Abstract
Background
Breastfeeding is considered as gold standard for infant nutrition and should be interrupted only when a compelling indication exists. Certain medical conditions such as abortion, stillbirth, HIV infection, or infant galactosemia and certain medications such as chemotherapy necessitate lactation inhibition to protect the health of mother and infant. Drug use evaluation (DUE) studies are done to explore the current practice in a setting and help to identify areas in which further information and education may be needed by clinicians.
Objective
The aim of this study was to conduct a DUE of cabergoline to assess indications for lactation inhibition, dosage regimen, and its safety.
Method
A retrospective cross-sectional DUE study was conducted over a period of 4 months from September 1, 2013, till December 31, 2013, at the Women’s Hospital, Qatar. All cabergoline prescriptions written for lactation inhibition within 10 days of delivery or abortion were included in the study. A descriptive data analysis was undertaken.
Results
Of the 85 patients included, stillbirth (50.6%) was considered as the main reason for lactation inhibition, followed by abortion (27.1%) and neonatal death (12.9%). The remaining 9.4% of the patients had live baby, and the majority of them were prescribed cabergoline for lactation inhibition because their maternal medical conditions required the use of drugs with insufficient safety data (n=6). Seventy-four percent of patients received cabergoline at accurate time and dose. However, 14% of the patients had preexisting hypertensive disorder and 58.3% of them were diagnosed as uncontrolled hypertension.
Conclusion
The current DUE study found that cabergoline was mainly used to inhibit lactation for patients with stillbirth, abortion, and neonatal death. In mothers who use medications for other medical conditions, benefits and risks of breastfeeding should be carefully balanced before prescribing cabergoline. Current prescribing pattern can be further enhanced through informing health care providers regarding appropriate cabergoline dosage regimen and its safety in patients with uncontrolled hypertension.
Introduction
Human milk is the normative standard for infant feeding and nutrition. It provides short- and long-term nutritional, cognitive, emotional, and immunologic advantages. Medical contraindications to breastfeeding are few. These include infant galactosemia or mother’s HIV infection, untreated and active tuberculosis, or use of some chemotherapy agents. Furthermore, some mothers avoid breastfeeding on personal or social grounds. Regardless of the reason for not breastfeeding, this decision can be associated with complications including milk secretion, breast pain, engorgement, or mastitis.Citation1–Citation7
Various approaches are used to suppress lactation in women. Severe breast pain can be encountered in women who do not breastfeed and use analgesics or use nonpharmacological approaches to suppress lactation such as breast binder or ice packs.Citation8 Furthermore, the use of pharmacological interventions such as estrogens alone, estrogen–androgen combination, pyridoxine, and dopamine agonists have been evaluated for efficacy in milk suppression and have shown variable results.Citation5
Cabergoline is a dopamine agonist used to suppress lactation despite the lack of approval from the United States Food and Drug Administration for this indication. It exerts its effect through the inhibition of prolactin release from the anterior pituitary gland. Compared to bromocriptine, the older dopamine agonist, cabergoline is better tolerated with significantly less rebound breast activity and adverse effects and with more simple administration schedule.Citation9,Citation10
A number of studies have been conducted to examine the dose-dependent effect of cabergoline.Citation11–Citation14 A range of oral doses between 0.4 mg and 1 mg was used, and the most effective dose for long-term suppression of lactation was found to be 1 mg.Citation15 Accordingly, a single dose of 1 mg during the first 24–27 hours postpartum is recommended for lactation inhibition,Citation10,Citation16 while a dose of 0.25 mg every 12 hours for four doses is used for suppression of established lactation.Citation16
Drug use evaluation (DUE) is a process of ongoing, systematic, criteria-based evaluation of drug use with the aim of ensuring that medications are used appropriately at the individual patient level.Citation17 DUE studies are a powerful tool to explore the pattern of prescribing medications, promote optimal medication therapy, and prevent medication-related problems. In addition, DUE studies help to identify areas in which further education or information may be needed by health care providers.Citation17
A valid indication is necessary for the use of cabergoline to inhibit lactation in nursing mothers. Physicians must be aware of the dose, indication, and frequency to prescribe cabergoline in nursing mothers. They also should review maternal medical conditions to ensure its safety. Therefore, we aimed to conduct a DUE of cabergoline to assess indications for lactation inhibition, dosage regimen, and its safety.
Method
Study setting and period
This DUE study was conducted at the Women’s Hospital, Qatar. This tertiary care facility has 234 beds including antenatal, postnatal, and high dependency units, an outpatient department followed by an emergency room, 16 labor rooms, and three operating theaters, as well as a level-3 neonatal intensive care unit. All cabergoline prescriptions for lactation inhibition written over a 4-month period between September 1, 2013, and December 31, 2013, were used in the study.
Study design
This retrospective cross-sectional study was conducted to evaluate the use of cabergoline in lactation inhibition. A literature review along with World Health Organization guidelines on conducting DUE studies was utilized for the DUE criteria selection.Citation17 The primary outcomes for the study were
Identify and assess indications for lactation inhibition.
Evaluate the appropriateness of cabergoline dosage regimen.
Evaluate the safe use of cabergoline in patients with existing medical comorbidities.
Eligibility criteria
Women who received a cabergoline prescription for lactation inhibition within 10 days of delivery or abortion were included in the study.
Data collection
A standardized data collection tool was developed utilizing the selected DUE criteria. Variables were designated to measure the prespecified outcomes. The tool was validated through piloting it on ten randomly selected files.
Outcome measures
The Centers of Disease Control and Prevention and American Academy of Pediatrics resources were utilized to identify the medical conditions that necessitate lactation inhibition.Citation18,Citation19 Drugs in Pregnancy and Lactation book and Drugs and Lactation Database (LactMed) by the US National Library of Medicine were used to identify the safety of medications with breastfeeding.Citation15,Citation20 A single dose of 1 mg of cabergoline within the first 24–27 hours of delivery/abortion is the recommended regimen for lactation inhibition.Citation10,Citation16 The main safety concerns for the use of cabergoline that were evaluated in this study were preexisting uncontrolled hypertension at the time of cabergoline administration and allergy with ergot alkaloid since the use of cabergoline in these populations could cause significant risk to patients.
Definitions
In this study, lactation inhibition refers to the prevention of milk formation within the first 27 hours postpartum before the mother starts breastfeeding, whereas lactation suppression pertains to suppression of established lactation. There is no clear definition and duration for controlled hypertension before cabergoline administration. So we defined controlled hypertension as having systolic blood pressure <150 mmHg and diastolic blood pressure <100 mmHgCitation21 within the previous 24 hours before prescribing cabergoline without intravenous antihypertensive medications to control the blood pressure.
Data processing and analysis
Data collection was done using patients’ medical records, and the collected data were manually sorted and categorized. A descriptive data analysis was carried out and evaluated against the preset criteria.
Ethical consideration
Waiver of informed consent and ethical approval were obtained from the Medical Research Center, Hamad Medical Corporation (approval number 13393/13).
Results
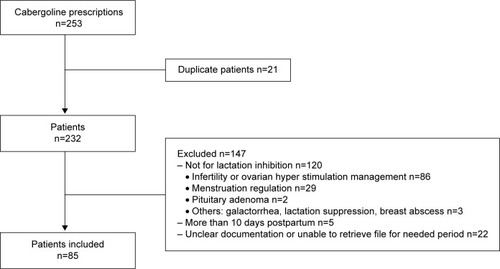
A total of 232 patients were prescribed cabergoline, of which 85 patients were included in this study (). Demographic characteristics of patients are shown in . Thirty-one percent of the patients were Qataris, and the majority of the patients had a singleton pregnancy (90.6%).
Table 1 Demographic characteristics
Indications for lactation inhibition
Stillbirth was found to be the main reason to inhibit lactation (50.6%), followed by abortion (27.1%) and neonatal death (12.9%). Only 9.4% of the patients who received cabergoline for lactation inhibition had live baby ().
Table 2 Indication for lactation inhibition
Appropriateness of indications for lactation inhibition
Following abortion, only one patient received cabergoline at gestational age <16 weeks as patient requested, whereas all the other patients received cabergoline at a higher gestational age.
In patients with live baby, no infant or maternal medical conditions that required lactation inhibition were encountered. One of the patients with severely inverted nipples requested cabergoline to suppress her lactation. Six patients who used medications for postkidney transplant, depression, epilepsy, and cardiomyopathy did not breastfeed their babies. All these patients were using combinations of medications (). Only one patient requested lactation inhibition with no clear reason.
Cabergoline dosage
The majority of the patients were prescribed cabergoline during hospital admission (83.5%), while the rest were prescribed cabergoline at discharge as home medication. Cabergoline was prescribed within the first 27 hours of delivery or abortion in >80% of the patients and was prescribed as a single dose of 1 mg in 71.8% of the patients. Appropriate prescribing pattern of both timing and dose was found in 74.1% of the patients (). It was noted that 50% of the patients with live baby received cabergoline after 27 hours of delivery.
Table 3 Dosage regimen of cabergoline
Safety of administration
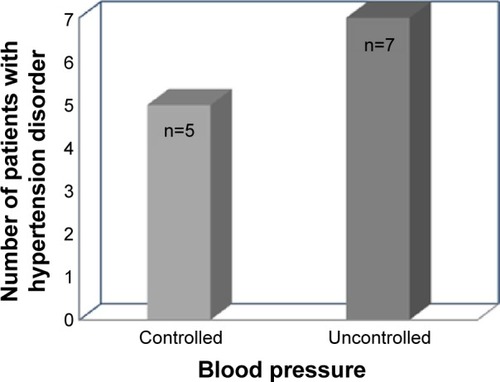
Fourteen percent of the patients had hypertensive disorders during current pregnancy. Fifty-eight percent of these patients had uncontrolled blood pressure 24 hours prior to prescribing cabergoline (). None of the patients who received cabergoline were allergic to ergot alkaloids ().
Discussion
This DUE study explores the current clinical practice and provides an evidence for the use of cabergoline in lactation inhibition with an emphasis on the indications and safety. To the best of our knowledge, no DUE studies on cabergoline use for lactation inhibition were published elsewhere.
Lactogenesis starts at ~16 weeks of gestation.Citation22,Citation23 Most of the patients received cabergoline at late gestational ages. Only 9.4% of the patients who received cabergoline had live baby. The main reason for that was the use of other medications by the mothers for their existing maternal comorbidities. Not all the received medications contraindicate breastfeeding,Citation15,Citation20 but may be the use of combinations of these medications led to the decision of lactation inhibition. Generally, for many of the medications that possess a low risk to the nursing baby, breastfeeding can be continued with close monitoring of the baby and with proper timing between medication administration and breastfeeding if applicable or a safer alternative can be used when appropriate.
Many studies assessed the prolactin inhibitory effect of different cabergoline doses, and the 1 mg single dose was shown to be the most effective dose.Citation13,Citation14 In our study, the 1 mg single dose was prescribed in 71.8% of the patients. Cabergoline administration should take place within the first 24–27 hours of delivery.Citation10,Citation16 Delay beyond this time period was noted in 50% of the patients with live baby, which might reflect delay in making the decision of stopping breastfeeding until weighing benefits and risks.
Cabergoline should not be used in women with pregnancy-induced hypertension, for example, preeclampsia or postpartum hypertension, unless the potential benefit is judged to outweigh the possible risk.Citation16 Hypertensive disorders were encountered in 12 patients, with 58.3% of them having uncontrolled blood pressure for the 24 hours prior to receiving cabergoline. Close monitoring of these patients would be required, especially for blood pressure.
One of the limitations of the study was its retrospective nature that does not provide information about all perspectives of the physicians and patients at the time of prescribing cabergoline. Moreover, unclear and incomplete documentation might reduce the amount of information that could be retrieved. Another limitation is that this DUE assesses the timing of writing the prescription, which might not reflect the exact time of administration, especially with patients who were planned to receive it at discharge as home medication.
Our study described different aspects of clinical practice regarding cabergoline use in lactation inhibition. Future work can be expanded to the evaluation of cabergoline in lactation suppression within the first 2–6 months postpartum, with a focus on patients with live nursing babies.
Conclusion
This DUE study found that cabergoline use in lactation inhibition was mainly for patients with stillbirth, abortion, and neonatal death. Maternal use of medications for other medical comorbidities requires careful evaluation of breastfeeding benefits and risks before prescribing cabergoline. To further enhance the current prescribing pattern, health care providers need to be informed regarding the appropriate dose, frequency, and safety concerns in patients with uncontrolled hypertension.
Disclosure
The authors report no conflicts of interest in this work.
Funding
This work received a grant from the Medical Research Center, Hamad Medical Corporation, Qatar.
References
- StuebeAThe risks of not breastfeeding for mothers and infantsRev Obstet Gynecol20092422223120111658
- MathurNBDhingraDBreastfeedingIndian J Pediatr201481214314923904066
- TchoffoPwebpage on the InternetTreatment for suppression of lactation: RHL commentary [last revised November 1, 2009]The WHO Reproductive Health LibraryGenevaWorld Health Organization http://apps.who.int/rhl/pregnancy_childbirth/care_after_childbirth/cd005937_Tchoffopa_com/en/Accessed April 23, 2015
- OladapoOFawoleBTreatments for suppression of lactationCochrane Database Syst Rev20129CD00593722972088
- NishaSUmaSVineetaSRole of newer drug cabergoline in lactation suppression as compared to estrogen-androgen combinationJ Obstet Gynecol India2009592152155
- OgbuanuCAProbstJLaditkaSBLiuJBaekJGloverSReasons why women do not initiate breastfeeding: a southeastern state studyWomens Health Issues200919426827819589476
- ColiMLactation after perinatal, neonatal, or infant lossClin Lactation20123394100
- SpitzMLeeCPetersonBTreatment for lactation suppression: little progress in one hundred yearsAm J Obstet Gynecol19981796 pt 1148514909855585
- WebsterJAComparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinaemia and inhibition of lactationDrug Safety19961442282388713691
- European Multicentre Study Group for Cabergoline in Lactation InhibitionSingle dose cabergoline versus bromocriptine in inhibition of puerperal lactation: randomised, double blind, multicentre studyBMJ19913026789136713711676318
- MelisBGambaccianiMPaolettiMDose-related prolactin inhibitory effect of the new long-acting dopamine receptor agonist cabergoline in normal cycling, puerperal, and hyperprolactinemic womenJ Clin Endocrinol Metabol1987653541545
- MelisBMaisVPaolettiMBeneventiFGambaccianiMFiorettiPPrevention of puerperal lactation by a single oral administration of the new prolactin-inhibiting drug, cabergolineObstet Gynecol1988713 pt 13113143279351
- Caballero-GordoALopez-NazarenoNCalderayMCaballeroJLMancheñoESghedoniDOral Cabergoline. Single-dose inhibition of puerperal lactationJ Reprod Med199136107177211683403
- Bravo-TopeteGMendoza-HernándezFCejudo-AlvarezJBriones-GarduñoCCabergoline for inhibition of lactationCir Cir20047215915087045
- BriggsGGFreemanRYaffeSDrugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal RiskPhiladelphia, PALippincott Williams & Wilkins2011
- Dostinex: Summary of Product Characteristics [webpage on the Internet]Medicines Compendium UK Available from: http://www.medicines.org.uk/emc/medicine/10003/spcAccessed May 11, 2013
- World Health Organization (WHO)Drug and Therapeutic Committee: A Practical Guide To Drug Use Evaluation (Drug Utilization Review)GenevaWorld Health Organization2003155
- Centers for Disease Control and Prevention (CDC) [webpage on the Internet]. Breastfeeding. [cited Mar 28, 2015] Available from: http://www.cdc.gov/breastfeeding/disease/Accessed April 23, 2015
- Section on BreastfeedingBreastfeeding and the use of human milk: policy statementPediatrics2012129e827e84122371471
- Drugs and Lactation Database (LactMed) [webpage on the Internet]US National Library of Medicine Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACTMEDAccessed March 5, 2015
- NICEHypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy NICE Clinical Guideline 107LondonNICE2011
- BensonABHaithMMSocial and Emotional Development in Infancy and Early Childhood1st edWaltham, MAAcademic Press2009
- KingTLBruckerMCKriebsJMFaheyJOVarney’s Midwifery5th edBurlington, MAJones & Bartlett Learning2013