Abstract
Current national and international asthma guidelines recommend treatment of children with asthma towards achieving and maintaining asthma control. These guidelines provide more stringent recommendations to increase therapy for patients with uncontrolled asthma in order to reduce asthma-related morbidity and mortality. Newer combination agents such as budesonide and formoterol have been shown to be safe and effective in treatment of asthma in children. Use of long-term controller agents like this in combination with improved compliance and treatment of co-morbid conditions have been successful in this endeavor. This review discusses control of pediatric asthma with focus on the use of budesonide in combination with formoterol.
Introduction
National and international asthma treatment guidelines have recently focused on control of asthma. These guidelines provide more stringent recommendations to increase therapy for patients with uncontrolled asthma in order to reduce asthma related morbidity and mortality. Further, they recommend different combinations of controllers for long term treatment of persistent asthma including addition of long-acting β2-agonists (LABA) to inhaled corticosteroid (ICS) therapy.Citation1,Citation2 This review discusses control of pediatric asthma with focus on the use of budesonide in combination with formoterol.
Diagnosis of asthma in children
Before long term controller medication can be initiated, the diagnosis of asthma must be made. Many studies show that asthma is under diagnosed particularly in children.Citation3–Citation5 Asthma is a chronic inflammatory disease of the airways characterized by episodic and reversible airflow obstruction. A clinical diagnosis of asthma is often prompted by symptoms of cough, shortness of breath, chest tightness, or wheezing. Spirometry is the recommended method of objectively measuring airflow obstruction and reversibility. Diagnosis of asthma is supported by reversible airflow obstruction as measured by forced expiratory volume at one second (FEV1) by more than 12% after bronchodilator therapy.Citation6,Citation7 There is no single diagnostic test for asthma, but careful review of symptoms along with physical examination and objective measurements of lung function can guide the clinician toward diagnosis. A detailed discussion on the differential diagnosis of asthma is beyond the scope of this review.
The diagnosis of asthma in younger children often presents a challenge for clinicians because episodic wheezing and cough are also common in children who do not have asthma. More than 50% of children in the United States and the United Kingdom wheeze in their first year of life.Citation8 The diagnosis of asthma is further complicated by the difficulty in obtaining objective measurements of lung function in children aged less than five years, the lack of definitive biomarkers, and the difficulty in discerning among the different asthma phenotypes.Citation9–Citation11
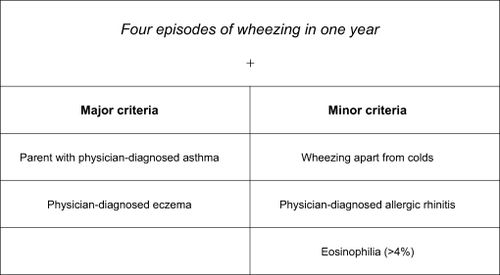
Castro-Rodriguez and colleagues developed the Asthma Predictive Index (API),Citation12 a practical tool that helps clinicians identify children who are more likely to have asthma. This simple clinical index based on the presence of wheeze before the age of three, and the presence of one major risk factor (parental history of asthma or a personal history of atopic dermatitis) or two of three minor risk factors (allergic rhinitis, wheezing apart from colds, and eosinophilia), has been shown to predict the presence of asthma in later childhood. The API has a positive predictive value of 76% and a negative predictive value of 95% ().
Figure 1 Asthma Predictive Index. History of early wheezing in a child with at least one major criteria or two minor criteria had a positive predictive index of 76% in children. Copyright © 2008, American Thoracic Society. Adapted from Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406.
Asthma control in children
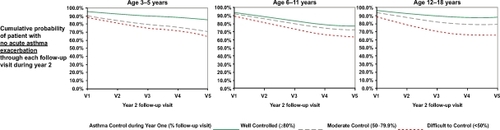
Currently most asthma guidelines measure asthma control using two domains consisting of 1) present asthma symptoms and lung function and 2) history of past asthma exacerbations.Citation1,Citation2 In the 2007 National Asthma Education Prevention Program Expert Review Panel 3 (NAEPP ERP 3) guidelines, assessment of a combination of current asthma symptoms (such as frequency of day and night symptoms), lung function, and asthma survey instruments such as the Asthma Control Test (ACT) are used to measure patient’s current impairment due to asthma. The number of prior asthma exacerbations requiring systemic steroid rescue is used to measure patient’s risk (). Impairment and risk are then used to determine the level of patient’s asthma control. Long term asthma control reduces asthma associated impairment and is believed to result in reduced risk of exacerbations.Citation13 We previously demonstrated that inner city asthmatic children who maintained a high degree of asthma control over the period of a year had a significantly lower risk of asthma exacerbations and emergency department visits/hospitalizations compared to similar children who had poor asthma control ().Citation14
Figure 2 Assessing asthma control in children aged 5–11 years. Refer to NAEPP guidelines for other age groups. Adapted from National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Management of Asthma: Expert Panel Report 3. Bethesda, MD: National Institutes of Health, National Heart, Lung and Blood Institute; 2007.
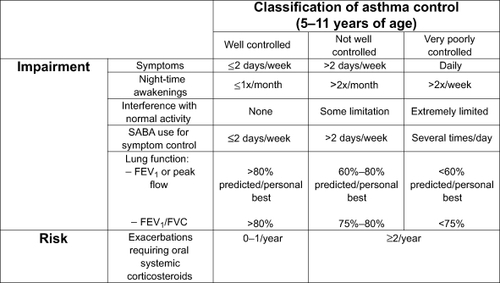
Figure 3 Degree of asthma control during year 1 was associated with probability of asthma exacerbation during year 2 in inner city children with asthma living in Los Angeles. Degree of asthma control was defined as the percentage of clinic visits during year 1 when asthma was rated as controlled based upon NAEPP ERP 2 asthma guidelines. Copyright © 2008, Elsevier. Adapted from Kwong KY, Morphew T, Scott L, Guterman J, Jones CA. Asthma control and future asthma-related morbidity in inner-city asthmatic children. Ann Allergy Asthma Immunol. 2008;101(2):144–152.
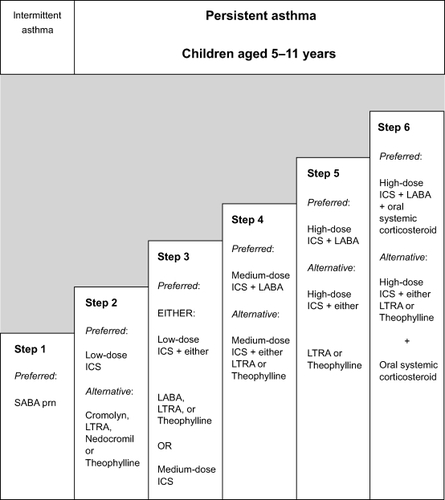
Previous asthma guidelines recommended classification of asthma severity upon initial assessment prior to controller treatment. This was an attempt to determine the baseline “controller naïve” asthma severity in patients and the level of controller therapy required.Citation1,Citation2 A recent update of those guidelines still recommends baseline classification of severity, but only as an initial starting reference point. Many patients initially seen by providers are not controller naïve and recent studies have demonstrated that asthma disease activity may vary over time and deviate significantly from the initial baseline classification. Patients initially classified with intermittent asthma may experience severe exacerbations at distal time points.Citation15,Citation16 For instance, it is well described that young children may have relatively mild asthma symptoms (impairment) throughout the year, but may have severe exacerbations during periods of viral infections (risk).Citation17,Citation18 Congruent with these observations, guidelines stress that ongoing monitoring of disease activity is essential to maintaining asthma control wherein asthma control is the measurement used during real time assessment of asthmatics at each provider encounter. Dynamic pharmacologic therapy using a stepwise approach is recommended with “step up” of controller agents when control is poor and “step down” of medications when control is maintained for extended periods of time ().
Figure 4 Stepwise Approach for managing asthma in children aged 5–11 years. Refer to NAEPP guidelines for other age groups. Adapted from National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Management of Asthma: Expert Panel Report 3. Bethesda, MD: National Institutes of Health, National Heart, Lung and Blood Institute; 2007.
Compliance and treatment of co-morbid conditions
Compliance and treatment of co-morbid conditions play major roles in achievement of asthma control in children. Asthma is a chronic disease and long term treatment is imperative to reduce risk of morbidity and mortality. Consistent with findings of other investigators, we have shown that compliance is a major independent variable that contributes towards maintaining asthma control in inner city asthmatic children once initial disease control was achieved.Citation19,Citation20 Long-term compliance is unfortunately suboptimal in many asthmatic children, which contributes to poor asthma control in these patients. Reasons for this include poor patient understanding of the disease, complexity of treatment, access to care and medications, and socioeconomic factors.Citation21,Citation22
Control of co-morbid conditions including allergic rhinitis, sinusitis, and gastroesophageal reflux disease (GERD) are important in achieving asthma control in children. This often allows asthma control using lower doses of controller agents, reducing risk of side effects. Pathology of the upper airway is believed to adversely impact the lower airways of asthmatics via mechanisms that are not completely understood. However, neuronal and inflammatory pathways have been implicated.Citation23,Citation24 Treatment of the upper airway with intranasal steroids, antihistamines, and leukotriene receptor antagonists (LTRA) have been shown to improve asthma control.Citation25–Citation27 Control of GERD with proton pump inhibitors, H2 blockers and surgery has also been shown to improve asthma symptoms and reduce doses of controller agents required.Citation28,Citation29
Controller agents recommended for long-term asthma control in children
Inhaled corticosteroids
Inhaled corticosteroids (ICS) remain the cornerstone of asthma treatment for children of all ages.Citation1,Citation2 They are the most potent and effective anti-inflammatory medication currently available. Corticosteroids block late-phase reaction to allergens, reduce airway hyperresponsiveness, and inhibit inflammatory cell migration and activation.Citation30–Citation32 ICS reduce asthma symptoms, improve quality of live, reduce frequency and severity of exacerbations, and reduce asthma mortality.Citation33–Citation35 The potential but small risk of adverse effects from ICS is well balanced by their efficacy. Local adverse effects include oropharyngeal candidiasis and dysphonia. Systemic adverse effects of long-term treatment with high doses of ICS include easy bruising,Citation36 adrenal suppression,Citation37,Citation38 and decreased bone mineral density.Citation39 The risk of systemic adverse effects depends upon its dose and potency. Low and medium doses of ICS appear to have no serious adverse effects on bone mineral density in children.Citation40 In addition, low and medium dose ICS have no significant effects on the incidence of subcapsular cataracts or glaucoma.Citation41 Perhaps the more significant adverse effect of ICS is growth velocity reduction in children. The available cumulative data suggest that even low-dose ICS have the potential for decreasing growth velocity, although the effects are small.Citation42,Citation43 Despite a small reduction in growth velocity, studies of early intervention with low- or medium-dose ICS showed significantly improved asthma outcomes.Citation33,Citation44
The efficacy and tolerability of the ICS, budesonide, is well established in the treatment of pediatric asthma.Citation45,Citation46 One of the largest and longest studies demonstrating the benefit of ICS in pediatric patients with asthma is the Childhood Asthma Management Program (CAMP).Citation47 1,041 children aged from 5 to 12 years with mild-to-moderate asthma were randomly assigned to receive either 1) budesonide, 2) nedocromil, or 3) placebo and were followed for four to six years (mean 4.3 years). Children given budesonide experienced fewer hospitalizations and urgent care visits, decreased symptoms, decreased need for albuterol rescue therapy, and fewer episodes of acute asthma requiring oral prednisone. However, neither budesonide nor nedocromil was better than placebo in terms of improving lung function in the long term. These findings suggest that continuous, long-term ICS treatment of children did not prevent deficits in lung function and did not alter the rate of progression of asthma. Side effects of budesonide included small, transient reduction in growth velocity. The mean increase in height in the budesonide group was 1.1 cm less than the mean increase in the placebo group. The difference between the budesonide and placebo groups in the rate of growth was evident primarily within the first year of treatment.
The CAMP investigators speculated that ICS did not affect the natural course of asthma because treatment did not begin early enough. The Prevention in Early Asthma in Kids study (PEAK) attempted to address this issue. The PEAK study was a multicenter, double-blind, randomized, placebo-controlled trial that investigated whether daily ICS could prevent the development of chronic asthma. 285 children aged two or three years with a positive asthma predictive index were randomized to receive either fluticasone propionate (88 μg twice daily) or masked placebo for two years, followed by a one-year period of observation without study medications.Citation42 During the treatment period, the children who received ICS had a greater proportion of episode-free days, a lower rate of asthma exacerbations, and decreased supplementary use of controller medications. However, during the observation period, no significant differences were seen between the two groups in the proportion of episode-free days, the number of exacerbations, or lung function. Therefore, in preschool children at high risk for asthma, two years of ICS therapy did not change the development of asthma symptoms or lung function during the third, treatment-free year. Congruent with findings from the CAMP study, the children who received ICS had a mean increase in height 1.1 cm less than the mean increase in the placebo group at 24 months. However, by the end of the trial, the height increase was only 0.7 cm less.
The START study evaluated the safety and tolerability of long-term treatment of both children and adults who have mild persistent asthma.Citation48 17.8 % and 27.8% of the patients in this study were aged 11–17 and 5–10 years, respectively. In the first three years of the START study, patients with mild persistent asthma who received low-dose daily budesonide had a reduction in their risk of a first severe asthma-related event by 44% compared to similar patients given placebo. Thus budesonide significantly reduced the overall risk of experiencing a severe asthma-related event in these pediatric patients. Similar to the CAMP study, children given budesonide compared to those receiving placebo experienced small reductions in growth velocity. However, low-dose daily budesonide was associated with significantly less effect on growth than with previously reported studies with high-dose budesonide.
A long-term placebo-controlled study by Agertoft and colleagues involving 211 children who received long-term treatment with budesonide (mean duration 9.2 years) reported no adverse effects on final height.Citation49 The mean daily dose of budesonide was 412 μg (range, 110 to 877) and the mean cumulative dose of budesonide was 1.35 g (range, 0.41 to 3.99). Growth rates were significantly reduced during the first years of budesonide treatment; however, these changes in early growth rate were not significantly associated with reductions in predicted final adult height. Other studies using long-term treatment with ICS show similar catch-up growth after the initial period of administration.Citation50–Citation53
Taken together, these studies demonstrate that ICS significantly improve symptoms, reduce impairment, and reduce the likelihood of asthma-associated morbidity in children with asthma. The significant benefit of this agent is balanced with the risk of linear growth reduction especially during initial periods of use which may be caught up over time.
Combination therapy in children
NAEPP EPR 3 recommend combination therapy for the majority of children with moderate to persistent asthma.Citation15 The most popular combinations are the use of ICS in combination with LABA and ICS in combination with LTRAs.
Inhaled corticosteroids in combination with long-acting β2-agonist
Numerous studies have established that the addition of LABA to ICS in adults is more effective at controlling symptoms, improving lung function, and reducing asthma exacerbations than doubling the dose of ICS. In the Formoterol and Corticosteroids Establishing Therapy (FACET) study, Pauwels and colleagues reported that in adult patients with moderate asthma, the addition of formoterol to low or moderate doses of budesonide reduced the frequency of asthma exacerbations, improved symptoms, and lung function.Citation54 In this double-blind parallel group study, 852 patients were randomly assigned to receive one of the following treatments for one year: 1) budesonide 100 μg bid, 2) budesonide/formoterol 100 μg/12 μg bid, 3) budesonide 400 μg bid, or 4) budesonide/formoterol 400 μg/12 μg bid. A post-hoc analysis of the FACET study confirmed that the addition of formoterol to low-dose budesonide increases the probability of well controlled asthma compared to a substantial increase in the dose of ICS.
The OPTIMA trial was a double-blind, randomized, and parallel-group study designed to determine whether regular treatment with low doses of inhaled budesonide, with or without doses of inhaled formoterol, would reduce severe asthma exacerbations and improve asthma control. In one arm of the trial, patients aged 12 years and older who were already taking budesonide at baseline were randomized to receive: 1) budesonide 100 μg bid, 2) budesonide/formoterol 100 μg/4.5 μg bid, 3) budesonide 200 μg bid, or 4) budesonide/formoterol 200 μg/4.5 μg bid.Citation55 The addition of formoterol reduced the risk for severe asthma exacerbations and improved asthma control more effectively than doubling the dose of budesonide.
Numerous other studies and meta-analysis of adults and children aged 12 years and older have established that the combination of ICS/LABA is more effective at controlling symptoms, improving lung function, and reducing asthma exacerbations than doubling the dose of ICS.Citation56–Citation59 However, there have been only a few studies of the efficacy of ICS/LABAs in asthmatics less than 12 years old, and to the best of our knowledge, none in children aged less than five years.
Tal and colleagues evaluated the effect on lung function and tolerability of budesonide/formoterol in a single inhaler in children (mean age 11 years) with moderate to persistent asthma.Citation60 This was a 12-week, double-blind, parallel-group, multicenter trial that randomized 286 asthmatic children to either budesonide/formoterol 90/9 μg bid or budesonide 200 μg bid. Children who received combination budesonide/formoterol had increased morning peak expiratory flow (PEF) relative to baseline compared to budesonide alone (7.22% predicted normal versus 3.45% predicted normal, respectively). Evening PEF also increased significantly with budesonide/formoterol compared to budesonide alone (6.13% predicted normal versus 2.73% predicted normal, respectively). Mean FEV1 and serial FEV1 measured over 12 hours increased significantly with budesonide/formoterol compared to budesonide alone. Combination budesonide/formoterol in a single inhaler provided rapid improvements in PEF and FEV1 compared to inhaled budesonide alone. The two treatment groups were similar in their adverse event profile and rates of discontinuation.
In a similar study by Pohunek and colleagues, investigators compared the efficacy and safety of combination therapy with budesonide and formoterol versus budesonide alone in children ages 4 to 11 years.Citation61 In a 12-week, double-blind study, 630 children were randomized to: 1) budesonide/formoterol 90/9 μg bid, 2) budesonide 200 μg bid, and 3) budesonide (90 μg) + formoterol (9 μg) in separate inhalers. The budesonide/formoterol combination significantly improved morning PEF, evening PEF, and FEV1 compared with budesonide alone. There was no significant difference between the budesonide/formoterol and budesonide + formoterol in separate inhalers. The adverse event profiles were similar in all groups and there were no serious asthma-related adverse events in any treatment group. Combination budesonide/formoterol significantly improved lung function in children aged 4 to 11 years with asthma compared to budesonide alone.
These studies, although small, suggest that budesonide/formoterol combination is a safe and effective treatment option for children with asthma.
Salmeterol and formoterol are highly selective, third generation LABAs that have been available for use since the early 1990s. Salmeterol and formoterol, however, differ in their pharmacological properties. One important difference is that the onset of action of formoterol is faster than that of salmeterol.Citation62,Citation63 A significantly greater improvement in FEV1 was noted within three minutes of dosing with formoterol than salmeterol and continued to have a more pronounced effect for the three hours following dosing. Formoterol produces bronchodilation as fast as the short-acting ß2-agonist salbutamol.Citation64,Citation65 In the Salmeterol Multicenter Asthma Research Trial, comparing daily treatment with salmeterol or placebo added to standard asthma therapy for adults resulted in an increased risk of asthma-related deaths in patients treated with salmeterol (13 deaths out of 13,176 patients treated with salmeterol versus three deaths out of 13,179 patients treated with placebo).Citation66 In addition, increased numbers of severe asthma exacerbations were noted in the trials submitted to the US Food and Drug Administration (FDA) for formoterol approval.Citation67 The association with severe asthma exacerbations and asthma-related deaths has resulted in a black box warning label on all preparations containing LABAs. A single nucleotide (Arg–Arg versus Gly–Gly) substitution in the β2-agonist receptor gene may be associated with worsening of asthma in this subset of patients taking salmeterol.Citation68,Citation69 It is worth noting that an increased risk of asthma-related deaths was not seen in the subset of patients reporting ICS use at baseline. Likewise, the evidence for increased risk of severe exacerbations from LABAs may stem from studies that compared monotherapy with LABAs versus monotherapy with ICS.Citation70,Citation71 Although it is still unclear whether salmeterol in combination with ICS still results in worsening of asthma in patients with the Arg–Arg genotype, a recent large retrospective study showed no such association.Citation72 LABA must never be used as monotherapy and should be always used in combination with ICS.
Inhaled corticosteroid in combination with leukotriene receptor antagonists
Use of LTRAs as adjunctive therapy to ICS has not been studied well in children aged 5–11 years and has not been studied at all in children aged less than four years. The majority of studies have been done using adult subjects. LTRAs may be added when asthma is not controlled on ICS alone. Lofdahl and colleagues showed that addition of montelukast to beclomethasone significantly improved FEV1 in asthmatics aged 15 years and older.Citation73 Furthermore, addition of LTRA to ICS may be an effective and well tolerated alternative to doubling the dose of ICS in patients with uncontrolled asthma. In the COMPACT study, adult asthmatics with inadequately controlled asthma showed similar improvements in symptoms, exacerbations, symptom-free days, peripheral eosinophil counts, and asthma-specific quality of life when given a LTRA in addition to their current dose of ICS or had their ICS dose doubled.Citation74 Finally addition of LTRAs to ICS may also allow reduction of ICS dose in asthmatics.Citation75 In a double-blind, placebo-controlled study, 26 adult patients with stable asthma on ICS were randomized to montelukast or placebo for up to 12 weeks. Compared to placebo, montelukast allowed significant reduction in the ICS dose. LTRAs may be used as monotherapy in patients with mild asthma (step 2 in NAEPP ERP 3 and 2006 GINA) although ICS are often the preferred choice.Citation1,Citation2
Currently most asthma guidelines recommend addition of LABA versus LTRA to ICS as the preferred strategy in most age groups of children with asthma. While several studies have compared LABA versus LTRA addition to ICS in terms of efficacy, a detailed discussion is beyond the scope of this review, and may be found elsewhere.Citation76–Citation78
New paradigm: combination therapy as maintenance and reliever
Periodic fluctuations in symptoms and airway inflammation are characteristic of asthma and treatment requirements can vary over time. Reliever medications such as short-acting β2-agonist provide rapid bronchodilation and symptom relief but do not treat the underlying inflammatory process. O’Byrne and colleagues challenged the treatment paradigm of fixed dosing of ICS/LABA and proposed that combination therapy could be used as both maintenance and reliever therapy.Citation79 In this pivotal study, the use of the combination of a rapid LABA (formoterol) and an inhaled glucocorticosteroid (budesonide) in a single inhaler both as a controller and reliever was effective in maintaining high levels of asthma control. This was a double-blind, parallel-group study where 2,760 patients aged 4 to 80 years were randomized to receive either: 1) budesonide/formoterol 80/4.5 μg bid as maintenance therapy plus budesonide/formoterol 80/4.5 μg as needed for reliever therapy, 2) budesonide/formoterol 80/4.5 μg bid as maintenance plus terbutaline 0.4 mg as reliever, or 3) budesonide 320 μg bid as maintenance plus terbutaline 0.4 mg as reliever. The patients who took budesonide/formoterol as both maintenance and reliever therapy had a reduced risk of severe asthma exacerbations, need for systemic steroids, improved asthma symptoms and improved lung function compared with the traditional fixed-dosing regimens. More importantly, the average daily dose of ICS received per patient was lower in the budesonide/formoterol groups and all of the medications were well tolerated.
Rabe and colleagues demonstrated that the combination budesonide/formoterol used for both maintenance and reliever improved asthma control with a lower steroid dose compared with higher doses of budesonide.Citation80 697 patients aged 12 years and older were randomized to receive either budesonide/formoterol 80/4.5 μg bid as maintenance therapy plus budesonide/formoterol 80/4.5 μg as needed for reliever therapy or budesonide 160 μg bid as maintenance therapy plus terbutaline 0.4 mg as needed for reliever therapy. Patients receiving the budesonide/formoterol combination for both maintenance and relief had greater lung function, decreased risk of exacerbations, fewer hospitalizations, and decreased asthma symptoms. The increased efficacy of budesonide/formoterol was achieved with less ICS than was used in the budesonide group (mean dose, 240 μg/day versus 498 μg/day) and with 77% fewer oral steroid treatment days versus budesonide alone (114 days versus 498 days, respectively).
In the Symbicort Maintenance and Reliever Therapy (SMART) trial, the combination therapy with budesonide and formoterol used both as maintenance and rescue was shown to be an effective and well tolerated treatment approach for pediatric asthma management.Citation81 This was a prospective study involving 41 centers in 12 countries. 341 children aged 4–11 years with uncontrolled asthma on current ICS therapy were randomized to receive either fixed-dose budesonide, fixed-dose combination budesonide/formoterol or budesonide/formoterol maintenance and as needed therapy (SMART group). Children receiving the SMART regimen had reduced asthma exacerbations, reduced nocturnal symptoms, and improved lung function. 14% of patients receiving the SMART regimen had an exacerbation, compared to 38% and 26% of patients in the fixed-dose combination and fixed-dose budesonide groups, respectively. Time to first exacerbation was significantly longer in the SMART group compared to fixed-dose combination and fixed-dose budesonide groups, respectively. This benefit was attained while using less than half the daily dose of budesonide compared with the fixed-dose budesonide group. In addition, yearly growth was higher by 1 cm for children in the budesonide/formoterol group versus those on higher fixed-dose daily budesonide. This approach to asthma management is currently not guideline based therapy but may provide a better alternative to improving control and decreasing risk. The benefit in preventing exacerbations and improving asthma control appears to be a consequence of early intervention and providing additional anti-inflammatory therapy and rapid symptom relief. The timing of the increased steroid dose in close proximity to asthma worsening may be the key to the effectiveness of this strategy, although other studies have shown that increasing the ICS dosing during an acute attack may not reduce risk of asthma related morbidity.Citation82–Citation84
The unique pharmacological properties of budesonide/formoterol in a single inhaler make it suitable for both maintenance dosing and as reliever therapy. Whether this approach can be employed with other combinations of controller and reliever requires further study. Currently, the budesonide/formoterol single combination inhaler is not licensed by the US FDA for use as reliever therapy for the treatment of asthma. However, the 2006 GINA guidelines do recommend that if a combination inhaler containing formoterol and budesonide is selected, it may be used for both rescue and maintenance therapy.Citation2
Finally, adherence to therapy is an important factor in the success of asthma treatment. Patients often link their poor adherence to prescribed ICS therapy to the lack of an immediate effect, unlike the rapid onset of effect experienced with short-acting β2-agonist.Citation85 Budesonide/formoterol maintenance and rescue therapy may provide a significant benefit for patients for whom adherence to two separate drugs may present serious difficulties or confusion.
Summary
Current asthma guidelines recommend treatment of asthma in children towards achieving and maintaining asthma control in order to reduce impairment due to asthma symptoms and to reduce risk of future asthma exacerbations. This approach likely reduces morbidity and mortality associated with uncontrolled asthma. Newer combination agents such as budesonide and fomoterol have been shown to be safe and effective in treatment of asthma in children. Use of long-term controller agents like this in combination with improved compliance and treatment of co-morbid conditions have been successful in this endeavor. Early studies even suggest that this combination can be used to reduce asthma exacerbations when used on a more intermittent basis in some patients. However, more studies have to be completed before this strategy can be universally recommended.
Disclosures
The authors report no conflicts of interest in this work. Dr. Kwong has been a Speaker for Sepracor, Genentech, and Novartis, and has received a research grant from Merck.
References
- National Asthma Education and Prevention ProgramGuidelines for the Diagnosis and Management of Asthma: Expert Panel Report 3Bethesda, MDNational Institutes of Health, National Heart, Lung and Blood Institute2007
- MasoliMFabianDHoltSBeasleyRGlobal Initiative for Asthma (GINA) ProgramGlobal Initiative for Asthma (GINA) program: the global burden of asthma: executive summary of the GINA Dissemination Committee reportAllergy200459546947815080825
- SierstedHCBoldsenJHansenHSMostgaardGHyldebrandtNPopulation based study of risk factors for underdiagnosis of asthma in adolescence: Odense schoolchild studyBMJ19983167132651655; discussion, 6556569522784
- SpeightANLeeDAHeyENUnderdiagnosis and undertreatment of asthma in childhoodBr Med J (Clin Res Ed)1983286637312531256
- NicholsBScottLJonesSKwongKYMorphewTJonesCADetection of undiagnosed and poorly controlled asthma in a hospital-based outpatient pediatric primary care clinic using a health risk assessment systemJ Asthma200946549850519544172
- American Thoracic SocietyStandardization of spirometry, 1994 updateAm J Respir Crit Care Med19951523110711367663792
- American Thoracic Society and European Respiratory Task ForceStandardization of lung function testingEur Respir J20052694896816264058
- TagerIBHanrahanJPTostesonTDLung function, pre- and post-natal smoke exposure, and wheezing in the first year of lifeAm Rev Respir Dis199314748118178466114
- MirandaCBusackerABalzarSTrudeauJWenzelSEDistinguishing severe asthma phenotypes: role of age at onset and eosinophillic inflammationJ Allergy Clin Immunol2004113110110814713914
- BelEHClinical phenotypes of asthmaCurr Opin Pulm Med2004101445014749605
- KelleyCFManninoDMHomaDMSavage-BrownAHolguinFAsthma phenotypes, risk factors, and measures of severity in a national sample of US childrenPediatrics2005115372673115741378
- Castro-RodriguezJAHolbergCJWrightALMartinezFDA clinical index to define risk of asthma in young children with recurrent wheezingAm J Respir Crit Care Med2000162(4 Pt 1): 1403140611029352
- BatemanEDBousheyHABousquetJGOAL Investigators GroupCan guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL studyAm J Respir Crit Care Med2004170883684415256389
- KwongKYMorphewTScottLGutermanJJonesCAAsthma control and future asthma-related morbidity in inner-city asthmatic childrenAnn Allergy Asthma Immunol2008101214415218727469
- ScottLNicholsBChoi KwongKYMorphewTJonesCALongitudinal patterns of predominant asthma disease activity in pediatric patients enrolled in an asthma-specific disease management programJ Asthma200845650150518612904
- CalhounWJSuttonLBEmmettADorinskyPMAsthma variability in patients previously treated with beta2-agonists aloneJ Allergy Clin Immunol200311261088109414657863
- SearsMREpidemiology of asthma exacerbationsJ Allergy Clin Immunol2008122466266819014756
- TanWCViruses in asthma exacerbationsCurr Opin Pulm Med2005111212615591884
- JonesCAClementLTMorphewTAchieving and maintaining asthma control in an urban pediatric disease management program: the Breathmobile ProgramJ Allergy Clin Immunol200711961445145317416407
- HowellGNonadherence to medical therapy in asthma: risk factors, barriers, and strategies for improvingJ Asthma200845972372918972285
- HaughneyJPriceDKaplanAAchieving asthma control in practice: understanding the reasons for poor controlRespir Med2008102121681169318815019
- BurgessSWSlyPDMorawskaADevadasonSGAssessing adherence and factors associated with adherence in young children with asthmaRespirology200813455956318422868
- BraunstahlGJOverbeekSEKleinjanAPrinsJBHoogstedenHCFokkensWJNasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airwaysJ Allergy Clin Immunol2001107346947611240947
- BraunstahlGJHellingsPWNasobronchial interaction mechanisms in allergic airway airways diseaseCurr Opin Otolaryngol Head Neck Surg200614317618216728896
- AdamsRJFuhlbriggeALFinkelsteinJAWeissSTIntranasal steroids and the risk of emergency department visits for asthmaJ Allergy Clin Immunol2002109463664211941313
- CorrenJManningBEThompsonSFHennessySStromBLRhinitis therapy and the prevention of hospital care for asthma: a case-control studyJ Allergy Clin Immunol2004113341541915007339
- Crystal-PetersJNeslusanCCrownWHTorresATreating allergic rhinitis in patients with comorbid asthma; the risk of asthma-related hospitalizations and emergency department visitsJ Allergy Clin Immunol20021091576211799366
- KiljanderTOSalomaaERHietanenEKTerhoEOGastroesophageal reflux in asthmatics; a double-blind, placebo-controlled crossover study with omeprazoleChest199911651257126410559084
- LittnerMRLeungFWBallardED2ndHuangBSamraNKLansoprazole Asthma Study GroupEffects of 24 weeks of lansoprazole therapy on asthma symptoms exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptomsChest200512831128113516162697
- BoothHRichmondIWardCGardinerPVHarkawatRWaltersEHEffect of high dose inhaled fluticasone propionate on airway inflammation in asthmaAm J Respir Crit Care Med1995152145527599861
- BusseWWWhat role for inhaled steroids in chronic asthma?Chest19931045156515718222825
- DuddridgeMWardCHendrickDJWaltersEHChanges in bronchoalveolar lavage inflammatory cells in asthmatic patients treated with high dose inhaled beclomethasone dipropionateEur Respir J1993644894978491298
- JuniperEFKlinePAVanzieleghemMARamsdaleEHO’ByrnePMHargreaveFEEffect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyper-responsiveness and clinical asthma in nonsteroid-dependent asthmaticsAm Rev Respir Dis199014248328362221590
- BarnesPJPedersonSEfficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992Am Rev Respir Dis1993148(4 Pt 2):S1S268214958
- SuissaSErnstPBenayounSBaltzanMCaiBLow-dose inhaled corticosteroids and the prevention of death from asthmaN Engl J Med2000343533233610922423
- CapewellSReynoldsSShuttleworthDEdwardsCFinlayAYPurpura and dermal thinning associated with high dose inhaled corticosteroidsBMJ19903006739154815512372620
- BrownPHGreeningAPCromptonLarge volume spacer devices and the influence of high dose beclomethasone dipropionate on hypothalamo-pituitary-adrenal axis functionThorax19934832332388497821
- LipworthBJSystemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysisArch Intern Med1999159994195510326936
- PauwelsRAYernaultJCDemedtsMGGeusensPSafety and efficacy of fluticasone and beclomethasone in moderate to severe asthma. Belgian Multicenter Study GroupAm J Respir Crit Care Med1998157(3 Pt 1):8278329517598
- RouxCKoltaSDesfougèresJLMininiPBidatELong-term safety of fluticasone propionate and nedocromil sodium on bone in children with asthmaPediatrics2003111(6 Pt 1)706713
- The Childhood Asthma Management Program Research GroupLong-term effects of budesonide or nedocromil in children with asthmaN Eng J Med20003431510541063
- GuilbertTWMorganWJZeigerRSLong-term inhaled corticosteroids in preschool children at high risk for asthmaN Engl J Med2006354191985199716687711
- LeoneFTFishJESzeflerSJSystemic review of the evidence regarding potential complications of inhaled corticosteroid use in asthma: collaboration of American College of Chest Physicians, American Academy of Allergy, Asthma, and Immunology, and American College of Allergy, Asthma, and ImmunologyChest200312462329234014665517
- PauwelsRAPedersonSBusseWWEarly intervention with budesonide in mild persistent asthma: a randomized double-blind trialLancet200336193631071107612672309
- BrogdenRNMcTavishDBudesonide. An updated review of its pharmacological properties, and therapeutic efficacy in asthma and rhinitisDrugs19924433754071382936
- ShapiroGBronskyEALaForceCFDose-related efficacy of budesonide administered via dry powder inhaler in the treatment of children with moderate to severe persistent asthmaJ Pediatr199813269769829627589
- The Childhood Asthma Management Program Research GroupLong-term effects of budesonide or nedocromil in children with asthmaN Eng J Med20003431510541063
- ShefferALSilvermanMWoolcockAJDíazPVLindbergBLindmarkBLong-term safety of once-daily budesonide in patients with early-onset mild persistent asthma: Results of the Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) studyAnn Allergy Asthma Immunol2005941485415702816
- AgertoftLPedersenSEffect of long-term treatment with inhaled budesonide on adult height in children with asthmaN Engl J Med2000343151064106911027740
- WoltersODImpact of inhaled and intranasal corticosteroids on the growth of childrenBioDrugs200013534735718034541
- InoueTDoiSTakamatsuIMurayamaNKamedaMToyoshimaKEffect of long-term treatment with inhaled belcometasone dipropionate on growth of asthmatic childrenJ Asthma199936215916410227266
- GulliverTMortonREidNInhaled corticosteroids in children with asthma: pharmacologic determinants of safety and efficacy and other clinical considerationsPaediatr Drugs20079318519417523699
- PedersonSClinical safety of inhaled corticosteroids for asthma in children: an update of long-term trialsDrug Saf200629759961216808552
- PauwelsRAOfdahlCGPostmaDSEffect of inhaled formoterol and budesonide on exacerbations of asthmaN Engl J Med199733720140514119358137
- O’ByrnePMBarnesPJRodriguez-RoisinRLow dose inhaled budesonide and formoterol in mild persistent asthmaAm J Respir Crit Care Med2001164(8 Pt 1):1392139711704584
- MatzJEmmettARickardKKalbergCAddition of salmeterol to low-dose fluticasone versus higher-dose fluticasone; an analysis of asthma exacerbationsJ Allergy Clin Immunol2001107578378911344343
- NiCMGreenstoneIRDucharmeFMAddition of inhaled long-acting beta2-agonist to inhaled steroids as first line therapy for persistent asthma in steroid-naïve adultsCochrane Database Syst Rev20052CD00530715846751
- SinDDManJSharpeHGanWQManSFPharmacological management to reduce exacerbations in adults with asthma: a systematic review and meta-analysisJAMA2004292336737615265853
- MasoliMWeatherallMHoltSBeasleyRModerate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthmaThorax200560973073416135679
- TalASimonGVermeulenJHBudesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthmaPediatr Pulmonol200234534235012357478
- PohunekPKunaPJorupCBoeckKDBudesonide/formoterol improves lung function compared with budesonide alone in children with asthmaPediatr Allergy Immunol200617645846516925692
- van NoordJASmeetsJJRaaijmakersJABommerAMMaesenFPSalmeterol versus formoterol in patients with moderately severe asthma: onset and duration of actionEur Respir J199698168416888866595
- PalmqvistMPerssonGLazerLRosenborgJLarssonPLötvallJInhaled dry-powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potencyEur Respir J19971011248424899426083
- RingdalNDeromEWåhlin-BollEPauwelsROnset and duration of action of single doses of formoterol inhaled via Turbuhaler®Respir Med1998928101710219893769
- SeberováEAnderssonAOxis® (formoterol given by Turbuhaler®) showed as rapid an onset of action as salbutamol given by a pMDIRespir Med200094660761110921767
- NelsonHSWeissSTBleeckerERYanceySWDorinskyPMSMART Study GroupThe salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterolChest20061291152616424409
- MannMChowdhuryBSullivanENicklasRAnthraciteRMeyerRJSerious asthma exacerbations in asthmatics treated with high-dose formoterolChest20031241707412853504
- IsraelEChinchilliVMFordJGUse of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trialLancet200436494441505151215500895
- WechslerMELehmanELazarusSCBeta-adrenergic receptor polymorphisms and response to salmeterolAm J Respir Crit Care Med2006173551952616322642
- BisgaardHEffect of long-acting beta2 agonist on exacerbation rates of asthma in childrenPediatr Pulmonol200336539139814520721
- LazarusSCBousheyHAFahyJVAsthma Clinical Research Network for the National Heart, Lung, and Blood InstituteLong-acting beta2 agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trialJAMA2001285202583259311368732
- BleeckerERPostmaDSLawranceRMEffect of ADRB2 polymorphisms on response to long-acting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studiesLancet20073702118212518156033
- LofdahlCGReiseTFLeffJARandomized, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patientsBMJ19993197202879010398629
- PriceDBHernandezDMagyarPRandomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthmaThorax200358321121612612295
- LofdahlCGReiseTFLeffJARandomized, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patientsBMJ19993197202879010398629
- NelsonHSBusseWWKerwinEFluticasone propionate/salmeterol combination provides more effective asthma control than low-dose inhaled corticosteroid plus montelukastJ Allergy Clin Immunol200010661088109511112891
- FishJEIsraelEMurrayJJSalmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapyChest2001120242343011502639
- RingdalNElirazAPruzinecRThe salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthmaRespir Med200397323424112645830
- O’ByrnePMBisgaardHGodardPPBudesonide/formoterol combination therapy as both maintenance and reliever medication in asthmaAm J Respir Crit Care Med2005171212913615502112
- RabeKFPizzichiniEStallbergBBudesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trialChest2006129224625616478838
- BisgaardHLe RouxPBjåmerDDymekAVermeulenJHHultquistCBudesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthmaChest200613061733174317166990
- Rice-McDonaldGBowlerSStainesGMitchellCDoubling daily inhaled corticosteroid dose is ineffective in mild to moderately severe attacks of asthma in adultsIntern Med J2005351269369816313543
- FitzGeraldJMBeckerASearsMRMinkSChungKLeeJCanadian Asthma Exacerbation Study GroupDoubling the dose of budesonide versus maintenance treatment in asthma exacerbationsThorax200459755055615223858
- GarrettJWilliamsSWongCHoldawayDTreatment of acute asthmatic exacerbations with an increased dose of inhaled steroidArch Dis Child199879112179771245
- JónassonGCarlsenKHSødalAJonassonCMowinckelPPatient compliance in a clinical trial with inhaled budesonide in children with mild asthmaEur Respir J199914115015410489843