Abstract
Background:
As proton pump inhibitors share CYP3A4 enzyme with tacrolimus for their hepatic elimination, they potentially affect its pharmacokinetics, most prominently in patients with CYP2C19 or CYP3A5 gene mutations. Our aim was to investigate the impact of omeprazole on tacrolimus pharmacokinetics in CYP3A5 non-expressors, kidney transplant recipients.
Methods:
Twelve patients (five males/seven females) were observed for 175 ± 92.05 days. Omeprazole (20 mg pos) was administrated for 75.83 ± 45.17 days. Immunosuppressant regimen consisted of tacrolimus (n = 12), methylprednisolone (n = 10), mycophenolate mofetil (n = 11), azathioprine (n = 1), and everolimus (n = 2). Patient’s body weight, coadministered drugs, and tacrolimus trough levels were monitored. Aspartate and alanine aminotransferase, γ-glutamyltransferase, and bilirubin were used for evaluating hepatic function. Tacrolimus kinetics were estimated with daily dose, concentration, dose adjusted concentration, and volume of distribution with and without coadministration of omeprazole. CYP3A5 genotyping was performed with PCR followed by restriction fragment length polymorphism analysis. Statistical analysis was performed with Prism 4 software (GraphPad Software, Inc).
Results:
No statistically significant difference was observed in tacrolimus kinetics and hepatic function during coadministration of omeprazole.
Conclusion:
Our results let us propose that there is no need for more frequent therapeutic drug monitoring of tacrolimus when coadministrated with omeprazole in CYP3A5 nonexpressors, though prospective studies with more patients and longer observation period are needed to confirm these findings.
Introduction
Proton-pump inhibitors (PPIs) are commonly administrated to transplanted patients to prevent stress-related gastric bleeding or gastrointestinal ulceration. In humans, PPIs are metabolized by cytochrome P450 (CYP) enzymatic system, mainly by CYP2C19 and CYP3A4.Citation1–Citation3 Systemic clearance of tacrolimus (TAC), the main immunosuppressant drug agent, is performed via CYP3A4 and CYP3A5.Citation4–Citation6 As PPIs and TAC share CYP3A4 enzyme for their hepatic elimination, PPIs may potentially affect TAC pharmacokinetics, most prominently in patients with CYP2C19 or CYP3A5 gene mutations.
The presence of clinically relevant pharmacokinetic drug interaction between omeprazole and TAC remains a matter of controversy.Citation2,Citation3,Citation7–Citation10 Omeprazole is metabolized into its major primary metabolites by CYP2C19 with a minor contribution of CYP3A4.Citation2,Citation3 In case of CYP2C19 gene mutation (poor metabolizers) or if high doses of omeprazole (40 mg) are given to extensive metabolizers, CYP3A4 becomes the main elimination enzyme for omeprazole.Citation9 In vivo studies using human liver microsomes have shown that omeprazole inhibits CYP3A4-mediated metabolism of TAC competitively.Citation7,Citation8 Takahashi et al proposed rabeprazole as a safer treatment option than omeprazole in kidney transplant recipients receiving TAC.Citation11 Lemahieu et al examined the impact of cimetidine and omeprazole on TAC exposure and on CYP3A4/PGP activity in vivo and concluded that switching treatment with cimetidine to omeprazole in renal transplant recipients is associated with a decrease of dose/weight normalized trough levels of TAC.Citation12 In contrast, Pascual et al estimated the potential interaction between omeprazole and TAC in renal transplant recipients and concluded to absence of important drug interaction.Citation10
Defective CYP3A5 genotype may reveal potential CYP3A4-mediated drug interaction between TAC and omeprazole. CYP3A5 may account for more than 50% of whole hepatic CYP3A in individuals who express it. Therefore, it is the main genetic factor responsible for the inter-individual variation of kinetics of drugs that are metabolized by CYP3A family.Citation13 Although CYP3A5 polymorphisms seem to have greater importance than those of CYP3A4, non-expressors may sometimes not be distinct because many drugs are metabolized by both CYP3A5 and CYP3A4.Citation14 The most important polymorphism in CYP3A5 gene is CYP3A5*1 (A6986G) which is situated in intron 3.Citation4–Citation6 Individuals carrying at least one CYP3A5*1 (g6986A) allele express CYP3A5 whereas subjects homozygotes for CYP3A5*3 (g6986G) allele do not express CYP3A5 protein.Citation13
The aim of the present study was to investigate the impact of omeprazole on TAC pharmacokinetics in CYP3A5 non-expressors, kidney transplant recipients.
Subjects and methods
Subjects
Twelve individuals (five males/seven females), who received a kidney transplant for end stage chronic renal insufficiency and attended the Outpatient Clinic of Nephrology in our institution, were selected to participate in the study. Informed consent was obtained from each participant.
Patients had received the kidney transplant 699.83 ± 969. 13 days ago, the average observation time was 175 ± 92.05 days and omeprazole treatment (20 mg pos) was administrated for 75.83 ± 45.17 days. The immunosuppressant regimen consisted of TAC (n = 12), methylprednisolone (n = 10), mycophenolate mofetil (n = 11), azathioprine (n = 1), and everolimus (n = 2). TAC was taken twice a day and its blood concentration was measured 12 hours post night dose at repeated time points. Patient’s body weight, coadministered drug agents, TAC trough levels, and blood chemistry were monitored during the study time. TAC dose adjusted concentration and volume of distribution (dose/concentration ratio) were calculated.
Concomitant medication was allowed only if it had been started 1 month before the initiation of the study especially if the administrated drugs influenced the kinetics of tacrolimus or omeprazole. Coadministrated treatment included drug agents for coronary heart disease, diabetes mellitus, thyroid gland disorders, hypertension, hyperuricemia, hypertriglyceridemia/hyperlipidemia, osteoporosis, anemia (iron-deficiency etc), magnesium disorders, and antibiotic prophylaxis.
CYP3A5 genotype determination
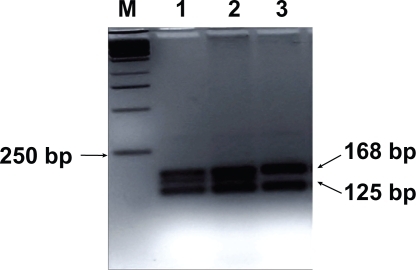
Genomic DNA was extracted from the 200 μL ethylene-diaminetetracetic acid-treated whole blood sample with the use of QIAamp DNA Blood kit (QIACEN GmbH). CYP3A5 genotyping was performed with PCR followed by restriction fragment length polymorphism analysis (RFLP). In accordance to van Schaik et al the forward primer used was 5′-CATCAGTTAGTAGACAGATGA-3′ and the reverse one 5′-GGTCCAAACAGGGAAGAAATA-3′.Citation13 These primers amplified a 293-bp fragment of CYP3A5. PCR conditions were 1 min at 94°C, 40 cycles of 1 min at 94°C, 1 min at 55°C, 1 min at 72°C, and a final extension of 7 min at 7°C. Digestion of PCR product was performed with the use of SspI endonuclease (New England BioLabs Inc.) and the digestion products were separated with 3.5% agarose/Trisborate EDTA gel electrophoresis and ethidium bromide staining (). CYP3A5*1/*1 genotype gave 148-, 125-, and 20-bp bands; CYP3A5*3/*3 genotype 168- and 125-bp bands; and CYP3A5*1/*3 genotype 168-, 148-, 125-, and 20-bp.Citation13
Figure 1 RFLP for CYP3A5. Lane M, base pair marker (250-bp DNA ladder); lanes 1–3, SspI-digested PCR products from three PCR products. CYP3A5*3/*3 genotype gives 168- and 125-bp bands (lane 3) and CYP3A5*1/*3 genotype gives 168-, 148-, 125-, and 20-bp (lanes 1 and 2). The 20-bp band is not visible. CYP3A5*1/*1 genotype is not seen in this picture. Analysis on a 3.5% agarose/Tris-borate-EDTA gel.
Statistical analysis
TAC pharmacokinetics were estimated with the use of TAC daily dose, concentration, dose adjusted concentration, and volume of distribution (dose/concentration ratio), with and without coadministration of omeprazole. Aspartate amin-otransferase, alanine aminotransferase, γ-glutamyltransferase, and bilirubin were used for the evaluation of hepatic function.
Statistical calculations and analyses were performed with the use of Prism 4 (GraphPad Software, Inc) statistical software package. Continuous data were tested with Kolmogorov’s normality test to estimate whether originated from normal distribution or not. Student’s t-test was used for testing the significance of difference of variables that passed the normality test. Mann-Whitney test was used for continuous variables that did not pass the normality test. All statistical tests performed were two-sided. The threshold of statistical significance was set at 5% (a = 0.05).
Results
The characteristics of the study population are presented in . The etiology of the primary kidney disease was unknown (n = 2), cystoureteral reflux (n = 2), polycystic kidney disease (n = 1), diabetic nephropathy (n = 1), Alport syndrome (n = 1), eclampsia (n = 1), antiphospholipidic syndrome/systemic lupus erythematosus (n = 1), nephrolithiasis (n = 1), nephronophthisis (n = 1), and IgA nephropathy (n = 1).
Table 1 Characteristics of patient population
No statistically significant difference was observed in TAC pharmacokinetics during the coadministration of omeprazole (). No statistically significant difference was observed in aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, and bilirubin during the concomitant use of omeprazole ().
Table 2 Tacrolimus pharmacokinetics and hepatic function with and without coadministration of omeprazole
Discussion
Defective CYP3A5 genotype in renal transplanted patients could reveal the CYP3A4-mediated drug interaction between TAC and omeprazole. The contribution of CYP3A5 to the interaction between TAC and omeprazole remains unclear. Seventy-five percent of whites and 50% of African Americans do not express functional CYP3A5.Citation14 In the Greek population, the CYP3A5*3 allele shows an allelic frequency of 94.35% whereas individuals homozygous for CYP3A5*3 show a frequency of 88.69%.Citation15 In our study, the participants were CYP3A5 nonexpressors to ensure that TAC is metabolized through CYP3A4 and thereby, share this cytochrome with omeprazole.
To our knowledge, up to now clinical significance of pharmacokinetic drug interactions between PPIs and TAC has mainly been investigated in case reports and inadequately estimated in clinical trials. Namely, no clear clinically relevant interaction between TAC and omeprazole has been reported and the results of the studies are controversial. Although omeprazole inhibits the in vitro metabolism of TAC by human liver microsomes, no clinical study is available on the relevance of such an interaction among transplant patients.Citation7,Citation8 Additionally, although interaction of omeprazole with TAC may involve inhibition of hepatic and intestinal CYP3A4, intestinal PGP, and pharmacokinetic characteristics of omeprazole, the only reported interactions between TAC and other PPI have been described in subjects with CYP2C19 gene variants.Citation16–Citation18 More specifically, increased blood concentration of omeprazole can cause CYP3A4 overload and result in interaction in this pathway in patients with CYP2C19 gene mutations as pharmacokinetic profiles of omeprazole and lanzoprazole depend on the CYP2C19 genotype status.Citation11,Citation17,Citation19
Because PPIs and TAC share the CYP3A4 system for their hepatic elimination, PPIs may potentially inhibit TAC metabolism via CYP3A4 thereby increasing drug blood concentrations, especially in patients with CYP2C19 gene mutations.Citation19,Citation20 In the Greek population, CYP2C19*2 allele show an allelic frequency of 13.07% whereas none of 283 subjects studied was found to possess the CYP2C19*3 allele.Citation15 Caucasian subjects with *1/*1 and *1/*17 genotype need stronger acid-suppression therapy compared to CYP2C19*2/*2, especially during the first days of treatment or with on-demand therapy.Citation21
Omeprazole is known to have the most pharmacokinetic interactions with TAC, compared to other PPIs. However, Pascual et al estimated the potential interaction between omeprazole and TAC in renal transplant recipients and concluded to absence of important drug interaction between omeprazole and TAC in the general transplant population.Citation10 Although other PPIs have fewer documented drug interactions than omeprazole, recently Takahashi et al showed important interactions between lansoprazole and TAC.Citation22
In our study, no statistically significant difference was found in the pharmacokinetic parameters of TAC with coadministration of omeprazole. Additionaly, we report no statistically significant difference in hepatic function. Hosohata et al reported that intestinal and graft liver CYP2C19 plays a relatively greater role in the metabolism of omeprazole than it does for lansoprazole, so that the effects of CYP3A5 on the metabolism of TAC might be masked by the interaction with omeprazole associated with the CYP2C19 genotype.Citation23 Studies in healthy volunteers suggest that a decrease of dose/weight normalized trough levels of TAC may be explained by an increase of intestinal CYP3A4 activity.Citation12
This was the first attempt to study TAC kinetics in Greek renal transplant patients. CYP3A5*3 is a common polymorphism among Greeks. Detection of CYP2C19 polymorphisms was not performed as a previous study showed that they are rare in Greeks and we assumed that our patients were not CYP2C19 polymorphic.Citation15 The absence of CYP3A5 expression was used to reveal the CYP3A4-mediated drug interaction between TAC and omeprazole. Although patient’s epigenetic characteristics (including age, dietary, and other habits) are known to affect TAC kinetics, these parameters were not studied here because of the small number of patients.
Since genetic information is not usually clinically available for each patient, careful monitoring of TAC trough levels is, still, needed to optimize the dosage regimen in patients receiving TAC. Our results let us propose that there is no need for more frequent therapeutic drug monitoring of TAC when coadministrated with omeprazole in CYP3A5 nonexpressors. However, due to restricted number of patients, prospective studies with greater number of patients and longer observation time are deemed necessary to further evaluate the clinical impact of interaction, if it exists, between TAC and omeprazole.
Acknowledgements
The authors would like to thank the personnel of the Department of Internal Medicine-Nephrology, University Hospital of Patras, Rion for their support.
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- IshizakiTHoraiYReview article: cytochrome P450 and the metabolism of proton pump inhibitors – emphasis on rabeprazoleAliment Pharmacol Ther199913273610491726
- RenbergLSimonssonRHoffmannKJIdentification of two main urinary metabolites of [14C]omeprazole in humansDrug Metab Dispos19891769762566473
- AnderssonTMinersJOVeroneseMEIdentification of human liver cytochrome P450 isoforms mediating omeprazole metabolismBr J Clin Pharmacol19933652153012959268
- MouradMWallemacqPDe MeyerMBiotransformation enzymes and drug transporters pharmacogenetics in relation to immunosuppressive drugs: impact on pharmacokinetics and clinical outcomeTransplantation200885S19S2418401258
- TsuchiyaNSatohSTadaHInfluence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipientsTransplantation2004781182118715502717
- HuYFHeJChenGLCYP3A5*3 and CYP3A4*18 single nucleotide polymorphisms in a Chinese populationClin Chim Acta200535318719215698606
- ChristiansUSchmidtGBaderAIdentification of drugs inhibiting the in vitro metabolism of tacrolimus by human liver microsomesBr J Clin Pharmacol1996411871908866917
- MatsudaHIwasakiKShiragaTTozukaZHataTGuengerichFPInteractions of FK506 (tacrolimus) with clinically important drugsRes Commun Mol Pathol Pharmacol19969157648824931
- RostKLRootsINonlinear kinetics after high-dose omeprazole caused by saturation of genetically variable CYP2C19Hepatology199623149114978675169
- PascualJMarcénROreaOEInteraction between omeprazole and tacrolimus in renal allograft recipients: a clinical-analytical studyTransplant Proc2005373752375316386527
- TakahashiKYanoIFukuharaYDistinct effects of omeprazole and rabeprazole on the tacrolimus blood concentration in a kidney transplant recipientDrug Metab Pharmacokinet20072244144418159131
- LemahieuWPMaesBDVerbekeKVanrenterghemYImpact of gastric acid suppressants on cytochrome P450 3A4 and P-glycoprotein: consequences for FK506 assimilationKidney Int2005671152116015698457
- van SchaikRHvan der HeidenIPvan den AnkerJNLindemansJCYP3A5 variant allele frequencies in Dutch CaucasiansClin Chem2002481668167112324482
- EvansWEMcLeodHLPharmacogenomics-drug disposition, drug targets, and side effectsN Engl J Med200334853854912571262
- ArvanitidisKRagiaGIordanidouMGenetic polymorphisms of drug-metabolizing enzymes CYP2D6, CYP2C9, CYP2C19 and CYP3A5 in the Greek populationFundam Clin Pharmacol20072141942617635181
- MoreauCDebrayDLoriotMATaburetAMFurlanVInteraction between tacrolimus and omeprazole in a pediatric liver transplant recipientTransplantation200681487488 Erratum in: Transplantation 2006;82:1382.16477241
- TakahashiKMotohashiHYonezawaALansoprazole-tacrolimus interaction in Japanese transplant recipient with CYP2C19 polymorphismAnn Pharmacother20048791794 Epub 2004 Mar 9.15010519
- ItagakiFHommaMYuzawaKFukaoKKohdaYDrug interaction of tacrolimus and proton pump inhibitors in renal transplant recipients with CYP2C19 gene mutationTransplant Proc2002342777277812431607
- FurutaTOhashiKKosugeKCYP2C19 genotype status and effect of omeprazole on intragastric pH in humansClin Pharmacol Ther19996555256110340921
- De MoraisSMWilkinsonGRBlaisdellJMeyerUANakamuraKGoldsteinJAIdentification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in JapaneseMol Pharmacol1994465945987969038
- HunfeldNGMathotRATouwDJEffect of CYP2C19*2 and *17 mutations on pharmacodynamics and kinetics of proton pump inhibitors in CaucasiansBr J Clin Pharmacol200865752760 Epub 2008 Jan 30.18241283
- van GelderTDrug interactions with tacrolimusDrug Saf20022570771212167066
- HosohataKMasudaSKatsuraTImpact of intestinal CYP2C19 genotypes on the interaction between tacrolimus and omeprazole, but not lansoprazole, in adult living-donor liver transplant patientsDrug Metab Dispos200937821826Epub 2009 Jan 12.19139162