Abstract
Artemether–lumefantrine is one of the artemisisnin-based combination therapies recommended for treatment of uncomplicated falciparum malaria. The drug combination is highly efficacious against sensitive and multidrug resistant falciparum malaria. It offers the advantage of rapid clearance of parasites by artemether and the slower elimination of residual parasites by lumefantrine. The combination can be used in all populations except pregnant mothers in the first trimester where safety is still uncertain. There are still concerns about safety and pharmacokinetics of the drug combination in children, especially infants, pregnant mothers and drug interactions with mainly non-nucleoside reverse transcriptase inhibitors and protease inhibitors used for HIV therapy.
Introduction
Malaria is a febrile illness caused by intracellular protozoa of the genus Plasmodium, and transmitted by the bite of an infected female mosquito of the genus Anopheles. Plasmodium species that cause disease in humans include: P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi. P. falciparum is the most prevalent and most virulent. Worldwide, malaria is one of the most important causes of morbidity and mortality. Approximately 2.2 billion people are exposed to malaria every year of whom about 300 to 500 million develop disease. In 2006, there were 247 million cases of malaria, causing nearly 1 million deaths, mostly among African children.Citation1 Malaria deaths are responsible for almost 3% of the world’s disability-adjusted life years, not counting the considerable and imprecisely quantified burden due to morbidity and disability.Citation2 In addition to causing significant morbidity and mortality, malaria significantly contributes to poverty through lost productivity and economic loss on antimalarial treatment. African countries spend US$12 billion annually on malaria, with individual African families spending up to 25% of their income on malaria prevention and control. Malaria has slowed economic growth in African countries by 1.3% per year. As a result of the compounded effect over 35 years, the gross domestic product for African countries is now up to 32% lower than it would have been in absence of malaria.Citation3
Reduction in malaria-associated morbidity and mortality largely depends on provision of prompt, effective, safe and affordable antimalarial drugs. Resistance to antimalarial drugs poses a significant challenge to malaria control programs in sub-Saharan Africa. Multi-drug resistance to sulfadoxine–pyrimethamine (SP) and chloroquine was described extensively in sub-Saharan Africa. The World Health Organization (WHO) recommends use of artemisinin-based combination treatments (ACT) as first-line therapy. The ACTs combine fast-acting artemisinins with another structurally unrelated and more slowly eliminated compound which permits elimination of residual malarial parasites.Citation4–Citation6 Of the 81 countries with endemic P. falciparum, 77 have now adopted the WHO recommendation.Citation7 Commonly used ACTs are artemether–lumefantrine (AL), amodiaquine–artesunate (AQAS), mefloquine–artesunate, dihydroartemisin–piperaquine (DP) and napthoquine–artemisinin. In this review we provide an update on efficacy, effectiveness and safety of AL for treatment of uncomplicated malaria.
Pharmacology of artemether–lumefantrine
A 6-dose regimen of artemether (20 mg) co-formulated with lumefantrine (120 mg) is recommended; with first and second doses taken 8 hours apart, the third dose taken 24 hours after the first and the remaining doses 12 hours apart. The 6-dose regimen is superior to the 4-dose regimen.Citation8,Citation9 Artemesinin from which artemether is derived is obtained from the Chinese herb sweet wormwood (Artemisua annua). Artemisinins have the most potent and rapid onset of antiparasitic activity against all Plasmodium species that infect humans.
Artemether acts rapidly with half-life of 1 to 3 hours, whereas lumefantrine has a half-life of 3 to 6 days and is responsible for preventing recurrent parasitemia.Citation10 Artemether and lumefantrine have different modes of action and act at different points in the parasite life cycle.Citation11,Citation12 Artemether interferes with parasite transport proteins, disrupts parasite mitochondrial function, inhibits angiogenesis and modulates host immune function.Citation13 Lumefantrine is an aryl-amino alcoholCitation14 that prevents detoxification of heme, such that toxic heme and free radicals induce parasite death.Citation12 Oral formulations of AL are available as tablet and dispersible formulations which have similar pharmacokinetic (PK) properties.Citation15,Citation16 Artemether and lumefantrine differ in rates of absorption and elimination. Artemether is rapidly absorbed reaching peak plasma concentrations within 2 hours post dose.Citation11,Citation17 It is metabolized rapidly by cytochrome P450 (CYP) 2B6, CYP3A4 and possibly CYP2A610 to dihydroartemisinin (DHA) which in turn is converted to inactive metabolites primarily by glucoronidation via UGT1A1, 1A8/9 and 2B7.Citation14 The metabolite DHA reaches peak plasma concentration within 2 to 3 hours post dosing.Citation11 Both artemether and DHA offer potent antimalarial properties causing significant reduction in asexual parasite mass of approximately 10,000-fold per reproductive cycle, with prompt resolution of symptoms.Citation18,Citation19
Lumefantrine is absorbed and cleared more slowly, acting to eliminate residual parasites that may remain after artemether and DHA have been cleared from the body and thus prevent recrudescence.Citation11,Citation12 Lumefantrine is highly lipophilic, thus absorption is enhanced with a fatty meal; its absorption occurs 2 hours after intake reaching peak plasma concentration after 3 to 4 hoursCitation20 with an elimination half life of 4 to 10 days.Citation20,Citation21
Food enhances absorption of both artemether and lumefantrine although this effect is more apparent for lumefantrine.Citation11,Citation20 Administration of AL with high-fat meal increased bioavailability of both artemether and lumefantrine by 2-fold and 16-fold respectively.Citation11 Premji et al in an evaluation of the typical fat content of African diets noted that total fat intake is 15 to 30 g/day during breast feeding, >10 g/day in the post weaning phase and 30 to 60 g/day in a normal diet and this is adequate for optimal efficacy of lumefantrine.Citation22
However, the effect of food on AL absorption is of concern because patients with malaria usually have anorexia, vomiting and low food intake. Lumefantrine is metabolized by N-debutylation mainly by CYP3A410 to desbutyllumefantrine with 5- to 8-fold higher antiparastic effect than lumefantrine. The key PK determinant of cure is the area under the concentration time curve (AUC) of the longer-acting lumefantrine.
Efficacy and effectiveness of AL
Efficacy of the 6 dose regimen of AL judged by elimination of malaria parasites using the 28-day polymerase chain reaction (PCR)-corrected cure rates and resolution of symptoms, has been demonstrated in semi-immune and non-immune populations in Asia and Africa to be consistently greater than 95%, with rapid parasite and symptom clearance and significant gametocidal effect.Citation15,Citation23–Citation27 Many studies in Africa and Asia have demonstrated AL to be as efficacious as other ACTs when used in pediatric and adult populations with differing immunity. PCR-corrected day 28 and day 42 cure rates range between 91% and 100% using evaluable patient analysis.Citation28–Citation62 Correction by PCR enables differentiation between recurrence and recrudescence of the initial infection from re-infection. A few cases of treatment failure were recorded after AL treatment, but these were mostly re-infections.Citation29,Citation32,Citation33,Citation38 This is of particular concern in areas with very intense malaria transmission where antimalarial drugs with longer half-life may offer the advantage of preventing re-infection. Lumefantrine with an estimated elimination half-life of 4 to 10 days offers post-treatment antimalarial prophylaxis of up to 4 weeks. Studies showed both AL and DP to be highly efficacious for treatment of uncomplicated malaria, although DP was superior to AL at preventing new malaria infections.Citation33,Citation34,Citation38,Citation50 In addition to excellent efficacy and effectiveness, AL has demonstrated significant gametocidal effects.Citation34,Citation42,Citation48,Citation51 A meta-analysis of 32 randomized trials showed AL to be one of the most effective ACTs with 28-day parasitological cure rates of 97.4%.Citation63
Effectiveness of AL may be influenced by poor adherence to the 3-day, 6-dose regimen and the food requirements for AL absorption. Clinical and parasitological responses to AL were similar with both supervised and unsupervised treatment in Uganda.Citation64 The supervised treatment arm received AL with fatty food while the unsupervised arm received AL as outpatient treatment with nutritional advice. Unsupervised treatment resulted in lower concentrations of lumefantrine with increased risk of early reinfection.Citation4,Citation64 In Uganda and Nigeria adherence to correct AL dose and duration prescribed to febrile children by community medicine distributors was greater than 80% and crude parasitological failure rates varied from 3.7% in Uganda to 41.8% in Nigeria and PCR-adjusted parasitological cure rate was 90.9% in Nigeria and 97.2% in Uganda.Citation6 Differences in crude rates may be due to differences in re-infection rates. A recent study of uncomplicated malaria in Uganda showed adherence to AL was 94.5% compared to that quinine of 85.4% with high unadjusted cure rates of AL of 96% vs 64% for quinine.Citation65
In multidrug-resistant areas, day 7 lumefantrine concentration was a useful surrogate marker for AUC and concentrations of less than 280 ng/mL predicted treatment failure.Citation17,Citation20 However, results from areas with lumefantrine-sensitive parasites showed no treatment failures despite day 7 concentrations less than 280 ng/mL in 45% of all patients, and re-infections occurred among patients with day 7 concentrations below 400 ng/mL and those who received a lower dose of lumefantrine per kilogram body weight.Citation4
Safety of AL
Safety and tolerability of AL has been assessed in clinical trials in Asia and Africa. Most adverse events are mild or moderate, mostly affecting gastrointestinal and nervous systems; however, most are typical of the symptomatology of malaria or concomitant infections.Citation15,Citation24–Citation27,Citation66 Serious adverse events were unlikely and were unrelated or most unlikely to be related to study medication.Citation15,Citation29–Citation31,Citation33,Citation34,Citation36,Citation38,Citation39,Citation41–Citation43,Citation46–Citation51,Citation53,Citation54,Citation67 Two meta-analysis concluded that AL is well tolerated, with mild or moderate adverse events mostly affecting gastrointestinal and nervous systems. Ototoxicity associated with AL has been reported recently in a few cases;Citation68,Citation69 however, this was not confirmed in a study that investigated hearing sensation following AL treatment.Citation31 Lumefantrine possesses a similar chemical structure to halofantrine which is known to cause cardiac arrhythmia; however, safety studies have not shown lumefantrine to be cardiotoxic or to prolong QTc interval.Citation67,Citation70 Other studies and a review of 15 trials concluded that AL did not cause hematological adverse events, although pre-clinical trials suggested the repeated exposure to AL may affect blood cell counts.Citation71
Safety assessment has been conducted during treatment of single episodes of malaria. Safety concerns become more important when AL is administered over the counter, which commonly results in overdiagnosis and overtreatment of malaria, and when patients get recurrent infections requiring repeated treatment. Overdiagnosis of malaria is common in malaria-endemic areas.Citation72 There are no standard guidelines for evaluating drug safety and tolerability in antimalarial trials.Citation64 Establishing systems for pharmacovigilance in areas where AL is frequently prescribed is of utmost importance and several challenges exist.Citation73
AL use in children
Vomiting, which may be due to disease-related nausea or taste of the medication, may influence drug intake especially in children. A more palatable dispersible formulation of AL is now available and has been shown to be as efficacious as the currently used crushed tablet in infants and children, and with similar safety and PK profile.Citation15 Pediatric dosing of AL is deduced from adult-based regimens adjusted for body weight, with little consideration for maturational effects on drug absorption and metabolism. Although diet and nutritional status are important determinants of PK processes, drug responses and toxicity, there are few relevant data for AL in this patient group. In resource-constrained areas, children may not be weighed at each clinic visit and dosing in such settings is usually based on age as a proxy measure for weight. Besides research on therapeutic dose levels based on body weight, there is urgent need for evidence-based translation of weight based dosing regimens to regimens that can be based on age, as the majority of fevers in malaria endemic areas are treated with over-the-counter antimalarial drugs without involvement of the formal health sector. Age-based dose regimens are more practical than weight-based regimens, but will inevitably result in a greater proportion of children receiving either too much or too little drug. This is a particular concern with lumefantrine, which has a narrow therapeutic margin between effective and toxic concentrations. This dosing consideration is especially important in malnourished, pre-school children and during onset of puberty when physiological variations in bodyweight by age are greatest. Earlier experience with SP and DP suggests that lack of clear guidance on age-based dosing as part of the regulatory process contributes to considerable variation in recommended age-based dose regimens,Citation74,Citation75 potentially resulting in poor, but widely used regimens, particularly for young rapidly growing children who bear the brunt of the malaria burden. Different age-based regimens are already being used in countries that have recently switched to ACTs. These concerns apply also to young infants <6 months old or of <5 kg body weight. Most ACTs are contra-indicated in this group because of lack of safety data, even though these children are at considerable risk. In western Kenya 50% of infants not protected by insecticide-treated mosquito nets had their first infection by 3 months.Citation76 In southern Mozambique, an estimated 9% of out-patient visits for uncomplicated malaria in children less than 5 years of age are children aged <6 months. Infants in endemic areas have the highest burden of severe malarial anemia, blood transfusions and death.Citation77,Citation78 Thus, programmatically implemented ACTs will end up being widely used in children <6 months even though the label does not provide guidance for this age group.
Malaria and AL use in pregnancy
Pregnant women with malaria, symptomatic and asymptomatic alike, should be treated without delay with effective and safe antimalarial drugs in order to reduce risks for adverse outcomes for both mother and fetus.Citation79 AL is a very attractive alternative because it is highly effective, acts rapidly and is well tolerated. However, there is insufficient information on safety and efficacy of ACTs in pregnancy, including exposure in the first trimester.Citation79,Citation80 Early data indicated that artemisinins were embryotoxic and potentially teratogenic in several animal species without maternal toxic effects or impaired fertility, and more recent studies have confirmed these findings.Citation79
Artemisinin derivatives have shown embryo-toxic effects in animal reproductive toxicology studies.Citation81 The mechanism of embryo-toxicity is thought to occur through depletion of embryonic erythroblasts causing severe anemia and cell damage and death due to hypoxia.Citation81 The most sensitive time window for embryo-toxicity in humans is between weeks 4 to 10. From these data ACTs are not indicated for malaria treatment in the first trimester of pregnancy unless no alternatives exist. There is increasing experience with artemisinin derivatives in second and third trimesters with no evidence of adverse outcomes in more than 1000 prospectively followed pregnancies.Citation82,Citation83 WHO Malaria Treatment Guidelines of 2006 recommend use of ACTs in pregnant women in the second and third trimester of gestation. None of the studies on AL use in pregnancy have reported increased risk of serious maternal adverse events, adverse birth outcomes or neuro-developmental deficits. However all these studies were underpowered to detect rare adverse outcomes.Citation84 Data from Sudan from a cohort of women who reported use of artemisinins in first trimester and were followed up until delivery and their babies followed up till 1 year of age showed that most delivered apparently healthy babies at full term with no congenital malformations and no maternal deaths, and none of the babies died during their first year of life.Citation85 A prospective observational study was conducted recently in Zambia which evaluated safety of AL and SP in pregnant women who received AL and SP to treat symptomatic falciparum malaria. Data from 1001 pregnant women and fetuses/newborns indicated that the incidence of perinatal death, spontaneous abortion, neonatal mortality, premature delivery, stillbirth and low birth weight is similar after pregnancy exposure to AL compared to SP.Citation86
Pregnancy has been associated with reduced plasma concentrations of AL which have a significant impact on treatment outcome since plasma concentrations of lumefantrine, after elimination of artemether, are an important determinant of cure.Citation87,Citation88 A study that evaluated PK of AL in pregnant women with recrudescent uncomplicated multidrug resistant falciparum malaria demonstrated that pregnant women in second and third trimester had lower concentrations of artemether, dihydroartemisinin and lumefantrine, and elimination of lumefantrine was more rapid than reported previously in non-pregnant adults.Citation87,Citation89 Another study that compared artesunate monotherapy to AL for treatment of uncomplicated falciparum malaria in second and third trimesters demonstrated that the standard 6-dose AL regimen was well tolerated and safe but efficacy was inferior to that of 7-day artesunate monotherapy and was unsatisfactory for general deployment in this geographic area. PK parameters measured in this study showed low drug concentrations in later pregnancy which could possibly explain the poor treatment outcomes.Citation89 There is need for further studies to determine the optimum dose regimen and efficacy of AL in pregnancy.
AL use in HIV-infected populations
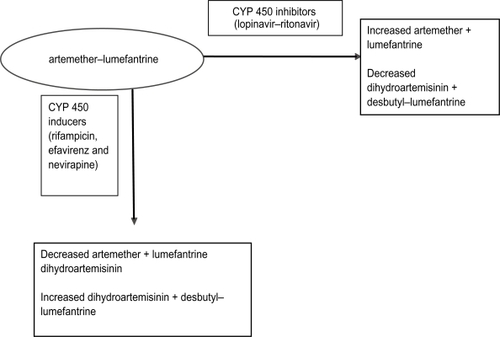
Human immunodeficiency virus (HIV)-infected individuals are at high risk for acquiring malaria parasitemia, with the risk increasing as immunity declines.Citation90–Citation93 Evidence for this interaction is more consistent in pregnant women of all gravidities.Citation94–Citation96 HIV-1 infected pregnant women have a higher prevalence of peripheral parasitemia and placental malariaCitation95,Citation96 and their infants experience higher postnatal mortality when both diseases are present.Citation97,Citation98 Therefore, offering adequate and efficacious antimalarial treatment and prevention is extremely important for this high risk group. Little is known about efficacy and safety of antimalarial drugs in HIV-infected individuals and much less on interaction between antimalarial and antiretroviral (ARV) drugs, and reliable data are urgently needed. Few studies have examined the effect of HIV infection on response to antimalarial treatment and these have yielded conflicting results.Citation99–Citation103 Most studies have shown that HIV-infected individuals have higher risk of experiencing antimalarial treatment failure due to re-infections.Citation101,Citation103 Birku et al demonstrated decreased clearance of parasites by artemisinin treatment in HIV-infected patients with malaria.Citation104 In Zambia, HIV-infected adult patients with CD4 counts of 300/μL and below had higher risk of getting recrudescent malaria than HIV-infected patients with higher CD4 counts and HIV-uninfected patients.Citation103 Recent studies, however, suggest that the threshold for an increased risk of malaria treatment failure (new infections or recrudescence) probably lies at 400 CD4 cells/μL.Citation105,Citation106 Following the latest WHO guidelines for sub-Saharan Africa this malaria vulnerable population should be protected by cotrimoxazole prophylaxis or highly active ARV therapy (HAART). There are concerns about safety of AL treatment in HIV-infected patients concomitantly receiving HAART. The standard first-line HAART regimens in many sub-Saharan countries where malaria is endemic are made up of a non-nucleoside reverse transcriptase inhibitor (NNRTI) backbone with 2 nucleoside reverse transcriptase inhibitors (NRTI). The second-line HAART regimen is made up of a protease inhibitor (PI) backbone and 2 NRTIs. Knowledge of the metabolism of ARVs and AL suggests that there is potential for PK drug–drug interactions.Citation107 For example, PIs like lopinavir/ritonavir (LPV/r) are among the most potent inhibitors of cytochrome P450 (typically CYP 3A4) metabolism, while NNRTIs (efavirenz and nevirapine) are also substrates of cytochrome P450 and usually these two induce but occasionally efavirenz inhibits some P450 isoforms. Although poorly studied the risk of clinically significant interactions involving AL and ARVs is considerableCitation108 and may result in high concentrations with excessive toxicity or reduced concentrations with reduced efficacy and risk for development of resistance to AL. The potential for interactions between ARVs and antimalarials have been shown in a study of healthy volunteers where AQAS was co-administered with the NNRTI efavirenz. In the first 2 study participants, the AUC for AQAS increased by 100% to 300% and alanine and aspartate transferase levels increased markedly above the upper limit of normal, suggesting hepatoxicity. This led to recommendations that AQAS should be avoided in patients receiving EFV. In a recent study of uncomplicated malaria in Uganda, treatment of HIV-infected children with AQAS was associated with markedly higher risk of neutropenia compared with treatment of HIV-uninfected children. The risk of neutropenia was higher in participants with concurrent ARV use, especially zidovudine, and in those with a history of repeated doses of AQAS.Citation109 These clinical observations demonstrate the need for thorough examination of the nature of interaction between ARVs and ACTs. An interaction is expected between lumefantrine and both EFV and PIs that could potentially lead to increased levels of lumefantrine (); no data are available. The potential interactions with NVP are less clear but co-administration could reduce lumefantrine levels. A study that investigated the PKs of AL when administered with LPV/r in HIV-uninfected healthy volunteers demonstrated that the PK of lumefantrine is influenced by LPV/r, resulting in 2- to 3-fold increases in lumefantrine AUC, and trends towards decreases in artemether maximum concentration (Cmax) and AUC were noted during co-administration. Decreases in DHA AUC were observed during co-administration without changes in DHA: artemether AUC ratios. The authors concluded that co-administration of AL and LPV/r can be carried out for patients co-infected with malaria and HIV.Citation110 This study did not address safety concerns with co-administration, which need to be considered in future studies among individuals living in malaria-endemic regions.
AL use in patients with co-morbidity
Treatment of tuberculosis is often a minimum of 6 months including 2 months of intense rifampicin-based treatment. Patients may concomitantly develop malaria requiring treatment with AL. There are currently no published data on interactions of rifampicin and AL. Rifampicin is a potent inducer of hepatic cytochrome and may influence the PKs of AL since both drugs are metabolized by CYP 450.Citation111 Theoretically co-administration of rifampicin with AL may result in decreased concentrations of AL resulting in decreased efficacy (). Data on these PK drug interactions are very scarce, thus the need for more studies. One study evaluated effects of concomitant administration of AL with a potent CYP 3A4 inhibitor. Artemether, DHA, and lumefantrine PKs were altered by ketoconazole. AUC and Cmax increased for all 3 compounds and terminal half-life increased for artemether and DHA. None of the changes in PK parameters were greater than the changes observed in healthy volunteers taking AL with a high-fat meal. There was no increase in observed side effects or electrocardiographic changes. The authors concluded that dosage adjustments of AL do not appear to be necessary with concomitant ketoconazole administration.Citation112
AL resistance
Antimalarial drug resistance has been defined as “the ability of a parasite strain to survive and/or multiply despite the administration and absorption of a drug given in doses equal to or higher than those usually recommended, but within the limits of tolerance of the subject.” This definition was later modified to specify that the drug in question must gain access to the parasite or the infected red blood cell for the duration of the time necessary for its normal action.”Citation113 Antimalarial drug resistance is heightened in individuals with lower immunity, such as children less than 5 years, pregnant women, non-immune immigrants to malarious areas, malnourished individuals and HIV-infected patients.Citation113 Reduced immunity allows the survival of a residuum of parasites that are able to survive treatment, and as such reduced immunity may further increase the development, intensification and spread of resistant strains.
Resistance to artemisinins has not been confirmed although reduced sensitivity has been reported in China and Vietnam.Citation114,Citation115 Treatment failures occurring after AL treatment are thought to be due to poor absorption with reduced concentrations.Citation116,Citation117 AL selects for the P. falciparum multidrug resistance gene (PfMDR1) N86, the chloroquine-susceptible allele which has been proposed as a marker for lumefantrine resistance.Citation118 In Tanzania, treatment with AL was associated with selection of newly infecting parasites containing the pfmdr1 86N allele,Citation118 which has been associated with decreased in vitro sensitivity to artemisinins and lumefantrine.Citation119
Factors that lead to development, intensification and distribution of antimalarial drug resistance can broadly be classified as: factors leading to treatment failure (incorrect dosing regimen, non-compliance, substandard drugs and misdiagnosis), human behavior, parasite and vector biology, and drug PKs.Citation113 In sub-Saharan Africa antimalarial drugs are readily available outside public health services, in pharmacies, drug shops and private practitioners’ clinics. Quality of antimalarials is a serious concern and counterfeits may be found in some of these units. In Southeast Asia half of the samples of artemisnins obtained from most countries were counterfeit.Citation11,Citation120 In sub-Saharan Africa substandard antimalarials were found in 7 countries.Citation121,Citation122
Conclusion
There is increasing evidence of very high efficacy and effectiveness of AL for treatment of uncomplicated malaria. Continued health education on correct use of AL and surveillance of effectiveness is necessary to prevent and detect emergence of drug resistance. There is need to develop strong systems for pharmacovigilance to increase the evidence base on safety of AL especially in pregnant mothers and infants weighing less then 5 kg. PK studies especially on drug interactions with ARV drugs are urgently needed.
Disclosures
None of the authors declare conflicts of interest.
References
- WHOhttp://wwwwhoint/mediacentre/factsheets/fs094/en/indexhtml Accessed July 2, 2009.
- BremanJGAlilioMSMillsAConquering the intolerable burden of malaria: what’s new, what’s needed: a summaryAm J Trop Med Hyg200471211515331814
- WHO/CDS/RBM/2000.17. The Abuja Declaration and the Plan of Action. 2000 April 25.
- ChecchiFPiolaPFoggCSupervised versus unsupervised antimalarial treatment with six-dose artemether-lumefantrine: PK and dosage-related findings from a clinical trial in UgandaMalar J20061955916854236
- MutabingwaTKAnthonyDHellerAAmodiaquine alone, amodiaquine + sulfadoxine-pyrimethamine, amodiaquine + artesunate, and artemether-lumefantrinefor outpatient treatment of malaria in Tanzanian children: a four arm randomised effectiveness trialLancet20053651474148015850631
- AjayiIOBrowneENBateganyaFEffectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan AfricaMalar J200827719018822170
- WHOWorld Malaria ReportGenevaWorld Health Organization2008
- OmariAAGambleCLGarnerPArtemether-lumefantrine (four-dose regimen) for treating uncomplicated falciparum malariaCochrane Database Syst Rev2006192CD00596516625646
- OmariAAGambleCLGarnerPArtemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malariaCochrane Database Syst Rev20054CD005564
- TravassosMarkALauferResistance to antimalarial drugs: molecular, pharmacologic, and clinical considerationsPediatr Res200965564R70R
- WhiteNJVan VugtMEzzetFEzzetFClinical Pharmacokinetics and pharmacodynamics of artemetherlumefantrineClin Pharmacokinet19993710512510496300
- KokwaroGMwaiLNzilaAArtemether/lumefantrine in the treatment of uncomplicated falciparum malariaExpert Opin Pharmacother20078759417163809
- GolenserJWaknineJHKrugliakMCurrent perspectives on the mechanisms of action of artemisininsInt J Parasitol2006361427144117005183
- AweekaFTGermanPIClinical pharmacology of artemisinin-based combination therapiesClin Pharmacokinet20084729110218193915
- AbdullaSBorrmannSD’AlessandroUEfficacy and safety of artemetherlumefantrine dispersible tablets compared with crushed commercial tablets in African infants and children with uncomplicated malaria: a randomised, single-blind, multicentre trialLancet20083721819182718926569
- AbdullaSSagaraIDispersible formulation of artemether/lumefantrine: specifically developed for infants and young childrenMalar J2009811186/475-2875-8-S1–S7.
- EzzetFMullRKarbwangJPopulation pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria parasitesBr J Clin Pharmacol1998465535619862244
- WhiteNPreventing antimalarial drug resistance through combinationsDrug Resistance Updates199813917092790
- DjimdéALefèvreGUnderstanding the pharmacokinetics of Coartem®Malar J2009811186/475-2875-8-S1–S4.
- EzzetFVan VugtMNostenFPharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malariaAntimicrob Agents Chemother20004469770410681341
- AshleyEAStepniewskaKLindegårdhNPharmacokinetic study of artemether–lumefantrine given once daily for the treatment of uncomplicated multidrugresistant falciparum malariaTrop Med Int Health20071220120817300626
- PremjiZGAbdullaSOgutuBThe content of African diets is adequate to achieve optimal efficacy with fixed-dose artemether-lumefantrine: a review of the evidenceMalar J2008724419032767
- Michael MakangaSKThe clinical efficacy of artemether/lumefantrine (Coartem®)Malar J2009811186/475-2875-8-S1–5.
- Van VugtMWilairatanaPGemperliBEfficacy of six doses of artemether/lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malariaAm J Trop Med Hyg19996093694210403324
- Van VugtMLooareesuwanSWilairatanaPArtemether/lumefantrine for the treatment of multi-drug resistant falciparum malariaTrans R Soc Trop Med Hyg20009454554811132386
- LefèvreGLooareesuwanSTreeprasertsukSA clinical and PK trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in ThailandAm J Trop Med Hyg20016456247256
- HatzCSotoJNothdurftHDTreatment of acute uncomplicated falciparum malaria with artemether/lumefantrine in non-immune populations: a safety, efficacy and PK studyAm J Trop Med Hyg20087824124718256423
- RojanawatsirivejCVijaykadgaSAmkladIMonitoring the therapeutic efficacy of antimalarials against uncomplicated falciparum malaria in ThailandSoutheast Asian J Trop Med Public Health20033453654115115123
- BukirwaHYekaAKamyaMRArtemisinin Combination Therapies for Treatment of Uncomplicated Malaria in UgandaPLos Clin Trials2006110010007
- DorseyGStaedkeSClarkTDCombination therapy for uncomplicated falciparum malaria in Ugandan childrenJAMA20072972210221917519410
- GürkovREshetuTMirandaIBOtotoxicity of artemether/lumefantrine in the treatment of falciparum malaria: a randomized trialMalar J2008717918796142
- KabanywanyiAMMwitaASumariDEfficacy and safety of artemisinin-based antimalarial in the treatment of uncomplicated malaria in children in southern TanzaniaMalar J2007614617996121
- KamyaMRYekaABukirwaHArtemether/lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trialPLoS Clin Trials20072e2017525792
- MårtenssonAStrömbergJSisowathCEfficacy of artesunate plus amodiaquine versus that of artemether/lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, TanzaniaClin Infect Dis2005411079108716163624
- MårtenssonANgasalaBUrsingJInfluence of consecutive-day blood sampling on polymerase chain reaction–adjusted parasitological cure rates in an antimalarial-drug trial conducted in TanzaniaJ Infect Dis200719559760117230421
- MohamedAOEltaibEHAhmedOAThe efficacies of artesunate-sulfadoxine-pyrimethamine and artemether/lumefantrine in the treatment of uncomplicated, Plasmodium falciparum malaria, in an area of low transmission in central SudanAnn Trop Med Parasitol200610051016417707
- MukhtarEAGadallaNBEl-zakiSEGA comparative study on the efficacy of artesunate plus sulphadoxine/pyrimethamine versus artemether/lumefantrine in eastern SudanMalar J200769217631681
- YekaADorseyGKamyaMRArtemether/lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in UgandaPLoS ONE20083e239018545692
- MulengaMVan geertruydenJPMwananyandaLSafety and efficacy of lumefantrine-artemether (Coartem®) for the treatment of uncomplicated Plasmodium falciparum malaria in Zambian adultsMalar J200657316923176
- TooveySEffectiveness of co-artemether in an unsupervised outpatient setting for the treatment of falciparum malariaTravel Med Infect Dis2008612293118342266
- AdjeiGOKurtzhalsJARodriguesOPAmodiaquine artesunate vs artemether/lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-upMalar J2008712718620577
- FayeBNdiayeJLNdiayeDEfficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in SenegalMalar J200768017570848
- KoramKAAbuakuBDuahNComparative efficacy of anti-malarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in GhanaActa Trop20059519420316054584
- MeremikwuMAlaribeAEjemotRArtemether/lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: randomized controlled trialMalar J200654316704735
- Owusu-AgyeiSAsanteKPOwusuRAn open label, randomised trial of artesunate + amodiaquine, artesunate + chlorproguanil-dapsone and artemether/lumefantrine for the treatment of uncomplicated malariaPLoS ONE20083e253018575626
- SagaraIDickoADjimdeAA randomized trial of artesunate-sulfamethoxypyrazinepyrimethamine versus artemether/lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in MaliAm J Trop Med Hyg20067563063617038684
- SowunmiAGbotoshoGOHappiCTTherapeutic efficacy and effects of artemether/lumefantrine and amodiaquine-sulfalenepyrimethamine on gametocyte carriage in children with uncomplicated Plasmodium falciparum malaria in southwestern NigeriaAm J Trop Med Hyg20077723524117690392
- SutherlandCJOrdRDunyoSReduction of malaria transmission to Anopheles mosquitoes with a sixdose regimen of co-artemetherPLoS Med20052e9215839740
- ZongoIDorseyGRouambaNArtemether/lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomized non-inferiority trialLancet200736949149817292769
- ZongoIDorseyGRouambaNRandomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether/lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicatedPlasmodium falciparum malaria in Burkina FasoClin Infect Dis2007451453146117990228
- FanelloCIKaremaCvan DorenWA randomised trial to assess the safety and efficacy of artemether/lumefantrine (Coartem®) for the treatment of uncomplicated Plasmodium falciparum malaria in RwandaTrans R Soc Trop Med Hyg200710134435017005222
- GuthmannJPCohuetSRiguttoCHigh efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central AngolaAm J Trop Med Hyg20067514314516837721
- NdayiragijeANiyungekoDKarenzoJEfficacité de combinaisons thérapeutiques avec des dérivés de l’artémisinine dans le traitement de l’accès palustre noncomplique au BurundiTrop Med Int Health2004967367915189457
- Van den BroekIKitzCAl AttasSEfficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of CongoMalar J2006511317125496
- KrudsoodSChalermrutKPengruksaCComparative clinical trial of two-fixed combinations dihydroartemisinin-napthoquine-trimethoprim (DNP®) and artemether/lumefantrine Coartem®/Riamet®) in the treatment of acute uncomplicated falciparum malaria in ThailandSoutheast Asian J Trop Med Public Health20033431632112971556
- StohrerJMDittrichSThongpaseuthVTherapeutic efficacy of artemether/lumefantrine and artesunate-mefloquine for treatment of uncomplicated Plasmodium falciparum malaria in Luang Namtha Province, Lao People’s Democratic RepublicTrop Med Int Health200491175118315548313
- MayxayMKhanthavongMLindegårdhNRandomized comparison of chloroquine plus sulfadoxine-pyrimethamine versus artesunate plus mefloquine versus artemether/lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People’s Democratic RepublicClin Infect Dis2004391139114715486837
- RatcliffASiswantoroHKenangalemETwo fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparisonLancet200736975776517336652
- HutagalungRPaiphunLAshleyEAA randomized trial of artemether/lumefantrine versus mefloquine-artesunate for the treatment of uncomplicated multi-drug resistant Plasmodium falciparum on the western border of ThailandMalar J200544616179089
- Van den BroekIVMaungUAPetersAEfficacy of chloroquine + sulfadoxine-pyrimethamine, mefloquine + artesunate and artemether + lumefantrine combination therapies to treat Plasmodium falciparum malaria in the Chittagong Hill Tracts, BangladeshTrans R Soc Trop Med Hyg20059972773516095643
- HaqueRThriemerKWangZTherapeutic efficacy of artemether/lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in BangladeshAm J Trop Med Hyg200776394117255226
- ThapaSHollanderJLinehanMComparison of artemether/lumefantrine with sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in Eastern NepalAm J Trop Med Hyg20077742343017827354
- JansenFHLesaffreEPenaliLKAssessment of the relative advantage of various artesunate-based combination therapies by a multi-treatment Bayesian random-effects meta-analysisAm J Trop Med Hyg2007771005100918165512
- PiolaPFoggCBajunirweFSupervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trialLancet200536594691467147315850630
- AchanJTibenderanaJKyabayinzeDEffectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trialBMJ200933927632763
- FaladeCMakangaMPremjiZEfficacy and safety of artemether/lumefantrine (Coartem®) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malariaTrans R Soc Trop Med Hyg20059945946715837358
- FaladeCManyandoCSafety profile of Coartem®: the evidence baseMalar J20098Suppl 1S619818173
- TooveySJamiesonAAudiometric changes associated with the treatment of uncomplicated falciparum malaria with coartemetherTrans R Soc Trop Med Hyg20049826126715109547
- MillerLGPanosianCBAtaxia and slurred speech after artesunate treatment for falciparum malariaN Engl J Med199733613289132599
- CousinMKummererSLefèvreGAdvisory Committee Briefing Book. Coartem® (artemether/lumefantrine) tablets for the treatment of malaria in patients with acute, uncomplicated infections due to Plasmodium falciparum or mixed infections includingP falciparumhttp://wwwfdagov/ohrms/dockets/ac/08/briefing/2008-4388b1-02-Novartispdf 2008
- BakshiRHermeling-FritzIGathmannIAn integrated assessment of the clinical safety of artemether-lumefantrine:a new oral fixed-dose combination antimalarial drugTrans R Soc Trop Med Hyg20009441942411127248
- Nankabirwa ZurovacDNjoguJNMalaria misdiagnosis in Uganda—implications for policy changeMalar J200916866
- TalisunaAOStaedkeSGD’AlessandroUPharmacovigilance of antimalarial treatment in Africa: is it possible?Malar J200655016780575
- BarnesKILittleFSmithPJSulfadoxine-pyrimethamine PKs in malaria: pediatric dosing implicationsClin Pharmacol Ther20068058259617178260
- PriceRNHasugianARRatcliffAClinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malariaAntimicrob Agents Chemother2007514090409717846129
- ter KuileFOTerlouwDJKariukiSKImpact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western KenyaAm J Trop Med Hyg200368687712749488
- SlutskerLTaylorTEWirimaJJIn-hospital morbidity and mortality due to malaria-associated severe anaemia in two areas of Malawi with different patterns of malaria infectionTrans R Soc Trop Med Hyg1994885485517992335
- SchellenbergDMenendezCKahigwaEAfrican children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for deathAm J Trop Med Hyg19996143143810497986
- NostenFWhiteNJArtemisinin-based combination treatment of Falciparum malariaAm J Trop Med Hyg200777618119218165491
- LongoMZanoncelliSManeraDEffects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos in vitroReprod Toxicol2006211839316026965
- DellicourSO ter KuileFStergachisAPregnancy exposure registries for assessing antimalarial drug safety in pregnancy in malaria-endemic countriesPLoS Med200859187
- NostenFMcGreadyRMutabingwaTCase management of malaria in pregnancyLancet Infect Dis20077211812517251082
- McGreadyRChoTKeoNKArtemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparumClin Infect Dis200133122009201611712093
- DellicourSHallSChandramohanDThe safety of artemisinins during pregnancy: a pressing questionMalar J20071461517300719
- AdamIElhassanEMOmerEMAbdullaMASafety of artemisinins during early pregnancy, assessed in 62 Sudanese womenAnn Trop Med Parasitol2009103320521019341535
- ManyandoCMkandawireRPumaLSafety profile artemether-lumefantrine (AL; Coartem®) compared sulfadoxine-pyrimethamine (SP) pregnant women symptomatic malaria: preliminary results of an observational studyAbstract 570, 57th Meeting of the American Society of Tropical Medicine and Hygiene (ASTMH)New Orleans, Louisiana, USADecember 2008
- TarningJMcGreadyRLindegardhNPopulation PKs of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated P. falciparum malariaAntimicrob Agents Chemother2009533837384619564366
- McGreadyRStepniewskaKLindegardhNThe pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malariaEur J Clin Pharmacol200662121021103117053895
- McGreadyRTanSOAshleyEAA randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancyPLoS Med2008235e25319265453
- WhitworthJMorganDQuigleyMEffect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort studyLancet200035692351051105611009139
- FrenchNNakiyingiJLugadaEIncreasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adultsAIDS200115789990611399962
- FrancesconiPFabianiMDenteMGHIV, malaria parasites, and acute febrile episodes in Ugandan adults: a case-control studyAIDS200115182445245011740196
- AtzoriCBrunoAChichinoGHIV-1 and parasitic infections in rural TanzaniaAnn Trop Med Parasitol19938765855938122920
- Van EijkAMAyisiJGTer KuileFOHIV increases the risk of malaria in women of all gravidities in Kisumu, KenyaAIDS200317459560312598780
- VerhoeffFHBrabinBJHartCAIncreased prevalence of malaria in HIV-infected pregnant women and its implications for malaria controlTrop Med Int Health19994151210203167
- SteketeeRWWirimaJJSlutskerLComparability of treatment groups and risk factors for parasitemia at the first antenatal clinic visit in a study of malaria treatment and prevention in pregnancy in rural MalawiAm J Trop Med Hyg199655117238702032
- ShulmanImportance and prevention of malaria in pregnancyTrans R Soc Trop Med Hyg2003971303512886801
- BlolandPBWirimaJJSRChilimaBMaternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infectionAIDS1995977217267546417
- Byakika-KibwikaPDdumbaEKamyaMEffect of HIV-1 infection on malaria treatment outcome in Ugandan patientsAfrican Health Sciences200772869217594285
- KamyaMRKigonyaCNMcFarlandWHIV infection may adversely affect clinical response to chloroquine therapy for uncomplicated malaria in childrenAIDS20011591187118811416726
- KamyaMRGasasiraAFYekaAEffect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based studyJ Infect Dis2006193191516323126
- ColebundersRBahweYNekweiWIncidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, ZaireJ Infect Dis1990212167173
- Van GeertruydenJPMulengaMMwananyandaLHIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malariaJ Infect Dis2006194791792516960779
- BirkuYMEBjörkmanAWoldayDDelayed clearance of Plasmodium falciparum in patients with human immunodeficiency virus co-infection treated with artemisininEthiop Med J20021172612802828
- PatnaikPJereCSMillerWCEffects of HIV-1 serostatus, HIV-1RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural MalawiJ Infect Dis200519298499116107950
- Van GeertruydenJPMulengaMKasongoWCD4 T-cell count and HIV-1 infection in adults with uncomplicated malariaJ Acquir Immune Defic Syndr200643336336717079994
- KhooSBackDWinstanleyPThe potential for interactions between antimalarial and antiretroviral drugsAIDS20051910995100515958830
- KamyaMoses RYekaAdokeBukirwaHasfaArtemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trialPLoS Clin Trials2007251371
- GasasiraAFKamyaMRAchanJHigh risk of neutropenia in HIV-infected children follwoing treatment with artesunate for uncomplicated malaria in UgandaCID200846985991
- GermanPParikhSLawrenceJLopinavir/ritonavir affects PK exposure of artemether/lumefantrine in HIV-uninfected healthy volunteersJ Acquir Immune Defic Syndr200951442442919506482
- SousaMPozniakABoffitoMPharmacokinetics and pharmacodynamics of drug interactions involving rifampicin, rifabutin and antimalarial drugsJ Antimicrob Chemother200862587287818713760
- LefevreGPK and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet®) with concomitant administration of ketoconazole in healthy subjectsBr J Clin Pharmacol20025448549212445027
- BlolandPBDrug resistance in malariaWHO/CDS/CSR/DRS/20014. 2001
- HuongNMHweittSDavisTMResistance of P. falciparum to anti-malarial drugs in a highly endemic area in southern Viet Nam: a study in vivo and vitroTrans R Soc Trop Med Hyg20019532532911491008
- YangHLiuDYangYChanges in susceptibility of Plasmodium falciparum to artesunate in vitro in Yunnan Province, ChinaTrans R Soc Trop Med Hyg20039722622814584382
- GordonPKellyRHedderwickSFailure of artemether-lumefantrine (Riamet) to clear Plasmodium falciparum in non immune travellerAbstract. First Interanational Conference of Travel Medicine and Infectious DiseaseLondon, UK2005
- YangsutakaMizunoYasuyukiKatoKoichiroKudoFirst case of Treatment failure of artemether-lumefantrine in a Japanese traveler with imported falciparum malariaJpn J Infect Dis20096213914119305055
- SisowathCStrombergJMartenssonAIn vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem)J Infect Dis20051911014101715717281
- DuraisinghMTJonesPSambouIvon SeidleinLPinderMWarhurstDCThe tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisininMol Biochem Parasitol2000108132310802315
- NewtonPNFernandezFMPlanconAA collaborative epidemiological investigation into the criminal fake artesunate trade in South East AsiaPLoS Med20085e3218271620
- AtemnkengMADe CockKPlaizier-VercammenJQuality control of active ingredients in artemisinin-derivative antimalarials within Kenya and DR CongoTrop Med Int Health200712687417207150
- BateRCoticelliPTrenRAntimalarial drug quality in the most severely malarious parts of Africa-a six country studyPLoS ONE20083e213218461128