Abstract
Purpose:
To evaluate the safety and efficacy of intravitreal injections of bevacizumab (Avastin®) as a treatment option for radiation maculopathy secondary to proton beam radiotherapy for choroidal melanoma.
Case:
A 61-year-old woman presented with a gradual decrease in left eye visual acuity (VA) 29 months after proton beam radiotherapy for choroidal melanoma. On presentation, her best-corrected VA (BCVA) was 2/10 in the left eye and the intraocular pressure was 15 mmHg. Fundoscopy revealed cystoid macular edema, intraretinal hemorrhages, epiretinal membrane in the posterior pole, and residual tumor scar with exudative retinal detachment and hard exudates in the periphery of the superotemporal quadrant. A treatment with intravitreal injections of bevacizumab (Avastin®) was recommended. The injections were performed on a six-weekly basis.
Results:
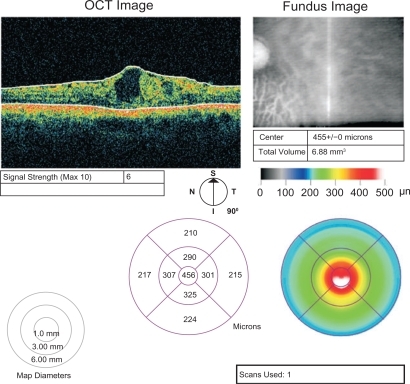
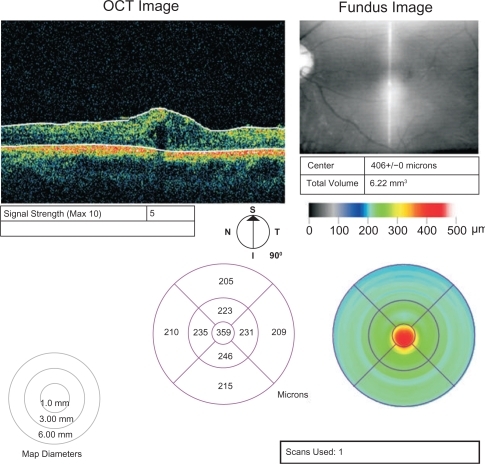
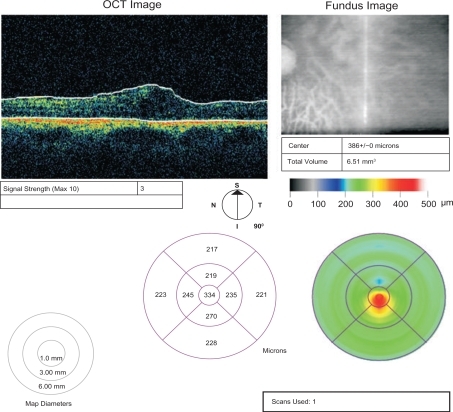
The central retinal thickness prior to the treatment was 458 μm. After the first intravitreal injection of bevacizumab, the retinal thickness at the centre of the fovea was reduced to 322 μm. After the third injection, the central retinal thickness was 359 μm and 18 months after presentation, it reduced to 334 μm. The BCVA increased to 3/10 after the intravitreal injections of bevacizumab and remained stable during the follow-up period. The intraocular pressure was within normal range during the follow-up period.
Conclusion:
Bevacizumab should be regarded as a treatment option for macular edema due to proton beam radiotherapy for choroidal melanoma. By reducing the central retinal thickness, intravitreal bevacizumab can improve VA or ameliorate further decline caused by radiation maculopathy.
Case report
A 61-year-old woman presented with a gradual decrease in left eye visual acuity (VA) 29 months after proton beam radiotherapy for choroidal melanoma. On presentation, her best-corrected visual acuity (BCVA) was 2/10 in the left eye and intraocular pressure was 15 mmHg. Ophthalmoscopy revealed cystoid macular edema, intraretinal hemorrhages and epiretinal membrane. Residual tumor with scar, exudative retinal detachment, and hard exudates were seen in the superotemporal periphery.
The patient was evaluated by fluorescein angiography and optical coherence tomography (OCT), and intravitreal injections of 1.25 mg bevacizumab (Avastin®) were recommended.
The patient was informed that the therapy was off-label and signed a relevant consent form.
The central retinal thickness before treatment was 458 μm () and a cystoid macular edema was evident on OCT and fluorescein angiography.
The procedure was carried out in the operation theatre. Topical proparacaine hydrochloride 0.5% was applied to the ocular surface followed by preparation with 5% povidone iodine. A cotton-tipped applicator soaked in proparacaine hydrochloride was applied to the injection site 4 mm posterior to the limbus. The intravitreal dose of bevacizumab (Avastin®) was 1.25 mg and we performed an intravitreal injection every six weeks. Indirect ophthalmoscopy was used to confirm uneventful intravitreal placement. Six weeks after the first intravitreal injection of bevacizumab (Avastin®), the central retinal thickness was reduced to 322 μm. The VA improved to 3/10 and the intraocular pressure remained stable and a second injection was suggested. Six weeks after the second injection, the central retinal thickness increased to 377 μm, and the patient was re-evaluated by ophthalmoscopy and OCT. A third intravitreal injection of bevacizumab was carried out and six weeks after the third injection, the central retinal thickness was reduced once again to 359 μm (). The VA was 3/10 and the intraocular pressure was 14 mmHg. At this point, treatment was discontinued and no further injections were recommended.
The patient was examined again 18 months after presentation. Her BCVA was 3/10 and intraocular pressure was 15 mmHg. Central retinal thickness was 334 μm, as documented by OCT (). The ophthalmoscopic findings were consistent with mild macular edema, epiretinal membrane, and residual tumor scar with exudative retinal detachment and hard exudates. The intraretinal hemorrhages have been absorbed.
Radiation retinopathy is a vision-threatening complication resulting from the therapeutic irradiation of ocular, orbital, periorbital, facial, nasopharyngeal, and cranial structures. It is a slow and progressive retinal microangiopathy with delayed-onset varying from 1 month to 15 years, but usually occurs within 3 years of treatment.Citation1,Citation2 Clinical manifestations of the disorder include macular edema and nonproliferative and proliferative retinopathy, similar to changes seen in diabetic retinopathy. The fundamental abnormality of chronic radiation damage is endothelial cell injury, primarily in capillaries, followed by capillary closure (this can be revealed by fluorescein angiography), subsequent retinal ischemia, necrosis of nerve tissue, and fibrovascular proliferation.Citation3
Consequently, increased concentrations of vascular endothelial growth factor (VEGF) were found in treated and untreated eyes with uveal melanoma.Citation4,Citation5 The radiation macular edema can be treated with laser photocoagulation, photodynamic therapy, and intravitreal triamcinolone acetonide.Citation6 The macular edema may also respond to anti-VEGF therapy.Citation7,Citation8 Bevacizumab is a recombinant humanized, full length, anti-VEGF monoclonal antibody that binds with all VEGF-A isoforms.
This case report suggests that cystoid macular edema, caused by proton beam radiotherapy for choroidal melanoma, responds to intravitreal injections of bevacizumab. This is shown by the reduction of the central retinal thickness on OCT. Also, anti-VEGF therapy seems to prevent further reduction and shows improved VA. The advantages of this treatment over the triamcinolone acetonide treatment are: the absence of intraocular pressure increase and cataract formation. Given that no serious side effects were observed, bevacizumab should be regarded as a treatment option for macular edema due to proton beam radiotherapy for choroidal melanoma.
Disclosures
The authors report no conflicts of interest in this work.
References
- BrownGCShieldsJASanbornGAugsburgerJJSavinoPJSchatzNJRadiation retinopathyOphthalmology198289149415017162794
- WaraWMIrvineARNegerREHowesELJrPhillipsTLRadiation retinopathyInt J Radiat Oncol Biol Phys197958183422419
- IrvineARAlvaradoJAWaraWMMorrisBWWoodISRadiation retinopathy: An experimental model for the ischemic-proliferative retinopathiesTrans Am Ophthalmol Soc1981791031227342397
- MissottenGSNottingICSchlingemannROVascular endothelial growth factor A in eyes with uveal melanomaArch Ophthalmol20061241428143417030710
- BoydSRTanDBunceCVascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic windowBr J Ophthalmol20028636836911914199
- ShieldsCLDemirciHDaiVIntravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanomaRetina20052586887416205566
- FingerPTRadiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin)Int J Radiat Oncol Biol Phys200815;70497497718313522
- MasonJO3rdAlbertMAJrPersaudTOVailRSIntravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanomaRetina200727790390717891015