range of patient activities and indoor air quality of the studied patients. Fourth, the information on the patients’ sunlight exposure per day was limited. Fifth, we are uncertain whether the previous 1-year average air-quality data for the patients’ living areas published by the Taiwan Environmental Protection Administration was sufficiently objective to represent the air quality around the patients. However, according to air-pollution studies of short-termCitation33 and long-termCitation34 exposure, we believe that our 1-year average air-pollution concentration data were adequately representative for this study.
Conclusion
In conclusion, the findings of this cross-sectional study indicated the presence of an association between ambient CO and SHPT in patients undergoing PD who did not have DM. Therefore, poor environmental air quality may be a risk factor for deterioration of SHPT in patients undergoing PD.
Acknowledgments
The authors thank the members of the Statistics Center in Chang Gung Memorial Hospital for their invaluable and dedicated assistance, and the members of the Peritoneal Dialysis Center in Chang Gung Memorial Hospital for their invaluable and dedicated assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- CouserWGRemuzziGMendisSTonelliMThe contribution of chronic kidney disease to the global burden of major noncommunicable diseasesKidney Int201180121258127021993585
- OwdaAElhwairisHNarraSToweryHOsamaSSecondary hyper-parathyroidism in chronic hemodialysis patients: prevalence and raceRen Fail200325459560212911164
- SlatopolskyEBrownADussoAPathogenesis of secondary hyperparathyroidismKidney Int Suppl199973S14S1910633458
- DouthatWGCastellanoMBerenguerLHigh prevalence of secondary hyperparathyroidism in chronic kidney disease patients on dialysis in ArgentinaNefrologia201333565766624089157
- AgarwalKSMughalMZUpadhyayPBerryJLMawerEBPuliyelJMThe impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, IndiaArch Dis Child200287211111312138058
- HosseinpanahFPourSHHeibatollahiMMoghbelNAsefzadeSAziziFThe effects of air pollution on vitamin D status in healthy women: a cross sectional studyBMC Public Health20101051920799984
- NakaiSAkibaTKazamaJEffects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in JapanTher Apher Dial2008121495418257812
- FouqueDKalantar-ZadehKKoppleJA proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney diseaseKidney Int200873439139818094682
- Environmental Protection AdministrationTaiwan Air Quality Monitoring Network Available from: http://taqm.epa.gov.tw/taqm/en/default.aspxAccessed August 25, 2014
- MartinezISarachoRMontenegroJLlachFA deficit of calcitriol synthesis may not be the initial factor in the pathogenesis of secondary hyperparathyroidismNephrol Dial Transplant199611Suppl 322288840307
- IshimuraENishizawaYInabaMSerum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failureKidney Int19995531019102710027939
- LambertPWSternPHAvioliRCEvidence for extrarenal production of 1α,25-dihydroxyvitamin D in manJ Clin Invest19826937227256895901
- WeismanYHarellAEdelsteinSDavidMSpirerZGolanderA1α,25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placentaNature19792815729317319551281
- TanakaYHalloranBSchnoesHKDeLucaHFIn vitro production of 1,25-dihydroxyvitamin D3 by rat placental tissueProc Natl Acad Sci U S A1979761050335035291919
- HowardGATurnerRTSherrardDJBaylinkDJHuman bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3J Biol Chem198125615773877406973569
- DussoASFinchJBrownAExtrarenal production of calcitriol in normal and uremic humansJ Clin Endocrinol Metab19917211571641986015
- TurnerRTPuzasJEForteMDIn vitro synthesis of 1α, 25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cellsProc Natl Acad Sci U S A19807710572057246934505
- LehmannBGenehrTKnuschkePPietzschJMeurerMUVB-induced conversion of 7-dehydrocholesterol to 1α,25-dihydroxyvitamin D3 in an in vitro human skin equivalent modelJ Invest Dermatol200111751179118511710930
- ZehnderDBlandRWilliamsMCExtrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylaseJ Clin Endocrinol Metab200186288889411158062
- KrauseRVitamin D and UV exposure in chronic kidney diseaseDermatoendocrinol20135110911624494043
- HolickMFEnvironmental factors that influence the cutaneous production of vitamin DAm J Clin Nutr1995613 Suppl638S645S7879731
- JacobsonMZIsolating nitrated and aromatic aerosols and nitrated aromatic gases as sources of ultraviolet light absorptionJ Geophys Res Atmos1999104D335273542
- DockeryDWPopeCA3rdXuXAn association between air pollution and mortality in six U.S. citiesN Engl J Med199332924175317598179653
- DockeryDWStonePHCardiovascular risks from fine particulate air pollutionN Engl J Med2007356551151317267912
- GanWQDaviesHWKoehoornMBrauerMAssociation of long-term exposure to community noise and traffic-related air pollution with coronary heart disease mortalityAm J Epidemiol2012175989890622491084
- MateenFJBrookRDAir pollution as an emerging global risk factor for strokeJAMA2011305121240124121427378
- TsaiSSGogginsWBChiuHFYangCYEvidence for an association between air pollution and daily stroke admissions in Kaohsiung, TaiwanStroke200334112612261614551399
- Raaschou-NielsenOAndersenZJHvidbergMLung cancer incidence and long-term exposure to air pollution from trafficEnviron Health Perspect2011119686086521227886
- CalabreseEJThe influence of ambient ozone on the incidence of bone fractures especially among the elderlyMed Hypotheses197952201207459973
- FegerMFajolALebedevaAEffect of carbon monoxide donor CORM-2 on vitamin D3 metabolismKidney Blood Press Res2013374–549650524247848
- GowAGElseREvansHBerryJLHerrtageMEMellanbyRJHypovitaminosis D in dogs with inflammatory bowel disease and hypoalbuminaemiaJ Small Anim Pract201152841141821797872
- BrownISoodACarterNDVitamin D binding globulin levels and affinity in various clinical conditionsJ Clin Pathol198033109669706893597
- KatsouyanniKTouloumiGSpixCShort-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air Pollution and Health: a European ApproachBMJ19973147095165816639180068
- PanasevichSLeanderKRosenlundMAssociations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sampleOccup Environ Med2009661174775319687019
