Abstract
Background
Epicardial adipose tissue (EAT), mean platelet volume (MPV), platelet-to- lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR) have been shown to be helpful in predicting adverse cardiovascular events. However, to date, in the literature, there have been no studies demonstrating the relationship between EAT, MPV, PLR, NLR, and thromboembolism risk in atrial fibrillation (AF). Therefore, we examined the relationship between EAT, MPV, PLR, NLR, and CHA2DS2-VASc score used for the evaluation of thromboembolism risk in patients with AF.
Methods
The study included 96 consecutive patients with AF and 52 age- and sex-matched control subjects. We calculated CHA2DS2-VASc risk score for each patient and measured baseline EAT thickness, MPV, PLR, NLR, left atrial volume index, and left ventricular ejection fraction.
Results
The group with high CHA2DS2-VASc score had higher EAT (7.2±1.5 vs 5.9±1.2 mm, P<0.001), MPV (9.1±1.1 vs 8.4±1.0 fL, P=0.004), PLR (152.3±28.4 vs 126.7±25.4, P=0.001), and NLR (4.0±1.6 vs 3.2±1.3, P<0.001) compared to group with low-intermediate CHA2DS2-VASc score. Moreover, CHA2DS2-VASc score was found to be positively correlated with EAT (r=0.623, P<0.001), MPV (r=0.350, P=0.004), PLR (r=0.398, P=0.001), and NLR (r=0.518, P<0.001).
Conclusion
Our study results demonstrated that EAT thickness, MPV, PLR, and NLR were associated with the thromboembolic risk exhibited by CHA2DS2-VASc score in patients with nonvalvular AF.
Introduction
Nonvalvular atrial fibrillation (AF) is the most frequently sustained arrhythmia in clinical practice and presents a high risk of thromboembolism.Citation1 Numerous scoring systems have been used in the stratification of thromboembolism risk in AF patients.Citation2 Of these, CHA2DS2-VASc is the most commonly suggested system. All the components of this system have been demonstrated to be associated with increased incidence of thromboembolism in patients with AF.Citation3 The genesis of AF is of a multifactorial nature, but the study has shown that inflammation plays a key role in the initiation and progression of AF.Citation4
Epicardial adipose tissue (EAT) functions as an active endocrine organ and has been demonstrated to be a significant source of inflammatory mediators and to play a major role in the pathophysiology of AF since it is located close to the heart.Citation5 Mean platelet volume (MPV), a measure of platelet activation and function, is a potential mediator of the relationship between inflammation and thrombosis.Citation6 MPV is higher in patients with AF compared to that in patients with sinus rhythm.Citation7 Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have recently been used as systemic inflammatory markers and prognostic indicators of adverse cardiovascular events.Citation8,Citation9 NLR is also used for predicting the left atrial (LA) thrombus in patients with nonvalvular AF.Citation10
The aim of this study was to investigate the association between EAT thickness, MPV, NLR, PLR, and the CHA2DS2‒VASc score used for the stratification of thromboembolism risk in patients with nonvalvular AF.
Materials and methods
Study population
The study was performed in compliance with the Declaration of Helsinki, and the study protocol was approved by the Yuzuncu Yil University, Faculty of Medicine Ethics Committee. The participants’ written informed consent was also obtained. The cross-sectional observational study included a total of 96 consecutive patients who presented to the outpatient clinics of our university hospital with nonvalvular AF and 52 age- and sex-matched control subjects. The inclusion criteria were patients over 18 years of age who presented to the cardiology clinic with at least one attack of AF diagnosed by electrocardiographic examination and assessed by a cardiologist. Exclusion criteria included autoimmune disease, significant congestive heart failure, renal or hepatic disease, clinically significant valvular heart disease, cancer, ongoing infection or systemic inflammatory conditions, comorbidities, and patients with poor echocardiographic windows.
A complete physical examination was performed in all the patients. Also, the medical histories and clinical features of the patients were recorded. AF was defined as the absence of P waves on electrocardiography and irregular R–R interval. The types of AF were defined in accordance with the European Society of Cardiology guidelines. According to these guidelines, a patient detected with AF for the first time is considered a patient with first diagnosed AF, regardless of the duration of arrhythmia or the presence and severity of AF-related symptoms. Paroxysmal AF is generally self-terminating and continues for up to 7 days. In persistent AF, the episode either lasts for >7 days or requires termination by cardioversion, either by medication or direct current cardioversion. In long-standing persistent AF, the episode lasts for ≥1 year, and a rhythm control strategy is adopted. On the other hand, if the patient presents with long-standing AF, in whom cardioversion has failed or has not been tried, the patient is considered to have permanent AF. In this study, we used the CHA2DS2-VASc scoring system, which is used to stratify the risk of long-term thromboembolic events related to AF (). Depending on this system, the AF patients were divided into two groups: 1) low-intermediate risk group and 2) high-risk group. Accordingly, a score of “0” was regarded as low risk, “1” as intermediate risk, and “≥2” as high risk.
Table 1 CHA2DS2-VASc score
Echocardiography
Each patient underwent transthoracic two-dimensional echocardiography at rest under standard procedures. The patient was placed in the left lateral decubitus position, and a commercial echocardiographic device (Vivid 3, General Electric, Chicago, IL, USA) with a 3.0 MHz transducer was used for the examination. The examinations were performed by two experienced cardiologists blinded to the study. In order to avoid interreader variability, the offline assessments of the EAT thickness were performed by two cardiologists blinded to the patient data. In order to assess the inter- and intraobserver variability, the echocardiograms of 30 patients were randomly selected and a second measurement of the EAT thickness was performed 2 weeks later. The inter- and intraobserver variability of the EAT thickness were 3.2% and 2.6%, respectively. The diameter of the left atrium was measured at end-systole in the parasternal long-axis view using the standard criteria. Left ventricular ejection fraction (LVEF) was also calculated. The epicardial fat was identified as an echo-free space in the pericardial layers on the two-dimensional echocardiography. By using the Simpson’s method, the maximum EAT thickness was measured at the point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus, which is used as the anatomic landmark at end-systole in three cardiac cycles.
Biochemical and hematological measurements
Blood sample was collected from the antecubital vein using a 21-gauge sterile syringe without stasis between 8 and 10 am, following a 12-hour fasting period. To assess complete blood count, a Coulter LH 780 Hematology Analyzer (Architect plus ci16200, Abbott, Lake Forest, IL, USA) was used for measuring the hematological parameters including platelet counts, neutrophil counts, lymphocyte counts, and MPV. Baseline NLR was measured by dividing neutrophil count by lymphocyte count. Baseline PLR was measured by dividing platelet count by lymphocyte count.
Statistical analysis
All the statistical data were analyzed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation, and the categorical data were expressed as percentage. When necessary, the Pearson’s or the Spearman’s correlation coefficient was performed to analyze the correlation between the variables. Statistical comparisons were performed using one-way analysis of variance, followed by Scheffé’s test. A P-value of <0.05 was considered significant.
Results
A total of 148 patients (96 patients with nonvalvular AF and 52 control subjects) were included in the present study. Baseline demographic, clinical, and laboratory characteristics of the nonvalvular AF and the control groups are presented in . The median CHA2DS2-VASc score was 2, and 53.1% of the patients were in the high CHA2DS2-VASc group. Within the nonvalvular AF group, there were 51 patients with high CHA2DS2-VASc score and 45 patients with low-intermediate CHA2DS2-VASc score. Of all the patients, 22.9% of the cases had paroxysmal, 16.7% had persistent, and 60.4% had permanent AF. Other baseline characteristics and previous medications are shown in . Patients with nonvalvular AF had significantly higher EAT (6.6±1.3 vs 4.9±1.0 mm, P<0.001), MPV (8.9±1.1 vs 7.8±1.0 fL, P<0.001), PLR (140.3±24.2 vs 119.3±21.9, P=0.002), and NLR (3.6±1.5 vs 2.9±1.3, P<0.001) compared to the control subjects. LA volume index (P<0.001) was significantly higher in the nonvalvular AF group compared to the controls. However, LVEF (P<0.001) was significantly lower in the nonvalvular AF group compared to the controls (). High CHA2DS2-VASc group had higher EAT (7.2±1.5 vs 5.9±1.2 mm, P<0.001), MPV (9.1±1.1 vs 8.4±1.0 fL, P=0.004), PLR (152.3±28.4 vs 126.7±25.4, P=0.001), and NLR (4.0±1.6 vs 3.2±1.3, P<0.001) compared to the low-intermediate CHA2DS2-VASc group. Moreover, age (P<0.001), LA volume index (P<0.001), hypertension (P<0.001), and diabetes (P=0.006) were significantly higher in the high CHA2DS2-VASc group compared to the low-intermediate CHA2DS2-VASc group. However, LVEF (P<0.001) was significantly lower in the high CHA2DS2-VASc group compared to the low-intermediate CHA2DS2-VASc group (). Moreover, CHA2DS2-VASc score was found to be positively correlated with EAT (r=0.623, P<0.001) (), MPV (r=0.350, P=0.004) (), PLR (r=0.398, P=0.001) (), and NLR (r=0.518, P<0.001) ().
Figure 1 Correlation plots between echocardiographic EAT thickness and CHA2DS2-VASc score.
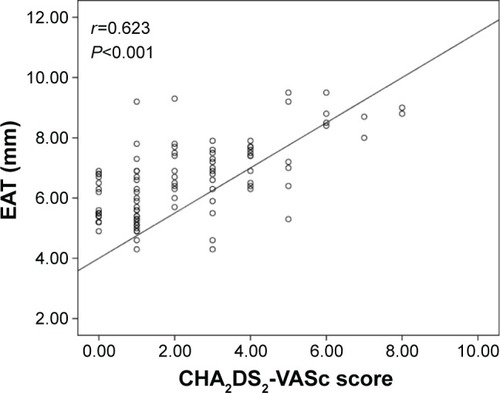
Figure 2 Correlation plots between MPV and CHA2DS2-VASc score.
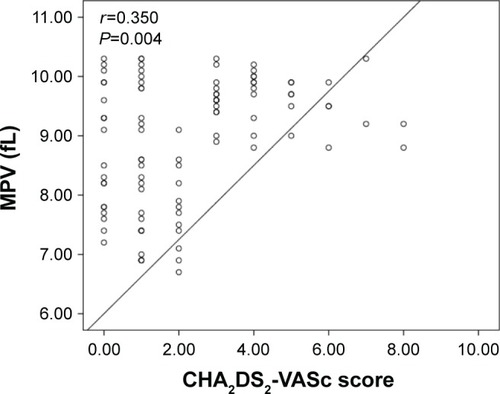
Figure 3 Correlation plots between PLR and CHA2DS2-VASc score.
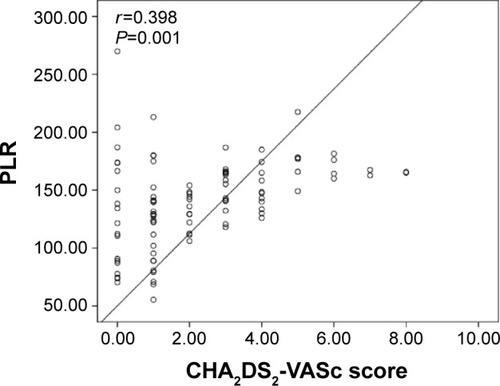
Figure 4 Correlation plots between NLR and CHA2DS2-VASc score.
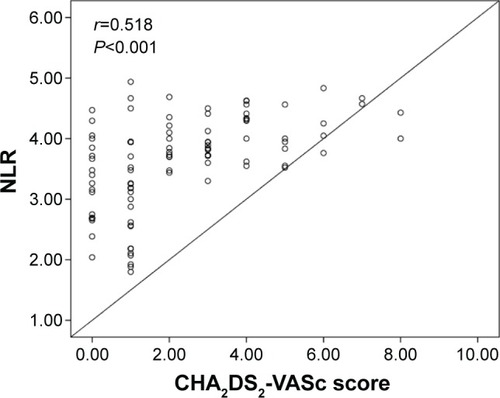
Table 2 Baseline characteristics of all patients with nonvalvular atrial fibrillation and control group
Table 3 Baseline clinical and laboratory characteristics according to CHA2DS2-VASc score in patients with nonvalvular AF
Discussion
There were three main findings of the present study. First, EAT thickness was found to be higher in the nonvalvular AF group compared to the control group. Second, inflammation markers including MPV, PLR, and NLR were significantly elevated in the nonvalvular AF group. Third, EAT thickness, MPV, PLR, and NLR were correlated with the CHA2DS2‒VASc score in patients with nonvalvular AF, which were also independent predictors of high CHA2DS2-VASc score.
Nonvalvular AF is the most frequently sustained arrhythmia in clinical practice and presents a high risk of stroke and thromboembolism.Citation1 The incidence of thromboembolism may vary according to the coexisting clinical, echocardiographic, and biochemical factors in AF patients. Therefore, numerous scoring systems have been developed for the stratification of thromboembolism risk in AF patients.Citation2 Of these systems, the CHA2DS2-VASc score is the most commonly suggested system.Citation11 This system categories the thromboembolism risk in AF patients into two levels: low risk and high risk. Accordingly, patients with high risk have a higher incidence of thromboembolic events.
In addition to the effects of well-known cardiovascular risk factors on the initiation and progression of AF, researchers have recently aimed at evaluating the role of inflammation on the progression of AF.Citation12 EAT has been shown to be effective on the progression of both coronary atherosclerosis and cardiac arrhythmia.Citation13 EAT is a true visceral fat deposited around the heart, particularly around the subepicardial coronary vessels. It has been demonstrated that EAT functions as an active endocrine organ, which generates several proinflammatory and proatherogenic cytokines and bioactive adipokines.Citation14 EAT thickness has recently emerged as a novel risk factor for cardiovascular disease in the general population.Citation14 A previous study has reported the autocrine and paracrine effects of EAT on cardiac physiology.Citation15 Moreover, a study also investigated the relationship between EAT thickness assessed by various methods and found a positive correlation between EAT thickness and AF.Citation13 Recently, Shin et alCitation16 also reported that computed tomography-measured epicardial fat volumes established a significant correlation with the presence and progression of AF. In addition, a relationship between the epicardial fat and long-term clinical outcomes following AF ablation has also been reported. Similarly, Nagashima et alCitation17 also reported greater epicardial fat volumes in patients with AF recurrence after radiofrequency ablation. In another study, the same authors reported a correlation between the dominant frequency sites and the epicardial fat locations. They also reported higher epicardial fat volumes and higher levels of serum inflammatory markers in patients with persistent AF when compared to the patients with paroxysmal AF.Citation18 Recently, EAT thickness as assessed by echocardiography has emerged as a practical method for the evaluation of the cardiometabolic risk and the risk of visceral adiposity. In this study, EAT thickness was measured using echocardiography, and a positive correlation was found between the CHA2DS2-VASc score and epicardial fat in the nonvalvular AF group.
MPV, PLR, and NLR, which are the parameters measured in routine blood samples, are inflammatory markers of cardiovascular disease.Citation6–Citation9 MPV is a measure of platelet size and is regarded as a marker and determinant of platelet function since the platelets with larger sizes are hemostatically more reactive than the platelets with normal sizes, thus increasing the predisposition to thrombosis and resulting in acute coronary syndromes or ischemic stroke.Citation19,Citation20 A study reported that the MPV level was higher in AF patients compared to subjects in sinus rhythm.Citation21 Additionally, Ha et alCitation19 reported that MPV functions as a predictive marker of stroke in AF patients. In the same study, MPV was found to add incremental predictive value to the clinical variables involved in the CHADS2 score. Very recently, a case–control study reported that stroke patients with AF exhibited higher MPV levels than those exhibited by AF patients with no history of stroke.Citation22 In our study, the MPV levels were higher in the nonvalvular AF group and correlation was established between the MPV levels and CHA2DS2-VASc score.
PLR and NLR have recently been used as significant inflammatory markers and novel predictors of major adverse outcomes in cardiovascular disease.Citation8,Citation9 Recent studies have also shown that high platelet count and low lymphocyte count have a close relationship with the poor prognosis in patients with coronary artery disease.Citation23–Citation25 Azab et alCitation24 reported that elevated PLR was an independent predictor for long-term mortality in patients with non-ST elevation myocardial infarction. Akkaya et alCitation25 found that PLR has a significant role in the increase in adverse clinical outcomes and mortality within the 6 months after percutaneous coronary intervention in patients with ST elevation myocardial infarction.
Increased PLR and NLR are associated with the severity of coronary atherosclerosis in patients with coronary artery disease and congestive heart failure.Citation9,Citation26 A study reported that increased NLR was associated with higher inpatient mortality in patients with advanced heart failure.Citation26 Another study found that NLR levels were associated with the increased risk of ventricular arrhythmias during percutaneous coronary intervention.Citation8 Moreover, NLR has recently emerged as a risk marker for AF progression following coronary artery bypass grafting and as a predictor for early recurrence of AF following radiofrequency catheter ablation.Citation27,Citation28 Also, elevated NLR has been shown to increase the risk of thromboembolic stroke in patients with nonvalvular AF.Citation29 In our study, PLR and NLR levels were higher in the nonvalvular AF group and a positive correlation was established between CHA2DS2-VASc score.
Stratification of the thromboembolism risk is of prime importance for the prevention of adverse outcomes in patients with nonvalvular AF. We propose that EAT thickness, MPV, NLR, and PLR, which can be measured by an inexpensive and simple method, may be significant predictors of the thromboembolism risk in patients with nonvalvular AF.
Limitations
Our study is limited since it had a relatively small number of patients. Echocardiographic EAT thickness is a linear measurement, and thus it may not assess the total epicardial fat volume that varies at various myocardial locations. Moreover, other cytokines or inflammatory markers were not evaluated and not compared with MPV, NLR, and PLR.
Conclusion
To our knowledge, this is the first study to determine the association of EAT thickness, MPV, PLR, and NLR with CHA2DS2-VASc score in patients with nonvalvular AF. The study revealed that EAT thickness, MPV, PLR, and NLR were associated with the thromboembolic risk exhibited by CHA2DS2-VASc score in patients with nonvalvular AF. On the other hand, it is of interest that we can infer from this article that there is a certain correlation between inflammatory markers and CHA2DS2-VASc scores, suggesting that anti-inflammatory therapy may be beneficial in reducing risk factors in AF patients. Moreover, these parameters may be beneficial in the stratification of the thromboembolic risk. However, further large-scale studies are needed in this regard.
Disclosure
The authors report no conflicts of interest in this work.
References
- WattigneyWAMensahGACroftJBIncreasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary preventionCirculation2003108671171612885749
- ChamberlainAMAgarwalSKFolsomARA clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study)Am J Cardiol20111071859121146692
- LipGYNieuwlaatRPistersRLaneDACrijnsHJRefining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillationChest2010137226327219762550
- BoosCJAndersonRALipGYIs atrial fibrillation an inflammatory disorder?Eur Heart J200627213614916278230
- RositoGAMassaroJMHoffmannUPericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham heart studyCirculation200811760561318212276
- GasparyanAYAyvazyanLMikhailidisDPKitasGDMean platelet volume: a link between thrombosis and inflammation?Curr Pharm Des201117475821247392
- ColkesenYAcilTAbayliBMean platelet volume is elevated during paroxysmal atrial fibrillation: a marker of increased platelet activationBlood Coagul Fibrinolysis20081941141418600091
- ChatterjeeSChandraPGuhaGPre-procedural elevated white blood cell count and neutrophil-lymphocyte (N/L) ratio are predictors of ventricular arrhythmias during percutaneous coronary interventionCardiovasc Hematol Disord Drug Targets2011112586022044033
- YukselMYıldızAOylumluMPlatelet/lenfosit oranı ile koroner arter hastalığının ciddiyeti arasındaki ilişki [The association between platelet/lymphocyte ratio and coronary artery disease severity]Anadolu Kardiyol Derg201515864064710.5152/akd.2014.5565 Turkish
- YalcinMAparciMUzONeutrophil–lymphocyte ratio may predict left atrial thrombus in patients with nonvalvular atrial fibrillationClin Appl Thromb Hemost201521216617124057399
- CammAJLipGYDe CaterinaR2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation developed with the special contribution of the European Heart Rhythm AssociationEur Heart J201233212719274722922413
- FriedrichsKKlinkeABaldusSInflammatory pathways underlying atrial fibrillationTrends Mol Med20111755656321763201
- GirerdNScridonABessiereFPeriatrial epicardial fat is associated with markers of endothelial dysfunction in patients with atrial fibrillationPLoS One20138e7716724143210
- MahabadiAABergMHLehmannNAssociation of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall StudyJ Am Coll Cardiol2013611388139523433560
- LinYKChenYJChenSAPotential atrial arrhythmogenicity of adipocytes: implications for the genesis of atrial fibrillationMed Hypotheses2011741026102920149554
- ShinSYYongHSLimHETotal and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillationJ Cardiovasc Electrophysiol20112264765521235672
- NagashimaKOkumuraYWatanabeIAssociation between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablationCirc J2011752559256521869533
- NagashimaKOkumuraYWatanabeIDoes location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation?Circ Arrhythm Electrophysiol2012567668322772897
- HaSIChoiDHKiYJStroke prediction using mean platelet volume in patients with atrial fibrillationPlatelets20112240841421599611
- MathurARobinsonMSCottonJMartinJFErusalimskyJDPlatelet reactivity in acute coronary syndromes: evidence for differences in platelet behaviour between unstable angina and myocardial infarctionThromb Haemost20018598999411434707
- YuceMCakiciMDavutogluVRelationship between mean platelet volume and atrial thrombus in patients with atrial fibrillationBlood Coagul Fibrinolysis20102172272520881479
- TurfanMErdoganEErtasGUsefulness of mean platelet volume for predicting stroke risk in atrial fibrillation patientsBlood Coagul Fibrinolysis2013241555823080368
- YilmazSSenFUnalSUsefulness of the platelet-to-lymphocyte ratio to predict bare metal stent restenosisScand Cardiovasc J2015491394425414124
- AzabBShahNAkermanMValue of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarctionJ Thromb Thrombolysis201234332633422466812
- AkkayaEGulMUgurMPlatelet to lymphocyte ratio: a simple and valuable prognostic marker for acute coronary syndromeInt J Cardiol2014177259759825220175
- UthamalingamSPatvardhanEASubramanianSUtility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failureAm J Cardiol201110743343821257011
- GibsonPHCuthbertsonBHCroalBLUsefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass graftingAm J Cardiol201010518619120102916
- ImSIShinSYNaJOUsefulness of neutrophil/lymphocyte ratio in predicting early recurrence after radiofrequency catheter ablation in patients with atrial fibrillationInt J Cardiol20131684398440023725815
- ErtasGSonmezOTurfanMNeutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillationJ Neurol Sci2013324495223084070
