Abstract
Background
To evaluate the respective or combinatory efficacy of locally delivered 2% minocycline (MO), and scaling and root planning (SRP) by assessing both clinical parameters and the loads of four main periodontal pathogens in treating chronic periodontitis (CP).
Methods
Seventy adults with CP were randomly assigned to the three treatment groups: 1) SRP alone; 2) MO alone; and 3) combinatory use of SRP and MO (SRP + MO). Before and 7 days after the treatments, we evaluated both clinical parameters (pocket depth [PD] and sulcus bleeding index [SBI]) and the gene load of four main periodontal pathogens (Aggregatibacter actinomycetemcomitans [Aa], Fusobacterium nucleatum [Fn], Porphyromonas gingivalis [Pg], and Prevotella intermedia [Pi]).
Results
The bacterial prevalence per patient was: Aa, 31.25%; Fn, 100%; Pg, 95.31%; and Pi, 98.44%. Seven days after treatment, the three treatments significantly reduced both PD and SBI, but not detection frequencies of the four pathogens. For PD, the reduction efficacy of SRP + MO was significantly higher than that of either MO or SRP. Only Pg responded significantly to SRP. Pg and Fn were significantly reduced in the presence of MO. Only SRP + MO showed a significant reduction effect on the gene load of Pi. The reduction of PD significantly correlated with the gene load of Pi (r=0.26; P=0.042) but not of the other bacteria.
Conclusion
SRP and MO reduced the load of Pi in an interdependent pattern, which correlated with symptomatic improvements of CP.
Introduction
Chronic periodontitis (CP), the most common periodontal disease, is an inflammatory disease leading to destruction of connective tissue and loss of the adjacent supporting bone. The initiation and progression of CP is a consequence of interaction between oral bacteria and the host immune responses. Bacteria including Aggregatibacter actinomycetemcomitans (Aa), Fusobacterium nucleatum (Fn), Porphyromonas gingivalis (Pg), and Prevotella intermedia (Pi) are currently thought to be highly associated with CP.Citation1 The primary goal of periodontal therapy is to reduce these periodontopathogens, so as to halt disease progression.
Scaling and root planning (SRP) is one effective mechanical treatment for most periodontal infections and remains an essential part of successful periodontal therapy.Citation2 SRP can directly remove the biofilm on the surface of the tooth root, and improves periodontal status, but it is rarely possible to completely remove periodontal pathogens with SRP.Citation3 The efficacy of SRP can be limited in cases with less access to deep pockets and furcations. In addition, there are well-documented secondary effects, such as gingival recession, loss of tooth substance, and dentin hypersensitivity.Citation4 Antimicrobial therapy is another regimen for treating periodontal disease by eradicating periodontopathic bacteria, especially for bacteria harbored at the bottom of deep pockets or in dentin tubules and not able to be removed by mechanical treatment. Antibiotics can be administered systemically or locally. A local drug delivery system has the advantage of possibly avoiding the side effect of increased bacterial resistance associated with systemic antibiotic therapy. Therefore, it has been used as a single therapy or as an adjunct to mechanical and surgical periodontal treatment, particularly in sites with deeper periodontal pockets.Citation5,Citation6
Minocycline (MO), one of the most active antibiotics against periodontopathogens, is a semisynthetic derivative of tetracycline with a broad antibacterial spectrum.Citation7 On one hand, some studies showed that CP patients responded favor-ably to SRP, but did not seem to benefit from an effect of local MO.Citation8,Citation9 On the other hand, many studies showed that MO provides additional clinical improvements when used as an adjunct to mechanical therapy.Citation10–Citation13 Moreover, the sensitivity of bacteria to a treatment option may vary according to the abundance and status of bacteria. The prevalence and abundance vary in different ethnicities.Citation14 The planktonic bacteria showed less sensitivity to antibiotics than those in biofilm. Moreover, the resistance of microorganisms to therapies can be induced in clinic if antibiotics are abused. Consequently, it is always of paramount importance to monitor the effectiveness of a therapy to reduce bacteria load and clinical symptoms of CP through well-performed clinical trials.
Hitherto, most of the clinical trials that evaluate the efficacy of SRP and MO focus on the clinical symptoms, while little data are available regarding quantifying bacteria counts in the local application of MO. Although the reduction of bacteria load may correlate with the improvement of clinical parameters, it is not elucidated whether the reduction of a certain bacterium may play a more important role than the others. In this randomized clinical trial, we evaluated the respective or combinatory efficacy of MO and SRP in the aspects of both clinical parameters (pocket depth [PD] and sulcus bleeding index [SBI]) and the loads of four main periodontal pathogens (Aa, Fn, Pg, and Pi). Real-time quantitative real-time PCR (qRT-PCR)Citation15,Citation16 was used as a powerful tool with high sensitivity and specificity to quantitatively assess target periodontal bacteria in a period of 7 days. We also tried to correlate the reduction of either total or respective bacteria with the improvements of clinical parameters, with an aim to uncovering the potential microbiological mechanism accounting for the efficacy of a therapy.
Materials and methods
Compliance with ethics guidelines
All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Stomatology of Zhejiang University on March 27, 2013. Informed, written consent was obtained from each participant for inclusion in the study. This clinical trial was registered on the website of US National Institutes of Health Clinical Trials Registry (ID number: NCT02355977). The registration of this study was performed after the enrollment of participants began, as the registration of clinical trials was not obligatory in the People’s Republic of China at that time. The authors confirmed that all ongoing and related trials for this drug/intervention were registered.
Subjects and randomization
All the subjects were recruited from the Affiliated Hospital of Stomatology of Zhejiang University from June 2013 to September 2014. All teeth of the subjects underwent periodontal examination. PD, clinical attachment, and bleeding on probing (BOP) were recorded at six sites (buccal-mesial, mid-buccal, buccal-distal, lingual-mesial, mid-lingual, and lingual-distal) of teeth. The inclusion criteria were: the CP group diagnosed with moderate or severe chronic periodontal disease who exhibited BOP and attachment loss, with radiographic alveolar bone loss in four or more teeth (PD ≥4 mm, clinical attachment ≥3 mm).Citation17 Patients were excluded if they were pregnant, had used antibiotics within the last 3 months, had periodontal therapy in the past 6 months, or had systemic diseases such as heart disease or hypertension. Two experienced periodontists performed the clinical examination in this selection procedure. The examiners were blinded to the assignment of interventions.
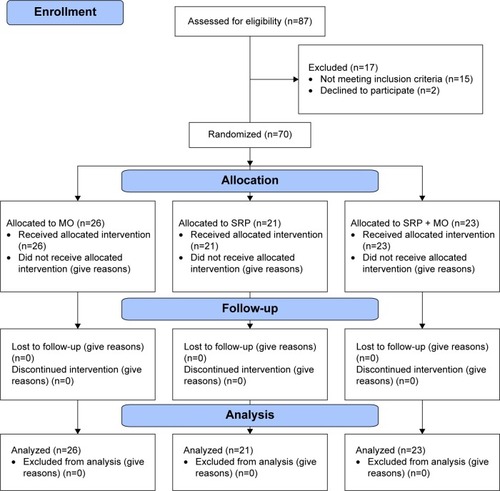
Participants were randomly assigned to one of the three treatment groups: 1) SRP treatment alone; 2) locally delivered 2% MO alone (Perio® ointment, Sunstar Inc, Osaka, Japan); or 3) SRP treatment with a following adjunctive use of locally delivered 2% MO (SRP + MO). The minimal inhibitory concentration of MO was <0.1 μg/mL for Pg, <0.39 μg/mL for Pi, and <0.78 μg/mL for Fn.Citation18 Pharmacological analysis revealed that the MO released from the MO ointment reached 1,400 μg/mL 1 hour postadministration in gingival crevicular fluid from periodontitic sockets. The concentration decreased with time and retained 1.59 μg/mL 5 days post-administration.Citation19 The randomization process was performed using SPSS 16.0 software (IBM, Chicago, IL, USA) by a computer-generated randomly permuted block. The randomized distribution resulted in comparable mean values of all investigated parameters in all groups. We estimated the sample size by examining previous publications with a similar clinical grouping. To ensure a sufficient power in this trial, we adopted around 21–26 participants per group, which was more than that in a previous report (13 participants per group).Citation8 We tested the power with the adopted sample size. For example, the mean PD changes (mm) of SRP, MO, and SRP + MO group were −0.65, −0.55, and −1.05. Basing on the given sample size in each group, the effect size f was 0.401. Using G*Power (version 3.1.9.2) softwareCitation20 to perform the calculation, the power for a one-way analysis of variance (ANOVA) analysis was then 0.845, which was higher than the required 0.80. Consequently, the power analysis for these parameters confirmed the adequacy of the sample size in our study. The CONSORT (Consolidated Standards of Reporting Trials) study flowchart is outlined in .
Treatment
For the SRP treatment, a single-visit, one-stage, full mouth removal of calculus and plaque that attached to the tooth surfaces was performed using periodontal ultrasonic scaler (Satelec, Mérignac, France). The SRP treatment was meticulously performed to achieve clean and smooth root surface. For the treatment of locally delivered 2% MO, MO was administered directly into the periodontal pocket up to the gingival margin of the selected teeth.
Before, and 7 days after treatment, we evaluated the following clinical parameters: PD and BOP. All clinical measurements were recorded by the same calibrated experienced periodontist. The examiner was masked to the experimental design. PD was measured using a standard CPI probe (Shanghai Medical Instruments, Shanghai, People’s Republic of China) and assessed to the nearest millimeter. BOP was evaluated for the treated tooth using the SBI by Muhlemann and Son with a range of 0 (no bleeding) to 5 (profuse bleeding).Citation21 Participants maintained their own routine oral hygiene during the 7-day study period. The periodontists for the clinical examination and the researchers for doing microbiological assessment were blinded to the assignment of interventions. After this study, SRP was subsequently performed on the participants in the group of MO. This was to ensure the clinical treatment efficacy in this group of participants, since SRP was considered to be the standard treatment option for CP.
Quantitative analysis of bacteria gene load using real-time PCR
Samples of subgingival plaques were collected from the targeted teeth using a standard procedure.Citation22 The sampled teeth were isolated with sterile cotton rolls, and underwent gentle air drying. Supragingival plaque samples were carefully removed by sterile explorers from the gingival margin to prevent contamination with subgingival plaque. A #30 sterile paper point was gently inserted into each periodontal pocket until resistance was felt. After 20 seconds, four paper points from a sampled tooth were immediately placed in a vial containing 1.5 mL of sterile reduced transfer fluid buffer. All samples were stored at −80°C immediately after collection.
DNA isolation from plaque samples and qRT-PCR was performed as previously described.Citation23 Briefly, DNAs from plaque samples and cultured Aa (ATCC 29523), Fn (ATCC 25586), Pg (ATCC 33277), and Pi (ATCC 25611) were extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The DNA concentrations of cultured bacterial strains were quantified by a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA), and the number of bacterial cell copies was quantified and calculated based on the molecular mass formula. Quantification of target species from unknown plaques was achieved by projecting them to standard curves of targeted bacteria based on counts of pure bacterial cultures with serial tenfold dilution from 102 to 107 cell copies. Bacteria-specific primer pairs () according to the literature based on the 16S rRNA gene, were used to quantify each target bacterium.Citation24 All samples were run in duplicate in 96-well plates in a LightCycler® 480 Real-Time PCR System (Roche Diagnostics, Mannheim, Germany). RT-PCR amplification was performed in a 10 μL reaction mixture containing 1 μL template DNA, 5 μL LightCycler® 480 SYBR Green I Master, 1 μL 2.5 μM bacterium-specific primer pair, and 3.5 μL ddH2O. The amplification cycling conditions were 95°C for 10 minutes; 40 cycles of 10 seconds at 95°C, 15 seconds at a bacterium-specific annealing temperature and 40 seconds at 72°C. Melting curve analysis was performed for each run to evaluate the specificity of the PCR products.
Table 1 PCR primer sequences for four main periodontal pathogens
Statistical analysis
We first used both Kolmogorov–Smirnov test and D’Agostino and Pearson omnibus normality test to comprehensively check the normality of each data. According to the results, we selected either parametric tests or non-parametric tests to analyze the data. To assess the efficacy of each treatment in changing the clinical parameters and microbial gene load before, and 7 days after treatment, we adopted either a paired t-test or a Wilcoxon signed ranks test accordingly. For the other comparisons, we adopted either one-way ANOVA with Bonferroni t-test for post hoc comparisons or Kruskal–Wallis H test accordingly. The correlations between the changes of clinical parameters and the bacteria gene load were evaluated using either Spearman’s rank correlation or Pearson’s correlation. All tests were two-sided with a significance level of 0.05. All analyses were conducted with SAS v9.2.
Results
Thirty-six male and thirty-four female patients with an age of 46.96±12.36 years (mean ± SD) were recruited for this study. In all, 21, 26, and 23 patients were assigned to the SRP, MO, and SRP + MO groups, respectively. One-way ANOVA test showed no significant differences in patients’ age, clinical parameters, and bacteria gene load among the three groups before treatments, which indicated the homogeneity of the three groups (). Analysis of t-test showed that sex or smoking history had no significant influence in SBI or PD. The prevalence of the bacteria per patient was Aa, 31.25%; Fn, 100%; Pg, 95.31%; and Pi, 98.44%.
Table 2 Distribution of demographic characteristics, clinical parameters, and bacteria gene load among the three groups before treatment
All SRP + MO three treatments significantly (P<0.001) reduced both PD () and SBI () 7 days after treatment. For PD, the reduction efficacy of SRP + MO was significantly higher than those of both MO (P=0.015) and SRP (P=0.009). For SBI, the reduction efficacy of SRP + MO was significantly higher than that of MO (P=0.007) but not of SRP. MO and SRP showed no significant difference in reducing PD or SBI.
Figure 2 The efficacy of three treatments (locally delivered 2% minocycline [MO], scaling and root planning [SRP], and their combinatory treatment [SRP + MO]) in improving the clinical symptoms ([A] pocket depth [PD]; and [B] sulcus bleeding index [SBI]) and reducing the gene loads of four main periodontal pathogens ([C] Aggregatibacter actinomycetemcomitans [Aa]; [D] Fusobacterium nucleatum [Fn]; [E] Porphyromonas gingivalis [Pg]; and [F] Prevotella intermedia [Pi]) for the patients with chronic periodontitis.
![Figure 2 The efficacy of three treatments (locally delivered 2% minocycline [MO], scaling and root planning [SRP], and their combinatory treatment [SRP + MO]) in improving the clinical symptoms ([A] pocket depth [PD]; and [B] sulcus bleeding index [SBI]) and reducing the gene loads of four main periodontal pathogens ([C] Aggregatibacter actinomycetemcomitans [Aa]; [D] Fusobacterium nucleatum [Fn]; [E] Porphyromonas gingivalis [Pg]; and [F] Prevotella intermedia [Pi]) for the patients with chronic periodontitis.](/cms/asset/daeb3ee8-e516-4eb5-863c-1052bc2a8152/dtcr_a_93982_f0002_b.jpg)
None of the three treatments could significantly reduced the gene load of Aa (). For Fn, MO and SRP + MO significantly reduced its gene load (). Such an effect of SRP + MO was significantly higher than that of SRP. All three treatments significantly reduced the gene load of Pg (). Such an effect of SRP was significantly lower than those of MO and SRP + MO. Only SRP + MO showed a significant reduction effect on the gene load of Pi (). Such an effect of SRP + MO was significantly higher than that of either MO or SRP.
We found that the reduction of PD was significantly correlated with the total load reduction of the four bacteria (Spearman’s r=0.25; P=0.049) (). We further found that the reduction of PD was significantly correlated with the gene load of Pi (Pearson’s r=0.26; P=0.042) but not of the other types of bacteria. SBI was significantly correlated with the reduction of PD (Pearson’s r=0.43; P<0.001) but not with the reduction of any bacteria.
Table 3 Correlation of the reduction of clinical parameters (pocket depth [PD] and sulcus bleeding index [SBI]) for all the cases (data from the three groups were combined) with the reduction of gene load of four main periodontal bacteria
Discussion
SRP is regarded as the gold standard in the treatment of periodontitis by mechanically removing periodontal pathogens.Citation25 Locally administrated antibiotics (eg, MO) can provide better access and improve clinical periodontal conditions by chemically killing bacteria. Therefore, SRP and locally administrated antibiotics are more frequently adopted as a combinatory therapy, which can further reduce bacteria load.Citation11,Citation26 In this randomized clinical trial, we assessed the respective or combinatory efficacy of MO and SRP in the aspects of both clinical parameters (PD and SBI) and the loads of four main periodontal pathogens (Aa, Fn, Pg, and Pi). We also, for the first time, showed a significant correlation between the reduction of PD with the reduction of Pi instead of the other selected bacteria.
To control the homogeneity of patients in the three groups, we statistically analyzed the characteristics in both clinical parameters and periodontal pathogens (). No significant difference was found among the three groups in both aspects, which indicated the homogeneous distribution of patients among the three groups through the predetermined randomization program.
The prevalence and distribution of periodontal pathogens are highly variable according to the selection of subjects, sampling sites, as well as the type of analytical procedures.Citation27 In addition, genetic analysis of bacteria has demonstrated an unanticipated diversity within species. Carriage rates and particular subsets of these species vary between ethnic groups.Citation28 Hagiwara et al, using a cultivable technique, reported the prevalence per patient of Aa, Fn, Pg, and Pi as 14.3%, 38.1%, 38.1%, and 42.9% respectively.Citation27 Gatto et al used real-time PCR to examine the prevalence of periodontal pathogens in subgingival samples of Italian patients with CP. He reported that Aa and Fn had the lowest and highest prevalence respectively (18.5% and 95%). The prevalence of Pg and Pi was 78% and 66%, respectively.Citation29 In this study, we showed a similar result as that of Gatto: Aa and Fn had the lowest and highest prevalence (Aa: 31.25% and Fn: 100%), while Pg and Pi also exhibited very high detection ratio (Pg 95.31% and Pi 98.44%). The 100% prevalence of Fn in this study was in line with reported prevalence rates (80%–100%).Citation29–Citation31 It was shown that Fn was one of the most abundant Gram-negative anaerobes in mature supragingival and subgingival plaques of both healthy subjects and patients with periodontitis.Citation32 Aa showed the least prevalence in among the four selected bacteria, which was consistent with previous reports.Citation31,Citation33,Citation34 This may be due to the fact that Aa is more associated with the aggressive periodontitis than CP.Citation35 The high prevalence detected for Pg (95.31%) agrees with previous results obtained in Spanish,Citation31 Norwegian,Citation33 Romanian,Citation36 and Italian populations.Citation29 Some authors have suggested that Pg is associated with disease progression and the proportion of this species increasing in deep pockets.Citation37,Citation38 We also found that Fn, Pg, and Pi had a high presence in Chinese CP patients, which was in accordance with the prevalence of these bacteria in Asian counties.Citation23
In this clinical trial, both MO alone and SRP alone significantly (P<0.001) resulted in significant reduction of PD (0.65 mm and 0.55 mm, respectively) () and SBI (0.58 and 0.41, respectively) (). No significant differences were found between MO and SRP in the effects of reducing PD or SBI. This result suggested that both MO alone and SRP alone were effective in improving clinical symptoms of CP with a similar efficacy. This was also consistent with a short-term study performed by Buchmann et al who found no difference at week 2.Citation39 In contrast, the combination of MO and SRP resulted in a significantly higher reduction of PD (1.05 mm) than either MO alone or SRP alone. The combinatory therapy also resulted in a significantly higher reduction of SBI than MO alone but not SRP alone. This result showed that the combinatory therapy of MO and SRP was more effective than MO alone or SRP alone, 1 week postoperation. This result was consistent with previous publications that combination of MO and SRP could result in significantly lower PD but not SBI in comparison with SRP alone.Citation8
Although both individual and combinatory therapies of MO and SRP were shown to significantly improve the clinical symptoms, bacteria did not respond accordingly. Different bacteria responded differentially to certain therapies (). For example, neither the individual nor the combinatory therapy of MO and SRP could significantly reduce the load of Aa (). This was consistent with previous studies that SRP and 2% MO ointment did not significantly change the level of Aa.Citation40 Although SRP was regarded as the gold standard treatment for CP, only Pg among the four selected periodontal pathogens showed a significant reduction in response to SRP alone (). While the sensitivity of Pg to SRP was significantly lower than that to locally administrated MO. Pg showed a superior sensitivity to MO than Fn, while Aa or Pi did not respond significantly (). Consistent with our result (), Maeda et al also found that bacterial loads of Pg and Pi were markedly decreased in subgingival plaques from eight patients post 1 week after the local drug delivery of MO.Citation41 The inability to reduce Pi by MO alone in this study may be due to the fact that Pi exhibited low susceptibility to MO HCl.Citation27 Consistently, the combinatory therapy resulted in a significantly higher reduction of Pg than SRP but not MO (). Interestingly, although Pi did not show good response to either MO alone or SRP alone, it responded very well to the combinatory therapy of MO and SRP (). This finding suggested that the effect of MO and SRP in reducing Pi was interdependent. The mechanism accounting for such a phenomenon remains to be elucidated.
Consistent with previous studies, the improvements in PD correlated with the total reduction of the four periodontal pathogens (). To further identify the contribution of each bacterium load reduction to symptomatic improvements, we tried to correlate the load reduction of each bacterium with PD or SBI (). We found the reduction of PD significantly correlated with the load reduction of Pi but not of the other three bacteria. This finding suggested that the reduction of Pi might give an important contribution to the reduction of PD. Since the load of Pi could be significantly reduced only by the combinatory but not the respective therapy of MO and SRP, it corroborated the necessity to adopt the combinatory therapy of MO and SRP to treat CP. Moreover, the sensitivity of Pi can also be used as an important parameter to evaluate the efficacy of a novel therapy. The reduction of SBI was found to be significantly correlated with the reduction of PD but not with load reduction of any bacteria. It seemed that the improvement in SBI was dependent on the reduction of PD.
Concerns may be raised on the adoption of MO alone as one treatment group since local MO application is commonly accepted as an adjunct therapy to SRP in the treatment of CP. However, we believe that such a group set-up is of both clinical and scientific significance. Firstly, in previously published clinical trials, locally administrated antibiotics could be used as a single treatment to treat periodontitis.Citation42–Citation44 Furthermore, local administration of antibiotics alone was proved to improve clinical symptoms of periodontitis, such as PD and SBI.Citation26 In our study, we did find both MO alone and SRP alone could significantly reduce clinical symptoms. MO alone was even more effective in reducing the bacterial load than SRP alone. Consequently, our data suggested more sensitivity of the selected bacteria to MO than SRP at least in this short term. Secondly, direct administration of SRP may potentially cause excessive bleeding and possibly transient bacteremia, which may potentially result in infection of other organs, such as the heart. The bacteremia can occur immediately after SRP in a rate of as high as 70%, with Pg showing the highest frequency in blood.Citation45 A sub sequent and adjunctive antibiotic can definitely not prevent the occurrence of bacteremia. Although such an application was not intensively investigated, studies indeed show that topical administration of antibacterial agents could reduce the incidence and magnitude of bacteremia caused by SRP or other dental surgery.Citation46–Citation48 Since the antibacterial effect of locally administrated antibiotics is expected to be much stronger than mouth rinsing, it is worth while to further investigate the effect of precedent local antibiotics on the prevention of bacteremia caused by SRP.
One limitation of this study was that we detected only four main periodontal pathogens. The role of other pathogens should be further investigated. In further study, the adoption of more advanced molecular microbiological methods for bacterial detection, such as sequencing, can be expected to provide more comprehensive knowledge to this field.
Acknowledgments
This study was supported by grants from Zhejiang Provincial Natural Science Foundation (LY13H140002), General Project of Health and Family Planning Commission of Zhejiang Province (2012KYB121, 2015KYB229), and the 2011 China State key Clinical Department.
Disclosure
The authors report no conflicts of interest in this work.
References
- LovegroveJMDental plaque revisited: bacteria associated with periodontal diseaseJ NZ Soc Periodontol2004721
- AmidRKadkhodazadehMFekrazadRHajizadehFGhafooriAComparison of the effect of hand instruments, an ultrasonic scaler, and an erbium-doped yttrium aluminium garnet laser on root surface roughness of teeth with periodontitis: a profilometer studyJ Periodontal Implant Sci20134310110523678394
- SgolastraFSeverinoMPetrucciAGattoRMonacoAEffectiveness of metronidazole as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysisJ Periodontal Res201449101923668676
- HaffajeeADCuginiMADibartSSmithCKentRLJrSocranskySSThe effect of SRP on the clinical and microbiological parameters of periodontal diseasesJ Clin Periodontol1997243243349178112
- BonitoAJLuxLLohrKNImpact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic reviewJ Periodontol2005761227123616101353
- KhatriPMKumarRUse of minocycline as systemic antimicrobial therapy in refractory periodontitis with chronic gingival enlargementJ Adv Pharm Technol Res20123757922470898
- van WinkelhoffAJAntibiotics in the treatment of peri-implantitisEur J Oral Implantol20125SupplS43S5022834393
- CortelliJRQueridoSMAquinoDRRicardoLHPallosDLongitudinal clinical evaluation of adjunct minocycline in the treatment of chronic periodontitisJ Periodontol20067716116616460239
- ZingaleJHarpenauLBruceGChambersDLunderganWThe effectiveness of scaling and root planing with adjunctive time-release minocycline using an open and closed approach for the treatment of periodontitisGen Dent20126030030522782041
- EhmkeBMoterABeiklerTMilianEFlemmigTFAdjunctive antimicrobial therapy of periodontitis: long-term effects on disease progression and oral colonizationJ Periodontol20057674975915898936
- Matesanz-PerezPGarcia-GargalloMFigueroEBascones-MartinezASanzMHerreraDA systematic review on the effects of local anti-microbials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitisJ Clin Periodontol20134022724123320860
- JungDYParkJCKimYTThe clinical effect of locally delivered minocycline in association with flap surgery for the treatment of chronic severe periodontitis: a split-mouth designJ Clin Periodontol20123975375922691058
- PanditNDahiyaRGuptaRBaliDKathuriaAComparative evaluation of locally delivered minocycline and metronidazole in the treatment of periodontitisContemp Clin Dent20134485323853452
- Human Microbiome Project ConsortiumStructure, function and diversity of the healthy human microbiomeNature201248620721422699609
- HaffajeeADSocranskySSIntroduction to microbial aspects of periodontal biofilm communities, development and treatmentPeriodontol 200020064271216930302
- TakahashiNIshiharaKKimizukaROkudaKKatoTThe effects of tetracycline, minocycline, doxycycline and ofloxacin on Prevotella intermedia biofilmOral Microbiol Immunol20062136637117064394
- RamseierCAKinneyJSHerrAEIdentification of pathogen and host-response markers correlated with periodontal diseaseJ Periodontol20098043644619254128
- NakashimaKSuidoHEguchiTNakamuraMSugiharaKMurayamaYAntibiotic therapy in periodontal disease. 1. Selection of antibioticsNihon Shishubyo Gakkai Kaishi1987294634713506022
- ChenWSunWGengXLiLIn vivo study of 2% minocycline-HCl ointment release profile in gingival crevicular fluidStomatology200525353354
- FaulFErdfelderELangAGBuchnerAG*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciencesBehav Res Methods20073917519117695343
- MuhlemannHRSonSGingival sulcus bleeding – a leading symptom in initial gingivitisHelv Odontol Acta1971151071135315729
- SerraESFWCasarinRCNicolela JuniorELPassosHMSallumAWGoncalvesRBMicrobial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patientsPLoS One20149e10976125329160
- HeJHuangWPanZQuantitative analysis of microbiota in saliva, supragingival, and subgingival plaque of Chinese adults with chronic periodontitisClin Oral Investig20121615791588
- SuzukiNYoshidaASaitoTKawadaMNakanoYQuantitative microbiological study of subgingival plaque by real-time PCR shows correlation between levels of Tannerella for sythensis and Fusobacterium sppJ Clin Microbiol2004422255225715131205
- HungHCDouglassCWMeta-analysis of the effect of scaling and root planing, surgical treatment and antibiotic therapies on periodontal probing depth and attachment lossJ Clin Periodontol20022997598612472990
- HanesPJPurvisJPLocal anti-infective therapy: pharmacological agents. A systematic reviewAnn Periodontol20038799814971250
- HagiwaraSTakamatsuNTominagaYUmedaMSubgingival distribution of periodontopathic bacteria in adult periodontitis and their susceptibility to minocycline-HClJ Periodontol19986992999527568
- KinaneDBouchardPPeriodontal diseases and health: Consensus Report of the Sixth European Workshop on PeriodontologyJ Clin Periodontol20083533333718724860
- GattoMRMontevecchiMPaolucciMLandiniMPChecchiLPrevalence of six periodontal pathogens in subgingival samples of Italian patients with chronic periodontitisNew Microbiol20143751752425387289
- PapapanouPNSellenAWennstromJLDahlenGAn analysis of the subgingival microflora in randomly selected subjectsOral Microbiol Immunol1993824298510980
- SanzMvan WinkelhoffAJHerreraDDellemijn-KippuwNSimonRWinkelEDifferences in the composition of the subgingival microbiota of two periodontitis populations of different geographical origin. A comparison between Spain and The NetherlandsEur J Oral Sci200010838339211037754
- PasterBJBochesSKGalvinJLBacterial diversity in human subgingival plaqueJ Bacteriol20011833770378311371542
- AliRWBakkenVNilsenRSkaugNComparative detection frequency of 6 putative periodontal pathogens in Sudanese and Norwegian adult periodontitis patientsJ Periodontol199465104610527853128
- Roman-TorresCVAquinoDRCortelliSCPrevalence and distribution of serotype-specific genotypes of Aggregatibacter actinomycetemcomitans in chronic periodontitis Brazilian subjectsArch Oral Biol20105524224820171609
- RylevMKilianMPrevalence and distribution of principal periodontal pathogens worldwideJ Clin Periodontol20083534636118724862
- AliRWVelcescuCJivanescuMCLofthusBSkaugNPrevalence of 6 putative periodontal pathogens in subgingival plaque samples from Romanian adult periodontitis patientsJ Clin Periodontol1996231331398849850
- ChenCWangTChenWOccurrence of Aggregatibacter actinomycetemcomitans serotypes in subgingival plaque from United States subjectsMol Oral Microbiol20102520721420536748
- FaveriMFigueiredoLCDuartePMMestnikMJMayerMPFeresMMicrobiological profile of untreated subjects with localized aggressive periodontitisJ Clin Periodontol20093673974919637996
- BuchmannRConradsGSculeanAShort-term effects of systemic antibiotics during periodontal healingQuintessence Int20104130331220305865
- EguchiTKoshyGUmedaMMicrobial changes in patients with acute periodontal abscess after treatment detected by PadoTestOral Dis20081418018418302679
- MaedaHFujimotoCHarukiYQuantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteriaFEMS Immunol Med Microbiol200339818614557000
- GarrettSJohnsonLDriskoCHTwo multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitisJ Periodontol19997049050310368053
- WennstromJLHeijlLDahlenGGrondahlKPeriodic subgingival antimicrobial irrigation of periodontal pockets (I). Clinical observationsJ Clin Periodontol1987145415503479456
- JavaliMAVandanaKLA comparative evaluation of atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: a clinical studyJ Indian Soc Periodontol201216434822628962
- WaghmareASVhanmanePBSavithaBChawlaRLBagdeHSBacteremia following scaling and root planing: a clinico-microbiological studyJ Indian Soc Periodontol20131772573024554880
- CherryMDalyCGMitchellDHighfieldJEffect of rinsing with povidone-iodine on bacteraemia due to scaling: a randomized-controlled trialJ Clin Periodontol20073414815517309589
- UgwumbaCUAdeyemoWLOdeniyiOMArotibaGTOgunsolaFTPreoperative administration of 0.2% chlorhexidine mouthrinse reduces the risk of bacteraemia associated with intra-alveolar tooth extractionJ Craniomaxillofac Surg2014421783173825028067
- RahnRSchneiderSDiehlOSchaferVShahPMPreventing post-treatment bacteremia: comparing topical povidone-iodine and chlorhexidineJ Am Dent Assoc1995126114511497560572
- SakamotoMTakeuchiYUmedaMIshikawaIBennoYRapid detection and quantification of five periodontopathic bacteria by real-time PCRMicrobiol Immunol200145394411270605
- BaumgartnerJCWatkinsBJBaeKSXiaTAssociation of black-pigmented bacteria with endodontic infectionsJ Endod19992541341510530240
- NadkarniMAMartinFEJacquesNAHunterNDetermination of bacterial load by real-time PCR using a broad-range (universal) probe and primers setMicrobiology200214825726611782518