Abstract
Glucocorticoids are essential in treating many disorders and they are widely used in spite of their negative impact on the skeletal system. As bisphosphonates reduce bone resorption through their action on osteoclasts, they play an important role in management of glucocorticoid-induced osteoporosis. Unlike other bisphosphonates, zoledronic acid is given by intravenous infusion and it has a potential advantage of increasing the compliance and adherence of patients when it is given 5 mg once a year. However, this treatment modality seems to be associated with more adverse events than oral administrations, and further studies with longer follow-up periods must be conducted to determine the safety and cost-effectiveness of long-term treatment with zoledronic acid.
Introduction
Glucocorticoids (GCs) are steroidal stress hormones which have many different Biological functions.Citation1,Citation2 Through binding to GC receptors, they exert most of their effects, such as regulating tissue differentiation during development, controlling intermediary metabolism and coordinating the adjustment of physiological processes in response to stress.Citation3–Citation5 Because of these effects, synthetic GCs have been developed which are widely used in the treatment of allergic, inflammatory, lymphoproliferative disorders, and autoimmune diseases.Citation6–Citation10 However, such a treatment is not without shortcomings: GCs exert a negative impact on the skeletal system as they result in a rapid, early phase of bone loss which is followed by a more chronic and progressive phase due to excessive bone resorption, in which finally the bone mass declines because of impaired bone formation.Citation11 The administration of GCs is the most frequent secondary cause of osteoporosis and it has been demonstrated that more than one third of individuals treated with GCs for 5 to 10 years will have an osteoporotic fracture.Citation12,Citation13
The purpose of this review is to discuss the pathophysiology of glucocorticoid-induced osteoporosis (GIO) and the efficacy and safety of zoledronic acid in the treatment of this disorder.
Pathophysiology of glucocorticoid-induced osteoporosis
Although it is estimated that, 30%–50% of patients using long-term GCs experience relevant bone loss, the exact prevalence of GIO is still unknown.Citation14,Citation15 The pathophysiology of GIO is complicated, as GCs may exert their effects on the skeletal system in many ways. In the beginning GCs cause an increase in bone resorption, however the most important effect of GCs is their action in decreasing bone formation by decreasing osteoblast proliferation and inhibiting osteoblasts from producing new bone.Citation16,Citation17 In addition, osteocyte apoptosis can be detected in GIO, which results in microarchitectural deterioration, as these cells are considered to determine bone strength independent of bone mineral density (BMD).Citation18 On the other hand, GCs also have systemic effects on bone metabolism as they have a negative effect on calcium homeostasis by decreasing absorption of calcium from the intestine and increasing calcium loss in the urine, as a result of defective vitamin D metabolism. This leads to secondary hyperparathyroidism, which results in increased bone resorption. Citation15,Citation16 Moreover, GCs affect bone metabolism indirectly by reducing levels of sex hormones as a result of suppressed pituitary gonadotrophin secretion.Citation16
Treatment of glucocorticoid-induced osteoporosis and bisphosphonates
As GIO is an important problem due to the increased risk of fracture, preventive measures are suggested in order to stabilize or increase the BMD in such patients, and consequently reduce the risk of fracture. It has been demonstrated that regular BMD assessment, and management of osteoporosis is suboptimal in patients using long-term GCs, despite the fact that screening and treatment rates have increased in recent years.Citation19 In an attempt to increase the rates of screening and treatment of osteoporosis in long-term GC users, Curtis et al employed an online continuing medical education program for physicians who prescribed long-term GC therapy.Citation20 Although the final results of this study revealed that the education provided did not yield any advantage when compared to the control group, a greater amount of education may be beneficial and the mode of education may affect the outcome.
Calcium and vitamin D supplementation, increasing BMD, and preventing high cortisol levels, are the main strategies in treating GIO. Moreover, numerous clinical trials have now demonstrated that bisphosphonates are highly effective at limiting bone loss in patients with GIO.Citation21 Furthermore, in GC-treated patients at high risk of fracture, bisphosphonate therapy has proved to be cost-effective.Citation22 Thus, nowadays bisphosphonates are considered to be the gold standard for the prevention and treatment of GIO, along with sufficient intakes of calcium and vitamin D as they increase BMD, and reduce vertebral fracture risk in patients beginning or continuing GC treatment.Citation23–Citation28 The nitrogen-containing bisphosphonates act by inhibiting farnesyl diphosphate (FPP) synthase, a key enzyme of the mevalonate pathway, which is critical to the production of cholesterol, other sterols, and isoprenoid lipids. The inhibition of osteoclasts by the nitrogen-containing bisphosphonates is very likely mediated by their action on the FPP synthase, which leads to protracted apoptosis of these giant cells.Citation29,Citation30
Although daily oral bisphosphonate therapy with alendronate or risedronate is effective for treatment of GIO, compliance and adherence with daily and weekly therapy has been shown to be suboptimal.Citation31–Citation35 This finding is important, as an association between poor adherence and increased fracture risk has been demonstrated in women with postmenopausal osteoporosis who were treated with oral bisphosphonate.Citation36 Therefore, studies were conducted in order to search for an alternative treatment regime which would increase the compliance and adherence of patients with GIO.Citation37
Zoledronic acid in the treatment of glucocorticoid-induced osteoporosis
Like other bisphosphonates, zoledronic acid binds to the calcium phosphate bone mineral hydroxyapatite, predominantly localizing at regions of high bone turnover.Citation38,Citation39 Additionally, the affinity of zoledronic acid for hydroxyapatite was shown to be higher than that of other bisphosphonates in an in vitro study.Citation38 In osteoporosis, zoledronic acid inhibits osteoclast-mediated resorption, therefore reducing bone turnover.
Zoledronic acid, which is available as an intravenous formulation, is approved for the treatment of osteoporosis in postmenopausal women and, more recently, in men at increased risk of fracture, and in patients in Europe with a recent low-trauma hip fracture.Citation40 Intravenous zoledronic acid 5 mg, administered via infusion, is the first once-yearly treatment approved for these indications.Citation41–Citation44
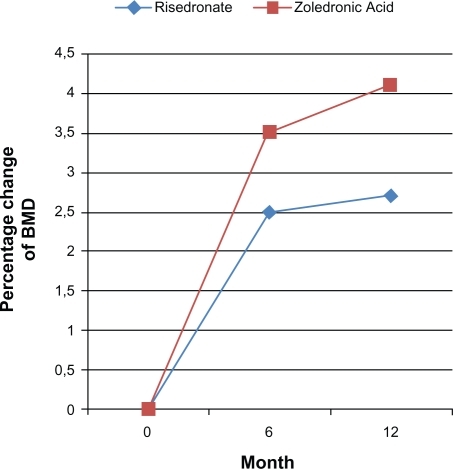
The efficacy of zoledronic acid for preventing and treating GIO was studied by HORIZON (Health Outcomes and Reduced Incidence with Zoledronic Acid Once) investigators in a large international study, where patients were randomized to receive either intravenous 5 mg zoledronic acid or oral 5 mg risedronate.Citation37 This study demonstrated that one 5 mg infusion of zoledronic acid annually increases bone mineral density of the lumbar spine and femoral neck, trochanter, and total hip more than the oral 5 mg risedronate daily, in patients who had recently started GCs (prevention group n:288) and in those who had been already taking them (treatment group n:545).Citation37 The patients were followed for 12 months and BMD was measured at 6th and 12th months. The primary endpoint of the study was the percentage change from baseline in BMD, while changes in bone turnover biomarker concentrations were measured as secondary end-points. Evaluation of this study indicated that the increase in BMD at 6 months was significantly higher with zoledronic acid than it was with risedronate (). It has also been shown that inhibition of the bone turnover markers was faster and more substantial in patients receiving zoledronic acid. Unfortunately, this study was not able to detect differences in fracture risk.Citation37 However, various studies have demonstrated that once-yearly administration of zoledronic acid 5 mg is effective in preventing fractures.Citation45–Citation47 Bearing this in mind, we may speculate that annual administration of 5 mg zoledronic acid will be efficacious in preventing bone fractures. However this speculation requires further studies to verify it.
Figure 1 Percentage changes in BMD of lumbar spine in GIO patients treated with either daily risedronate or annual zoledronic acid (HORIZON).Citation37
Severe adverse effects associated with the use of intravenous bisphosphonates are uncommon, however approximately 10% to 30% of patients receiving their first nitrogen-containing bisphosphonate infusion will experience an acute phase reaction within the first 3 days of treatment. This is most commonly characterized by transient pyrexia with associated myalgias, arthralgias, headaches, and influenza-like symptoms. Citation37 This acute phase response is believed to be the result of proinflammatory cytokine production by peripheral blood T cells.Citation48 The reactions are typically self-limited and resolve completely within 24 to 48 hours. In addition to the administration of adequate fluid, NSAIDs or acetaminophen may be useful supportive treatments. The frequency of pyrexia within the first days of treatment was 15% in a study where no co-drug was used during the administration of zoledronic acid, however in a study where NSAIDs or paracetamol was administered with zoledronic acid the rate was 8.7% ().Citation37,Citation45 Fortunately, the frequency of these reactions is rare in subsequent infusions of nitrogen-containing biphosphonates.Citation37,Citation49
Ocular complications, nephrotoxicity, and/or electrolyte abnormalities such as hypocalcemia, hypophosphatemia, and hypomagnesemia are also reported.Citation31 The most frequent observed ocular complication is nonspecific conjunctivitis, which is usually self-limited.Citation50 On the other hand uveitis can also be seen after the administration of zoledronic acid and this requires medical treatment.Citation51 The physician administering intravenous bisphosphonates should be alert for such situations where patients present with ocular pain, itching, or decrease in vision, and in such cases they should promptly direct the patient to be examined by an opthalmologist.Citation50
Previous studies have proved that the effect of risderonates on renal functions is comparable with placebo even in patients with impaired renal functions.Citation52 It is well known that all intravenous bisphophonates have the potential to affect renal functions, especially in patients whose renal function is compromised.Citation53 However the findings of the HORIZON study did not demonstrate a higher rate of renal impairment in patients receiving zoledronic acid treatment than in patients in the risedronate arm.Citation37 When we take the previously-mentioned studies’ results into consideration it may be further speculated that zoledronic acid may be comparable with placebo with respect to renal complications.
Electrolyte abnormalities can also be a problem in zoledronic acid treatment. In a multicenter review hypocalcemia was reported in 35% of patients after zoledronic acid infusion whereas symptomatic hypocalcemia requiring treatment was observed in 8% of patients despite prophylactic measures.Citation54
Bisphosphonates have been linked to osteonecrosis of the jaw which can be prevented with pretherapy dental care.Citation55 It has been shown that prolongation of bisphosphonate treatment increases the frequency of osteonecrosis of the jaw.Citation56 The management of this situation is a matter for debate and depends on the stage at which it is diagnosed.
In the HORIZON-PFT (Health Outcomes and Reduced Incidence with Zoledronic Acid Once Pivotal Fracture Trial), where patients were treated annually with intravenous zoledronic acid, a statistically-significant increase in the incidence of serious atrial fibrillation (defined as events resulting in hospitalization, or disability, or judged to be life-threatening) was noted.Citation42 The etiology of this electrophysiologic abnormality is not clarified yet. Unfortunately, we have limited data regarding other bisphosphonate preparations which may have the potential to produce similar rates of atrial fibrillation. One large population-based case-control study suggested a correlation between alendronate administration and a slightly-increased incidence of atrial fibrillation, whereas another population-based case-control study showed no evidence of an increased risk of atrial fibrillation or flutter with alendronate use.Citation57,Citation58 On the other hand, no increase was detected in the rate of atrial fibrillation seen in patients received IV zoledronic acid after a hip fracture (the HORIZON Recurrent Fracture Trial).Citation45,Citation59 Apparently, more studies examining the potential relationship between bisphosphonate use and atrial fibrillation are required.
Conclusion
Since GCs are essential in treating many disorders, physicians should consider their negative effects on skeletal health, and they must be aware of GIO prior to administering these drugs. As bisphosphonates reduce bone resorption through their actions on osteoclasts, they play an important role in management of GIO. However awareness rates are much below those expected. Future studies focusing on improving the understanding of GIO and its management should be conducted, as it may be beneficial to increase the awareness of physicians about GIO and its treatment options through rigorous education programs.
Zoledronic acid, which is given by intravenous infusion, has a potential advantage of increasing the compliance and adherence of patients when it is given 5 mg, once a year. Zoledronic acid is effective in preventing and treating GIO, with a overall high safety and tolerability rate, and a low rate of serious adverse events. Future studies concentrating on the long-term effects and cost-effectiveness would be beneficial. In addition, research focusing on new targets such as anabolic drugs which stimulate bone formation by acting on osteoblasts and osteocytes could lead to a more compelling rationale for use than bisphosphonates.
Disclosures
The authors report no conflicts of interest in this work.
References
- KinoTTissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoidsHorm Metab Res200739642042417578758
- ViegasLRHoijmanEBeatoMPecciAMechanisms involved in tissue-specific apopotosis regulated by glucocorticoidsJ Steroid Biochem Mol Biol20081093–527327818424036
- BaxterJDRousseauGGGlucocorticoid Hormone Action: an overviewMonogr Endocrinol197912124386083
- ColeTJBlendyJAMonaghanAPTargeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturationGenes Dev1995913160816217628695
- BergerSBleichMSchmidWMineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolismProc Natl Acad Sci U S A19989516942494299689096
- McCabeBFAutoimmune sensorineural hearing lossAnn Otol Rhinol Laryngol1979885 Pt 1585589496191
- DodsonKMSismanisAIntratympanic perfusion for the treatment of tinnitusOtolaryngol Clin North Am2004375991100015474106
- DodsonKMWoodsonESismanisAIntratympanic steroid perfusion for the treatment of Ménière’s disease: a retrospective studyEar Nose Throat J200483639439815266874
- TaitASButtsCLSternbergEMThe role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious diseaseJ Leukoc Biol200884492493118664528
- TruneDRKemptonJBHarrisonARWobigJLGlucocorticoid impact on cochlear function and systemic side effects in autoimmune C3.MRL-Faslpr and normal C3H/HeJ miceHear Res20072261–220921717098384
- CanalisEBilezikianJPAngeliAGiustinaAPerspectives on glucocorticoid-induced osteoporosisBone200434459359815050888
- BaltzanMASuissaSBauerDCCummingsSRHip fractures attributable to corticosteroid useLancet19993539161132710218535
- CenterJRNguyenTVSchneiderDSambrookPNEismanJAMortality after all major types of osteoporotic fracture in men and women: an observational studyLancet199935391651707
- CooperCCouplandCMitchellMRheumatoid arthritis, corticosteroid therapy and hip fractureAnn Rheum Dis199554149527880122
- ReidIRGlucocorticoid osteoporosis – mechanisms and managementEur J Endocrinol199713732092179330580
- McIlwainHHGlucocorticoid-induced osteoporosis: pathogenesis, diagnosis, and managementPrev Med200336224324912591000
- DelanyAMDongYCanalisEMechanisms of glucocorticoid action in bone cellsJ Cell Biochem19945632953027876321
- van StaaTPThe pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosisCalcif Tissue Int200679312913716969593
- CurtisJRWestfallAOAllisonJJLongitudinal patterns in the prevention of osteoporosis in glucocorticoid treated patientsArthritis Rheum200552814851494
- CurtisJRWestfallAOAllisonJChallenges in improving the quality of osteoporosis care for long-term glucocorticoid users: a prospective randomized trialArch Intern Med2007167659159617389291
- de NijsRNJacobsJWLemsWFAlendronate or alfacalcidol in glucocorticoid-induced osteoporosisN Engl J Med2006355767568416914703
- van StaaTPGeusensPZhangBLeufkensHGBoonenACooperCIndividual fracture risk and the cost-effectiveness of bisphosphonates in patients using oral glucocorticoidsRheumatology (Oxford)200746346046616899499
- CohenSLevyRMKellerMRisedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group studyArthritis Rheum199942112309231810555025
- ReidDMHughesRALaanRFEfficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment StudyJ Bone Miner Res20001561006101310841169
- SaagKGEmkeyRSchnitzerTJAlendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study GroupN Engl J Med199833952922999682041
- CompstonJEEmerging consensus on prevention and treatment of glucocorticoid-induced osteoporosisCurr Rheumatol Rep200791788417437672
- CompstonJUS and UK guidelines for glucocorticoid-induced osteoporosis: similarities and differencesCurr Rheumatol Rep200461666914713404
- AdlerRAHochbergMCSuggested guidelines for evaluation and treatment of glucocorticoid-induced osteoporosis for the Department of Veterans AffairsArch Intern Med2003163212619262414638562
- WeinsteinRSRobersonPKManolagasSCGiant osteoclast formation and long-term oral bisphosphonate therapyN Engl J Med20093601536219118304
- RoelofsAJThompsonKGordonSRogersMJMolecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res20061220 Pt 262226230
- TanvetyanonTStiffPJManagement of the adverse effects associated with intravenous disphosphonatesAnn Oncol200617689790716547070
- HamiltonBMcCoyKTaggartHTolerability and compliance with risedronate in clinical practiceOsteoporos Int200314325926212730745
- CramerJALynchNOGaudinAFWalkerMCowellWThe effect of dosing frequency on compliance and persistence with bisphosphonate therapy in postmenopausal women: a comparison of studies in the United States, the United Kingdom, and FranceClin Ther200628101686169417157124
- CramerJAGoldDTSilvermanSLLewieckiEMA systematic review of persistence and compliance with bisphosphonates for osteoporosisOsteoporos Int20071881023103117308956
- SeemanECompstonJAdachiJNon-compliance: the Achilles’ heel of anti-fracture efficacyOsteoporos Int200718671171917245547
- SirisESHarrisSTRosenCJAdherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databasesMayo Clin Proc20068181013102216901023
- ReidDMDevogelaerJPSaagKZoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trialLancet200937396711253126319362675
- NancollasGHTangRPhippsRJNovel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatiteBone200638561762716046206
- DunfordJEThompsonKCoxonFPStructure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonatesJ Clin Pharmacol Exp Ther20012962235242
- European Medicines AgencyAclasta: summary of product characteristics [online]. April 14, 2010. Available from: http://www.emea.europa.eu/humandocs/Humans/EPAR/aclasta/aclosta.htm Accessed on April 26, 2010
- Food and Drug AdministrationReclast® (zoledronic acid) injection: US prescribing information [online]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021817s001lbl.pdf
- BlackDMDelmasPDEastellROnce-yearly zoledronic acid for treatment of postmenopausal osteoporosisN Engl J Med2007356181809182217476007
- McClungMReckerRMillerPIntravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronateBone200741112212817468062
- ChenTBerensonJVescioRPharmacokinetics and phamiacodynamics of zoledronic acid in cancer patients with bone métastasesJ Clin Pharmacol200242111228123612412821
- LylesKWColon-EmericCSMagazinerJSZoledronic acid and clinical fractures and mortality after hip fractureN Engl J Med2007357181799180917878149
- BoonenSBlackMDColon-EmericSCEfficacy and safety of a once yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and olderJAGS201058292299
- ErkisenEFLylesKWColon-EmericCSAntifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fractureJ Bone Miner Res20092471308131319257818
- HewittRELissinaAGreenAESlayESPriceDASewellAKThe bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statinsClin Exp Immunol2005139110111115606619
- ContePFGuarneriVSafety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist9Suppl 42004
- FraunfelderFWFraunfelderFRJensvoldBScleritis and other ocular side effects associated with pamidronate disodiumAm J Opthalmol135219222
- ColucciAModoratiGMiserocchiEAnterior uveitis complicating zoledronic acid infusionOcul Immunol Inflamm200917426726819657981
- MillerPDRouxCBoonenSSafety and efficacy of risedronate in patients with age related reduced renal functions as estimated by the Cockcroft and Gaukt method: a pooled analysis of nine clinical trialsJ Bone Min Res20052021052115
- LawanskySKomaroffECavanaughPFRelationship between age, renal function and bone mineral density in the US populationOsteoporosis Int200314570576
- ChennuruSKoduriJBaumannMARisk factors for symptomatic hypocalcaemia complicating treatment with zoledronic acidIntern Med J200838863563718284458
- MarxRESawatariYFortinMBroumandVBisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatmentJ Oral Maxillofac Surg200563111567157516243172
- KhoslaSBurrDCauleyJBisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American society for bone and mineral researchJ Bone Miner Res200722101479149117663640
- HeckbertSRLiGCummingsSRSmithNLPsatyBMUse of alendronate and risk of incident atrial fibrillation in womenArch Intern Med2008168882683118443257
- SorensenHTChristensenSMehnertFUse of bisphosphonates among women and risk of atrial fibrillation and flutter: population based case-control studyBMJ2000336764881381618334527
- KaramRCammJMcClungMYearly zoledronic acid in postmenopausal osteoporosisN Engl J Med2007357771271317703529