Abstract
Background:
Despite the well recognized protective effect of cruciferous vegetables against various cancers, including human colorectal cancers, little is known about how this effect is conferred. It is thought that some phytochemicals found only in these vegetables confer the protection. These compounds include the glucosinolates, of which indole-3-carbinol is one. They are known to induce carcinogen-metabolizing (phase II) enzymes, including the glutathione S-transferase (GST) family. Other effects in humans are not well documented. We wished to assess the effect of indole-3-carbinol on GST enzymes.
Methods:
We carried out a placebo-controlled human volunteer study. All patients were given 400 mg daily of indole-3-carbinol for three months, followed by placebo. Serum samples were tested for the GSTM1 genotype by polymerase chain reaction. Serum GST levels were assessed using enzyme-linked immunosorbent assay and Western Blot methodologies.
Results:
Forty-nine volunteers completed the study. GSTM1 genotypes were obtained for all but two volunteers. A slightly greater proportion of volunteers were GSTM1-positive, in keeping with the general population. GST was detected in all patients. Total GST level was not affected by indole-3-carbinol dosing compared with placebo. Although not statistically significant, the GSTM1 genotype affected the serum GST level response to indole-3-carbinol.
Conclusion:
Indole-3-carbinol does not alter total serum GST levels during prolonged dosing.
Introduction
There is strong evidence that diets including a large vegetable component confer protection against various human cancers including colorectal cancer.Citation1–Citation6 The underlying mechanism(s) for this protective effect is poorly understood. Vegetables of the brassica genus are known to provide significant protection. Compounds from these vegetables, including the glucosinolates and their metabolites, have been shown in in vitro studies to inhibit the proliferation of and induce apoptosis in various human cancer cell lines, act as strong antioxidants, and induce the production of both phase I and phase II carcinogen-metabolizing enzymes.Citation7 The glucosinolates are found in several plant groups, but the only human dietary source is from cruciferous vegetables.Citation8–Citation10 We wished to quantify the effect of one of these compounds, indole-3-carbinol, on human serum glutathione transferase levels as a logical progression of our in vitro work,Citation11,Citation12 in a manner similar to that suggested by Kristal and Lippman.Citation13
Materials and methods
Volunteers
Fifty-six volunteers consented to take part in the double-blind pilot study. They were recruited from advertisements in the local press, from family members of patients attending the Hunter Family Cancer Service, and as a result of media interest in our laboratory studies. Because this was a pilot study, no sample size or power calculation was made. Ethics approval was obtained from the Hunter Area Research Ethics Committee.
Dosing and blood collection
A dose of 400 mg of indole-3-carbinol was to be taken each day based on our previous laboratory work and other studies. Citation14 At the initial visit, volunteers provided a baseline blood sample and were provided with sufficient tablets until the following meeting. Venous blood was obtained in the usual manner, mostly from the antecubital fossa, using the Vacuette® system (Greiner Bio-One, Interpath, West Heidelberg, Australia). Blood suitable for genotyping was retrieved in standard specimen bottles containing ethylenediamine tetra-acetic acid. A total of 50 mL of blood was obtained. A further five meetings occurred, during which additional blood samples were taken and further tablets provided. After the third visit, volunteers changed from indole-3-carbinol to placebo tablets for the following three months. No tablets were given at the final appointment. Volunteers were advised to continue their normal diet throughout the study period.
Genotyping for glutathione transferase Mu
Whole blood was stored at −20°C and the genotype assessed using the polymerase chain reaction (PCR). The following primers were utilized (Life Technologies, Melbourne, Australia):
DNA was extracted from a whole blood sample using reagents specified for the BioRobot M48 (Qiagen, Doncaster, Australia). This was then mixed with 30.55 μL of H2O, 4 μL dNTPs (2.5 mM) 200 mM (Promega, Annandale, Australia), 5 μL buffer (10×) (Promega), 1.25 μL of primers, 0.2 μL Taq (5 U/mL) (Promega) up to a total volume of 50 μL. An automated PCR was carried out using the Eppendorf Mastercycler (EpGradient S, Sydney, Australia). Initially the samples were denatured for five minutes at 94°C, with a further 14 cycles, each cycle comprising 30 seconds at 94°C, 45 seconds at 60–54.5°C (step-down 0.5°C/two cycles), and one minute at 72°C. This was then followed by 30 cycles, each cycle consisting of 94°C for 30 seconds, 53.5°C for 45 seconds, and 72°C for 60 seconds. Once the cycling reaction was complete, a final extension period of 10 minutes at 72°C was performed. The resulting 132bp GSTM1 samples were digested with Hae11 enzyme (10 U/mL) at 37.5°C for 3.5 hours. This revealed the homozygous, heterozygous, or null genotype (alleles) of GSTM1. The polymorphism was evaluated by gel electrophoresis (2% agarose), and the DNA levels were checked using a spectrophotometer.
Phenotyping of glutathione transferase Mu
Serum was initially separated from the whole blood and stored at −20°C. The degree of glutathione S-transferase (GST) expression was assessed using both an enzyme-linked immunosorbent assay (ELISA) and Western Blot technique.
ELISA methodology
The wells of 96-well Polysorb ELISA plates (NUNC, Sydney, Australia) were coated with 10 μL of serum from volunteers. These were then incubated at 37°C for 2–3 hours. The plates were then washed and incubated following administration of blocking buffer (10% skim milk, 1% Tween 20) for a further 60 minutes at 37°C. The primary antibody (rabbit anti-human GST, donated by Professor Clancy, University of Newcastle, Australia) was added and incubated for another 120 minutes at 37°C. The plates were washed again and more blocking buffer applied prior to further incubation for 60 minutes at 37°C. Following this, the secondary antibody (anti-rabbit IgG-HRP conjugated, donated by Professor Clancy, University of Newcastle, Australia) was applied and reincubated at 37°C for two hours. The plates were then read in a microplate reader at 405 nm after the horseradish peroxidase (HRP) substrate (ABST) was fixed. A control plate of pure GST was run in a similar fashion for reasons of comparison.
Western Blot methodology
The serum samples were subjected to polyacrylamide gel (12.5%) electrophoresis. The gel was run at 110 V for 75 minutes. Following this, the gel was placed over a sheet of nitrocellulose and the protein in the gel was transferred to the nitrocellulose electrophorectically in transfer buffer (1× Tris, glycine plus 20% methyl alcohol). Blocking buffer was then prepared by adding 0.2% Tween 20 and 10% skim milk powder to a solution of 10 mM Tris and 100 mM NaCl. The nitrocellulose was then placed in this buffer solution for 120 minutes with shaking. The blocking buffer was removed and a 1/10000 dilution of the first antibody was prepared and added to the membrane. The reaction was incubated for 90 minutes at room temperature and removed. A further application of blocking buffer was applied for 90 minutes. The nitrocellulose was then placed in the secondary antibody (1/5000 or 1/10000 in blocking buffer) and incubated for another 90 minutes at room temperature (shaking). Two milliliters of ECL developing reagent (Amersham Biosciences, Rydalmere, Australia) was prepared and spread onto two glass slides, according to the manufacturer’s instructions. The resultant membrane was exposed to Hyperfilm ECL (Amersham Biosciences) for five to 10 minutes, followed by developing and visualization. This was done primarily to validate the results of the ELISA tests.
Statistics
The outcome measure of serum glutathione transferase level was measured for both treatment and placebo. These measures were analysed as continuous variables and the effect of the predictor variables (ie, age, sex, diet) on the mean value for the outcome variables was determined with regression or analysis of variance (ANOVA), (unpaired t-test if the predictor had only two levels). To determine the effect of indole-3-carbinol, the difference between the treatment and placebo groups was analyzed to determine the impact on the mean change. The null hypothesis of no change was assumed, and statistical significance was taken as P < 0.05.
Results
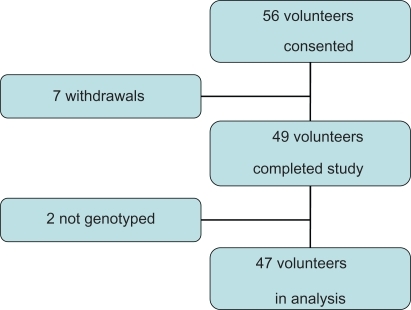
Of the 56 volunteers who consented to take part in the study, seven withdrew. The reasons given were inconvenience (n = 3), a newly diagnosed medical condition (1), tiredness (1, whilst on the trial preparation), and diarrhea (2, whilst on the trial preparation). The GSTM1 genotype could not be determined in two volunteers, who were excluded from further analysis. Glutathione transferase was detectable in all the remaining volunteers ().
Demographics of volunteers
The median age of volunteers who completed the trial was 58 years (range 20–79). Slightly more females took part (57.1% versus 42.9%) and the majority were “never” smokers (never, 55.1%; no longer, 38.8%; current, 6.1%).
Results of genotyping
Of the 49 volunteers who completed the trial in the two phases, a GSTM1 genotype was obtainable for all but two volunteers (one male and one female). Of the remaining 47 volunteers who completed the study, 25 (53.2%) were positive for GSTM1, which is comparable with the normal population in other studies.Citation15–Citation20 Of these, six were 1a/1a or 1a/null-positive and the remainder were 1b/1b or 1b/null. There was no significant difference between GSTM1 genotype for volunteers by age (GSTM1-null: <50 years, 60.0% versus >50 years, 40.6%; P = 0.21) nor by gender (GSTM1-null: male, 45.0% versus female 48.1%; P = 0.82).
Effects of indole-3-carbinol on serum GST
When all results were considered, there was no significant difference in mean serum GST levels for volunteers receiving indole-3-carbinol or placebo (indole-3-carbinol, 0.168; placebo, 0.168; P = 0.84; ).
The results were then analyzed as matched pairs; that is, the difference in the mean serum GST level at times 1 and 2 for indole-3-carbinol against the mean serum GST level at times 1 and 2 for placebo. There was no statistically significant mean difference (indole-3-carbinol, 0.173; placebo, 0.170; difference, 0.003; P = 0.67).
We then considered the matched pairs for all the serum GST levels on indole-3-carbinol against the baseline serum GST levels and the matched time equivalent on placebo. In general, indole-3-carbinol did not affect serum GST levels (); however, there was nonstatistically significant increase in serum GST level following the three months of treatment with indole-3-carbinol against the baseline.
Table 1 Results of matched pairs t-tests for all samples
Effect of indole-3-carbinol on serum GST by GSTM1 genotype
We also compared the effect of indole-3-carbinol on serum GST levels according to GSTM1 genotype. Although not statistically significant, indole-3-carbinol caused a decrease in GST levels in GSTM1-null volunteers and an increase in GST levels in GSTM1-positive volunteers (P = 0.48; ).
Table 2 The effect of indole-3-carbinol on serum GST levels by GSTM1 genotype
Discussion
We have successfully completed a double-blind, placebo-controlled, pilot study using indole-3-carbinol, without undue or intolerable adverse events. In this study, indole-3-carbinol had no effect on serum glutathione transferase levels when compared with placebo. Using the matched-pairs analysis again there was no difference in serum glutathione transferase levels. Against the baseline serum glutathione transferase levels, however, treatment with indole-3-carbinol did increase serum glutathione transferase levels, although not to a level of statistical significance. Such differences were not seen when treatment was compared with the equivalent placebo period. These differences between treatment and placebo periods and treatment and baseline periods are not easily explained, but they may be accounted for by the distribution and function of glutathione transferase and its isoenzymes.
Cytosolic glutathione transferases “mop up” the byproducts of normal oxidative stress, such as lipid peroxidation as well as xenobiotics.Citation21–Citation24 The enzymes do this primarily by attaching a glutathione molecule to electrophiles, making them water-soluble, which facilitates urinary or biliary excretion.Citation19,Citation25–Citation27 This is the first step in the formation of mercapturic acids, a pathway which results in the elimination of potentially toxic compounds from the body.Citation19,Citation28–Citation30 Within mammalian cells, at least five different isoenzymes of glutathione transferases have been discovered. The most widely studied to date have been named as follows: GSTα, GSTμ, GSTπ, GSTθ and GSTγ.Citation20,Citation22 They all have different substrate specificity, but there is significant crossover of function.Citation22,Citation28,Citation31 The regulation of cytosolic glutathione transferases is subject to a complex set of endogenous and exogenous parameters including developmental-, gender-, and tissue-specific factors, as well as a large number of xenobiotic agents, such as polycyclic aromatic hydrocarbons, reactive oxygen species, isothiocyanates, and drugs.Citation31 The genetic and sporadic loss of glutathione transferase isoenzymes is known to cause upregulation of the remaining transferases.Citation22 Persons deficient in certain isoenzymes are thought to be more susceptible to cancers of the lung, bladder, prostate, colon, and rectum.Citation17,Citation20,Citation32–Citation35
The vast majority of glutathione transferases in the human body are found in the liver and small intestine. GSTμ is less organ-specific than the other glutathione transferases.Citation19 It is found in relatively low levels in lung and colon tissue Citation36,Citation37 and in high levels in the liver.Citation36
The mechanism and site of action for the chemoprotective effects of cruciferous vegetables is not known. It has been postulated that the effects are localized within the gut lumen. This may be as a result of direct inhibition of proliferation/induction of apoptosis in abnormal/cancer cells or the induction of local carcinogen-metabolizing enzymes. This does not explain the regression of carcinoma in situ of the cervix when indole-3-carbinol is administered orally.Citation14
The evidence for an increase in overall GST activity and that within specific tissues is equivocal. Studies in rats fed brussels sprout extract or cabbage-substituted diets showed an overall increase in GST activity.Citation38,Citation39 In human studies, no effect on overall GST activity was noted in subjects fed test diets of brussels sproutsCitation40,Citation41 or broccoli pills.Citation25 Other studies of rats which were fed brussels sprouts have shown an increase in the glutathione transferase activity of intestinal cells.Citation42
Several studies have looked at cancer risk in GSTM1-null patients in relation to brassica consumption. These studies would suggest that a greater intake of brassica vegetables or extracts could confer greater protection in GSTM1-null patients. These results have been explained by the slower metabolism of the isothiocyanates in the absence of the GSTμ, prolonging their chemoprotective effect.
In our study, indole-3-carbinol was found to cause an increase in serum glutathione transferase levels in those who were GSTM1-positive and, conversely, a decrease in those who were GSTM1-null. One might expect such a pattern because GSTμ expression cannot be induced in null individuals. This may also indicate that indole-3-carbinol induces GSTμ specifically and has an inhibitory or no effect on the other isoenzymes, despite the observed compensatory mechanism described above. It may be that isoenzyme activity in specific tissues is induced following administration of brassica vegetables, unrefined extracts, or specific glucosinolate metabolites. This induction may be at a level not detectable when overall glutathione transferase activity is considered.Citation43 Glutathione transferases are abundant in the liver and much less so in other tissues,Citation44 such that induction of already saturated cells is futile. In extrahepatic tissues, however, a modest induction in specific glutathione transferase isoenzymes is achievable,Citation31,Citation41,Citation43,Citation45 and may be of biologic significance.
GSTM1-positive volunteers with a 1a/1a or 1a/null allele status had higher serum levels of GST (not significant, data not shown) than the rest. It may be that the 1a genotype gives rise to greater expression of the GSTμ isoenzyme, increasing overall GST levels, or 1a may in fact be relatively inactive so there is greater compensatory expression of the other isoenzymes. Different phenotypes have been found between homozygous and heterozygous GSTM1-positive individuals. In a study by Moore et al, individuals who were GSTM1+/+ were found to be at greater risk of developing colorectal adenomas than either GSTM1+/− or −/− individuals.Citation46 Some authors have suggested that these phenotypic differences may account for the lack of evidence for any increased cancer risk attributable to glutathione transferase deficiency, as most studies do not differentiate homozygous from heterozygous positive individuals.Citation22
These results raise many questions. The relatively small sample size may have reduced the statistical power of some of the observations, and a larger trial looking particularly at specific isoenzymes should answer some of these. The differences observed for indole-3-carbinol against the baseline tests implies that indole-3-carbinol has some effect in the manner to be expected from the previous work by ourselves and others. The lack of any difference against placebo might suggest that the placebo tablets, which only contained rice flour, may themselves have had some effects on the systems we were investigating. Measurement of individual GST isoenzymes was not available in this study. We intended to measure GST Mu levels using the formerly available MuKit (Biotrin, Ireland). However, this was not available in sufficient quantities to enable testing of all samples. More enzyme- or tissue-specific testing may provide very different results from those we found.
The results also question the perceived role that indole-3-carbinol plays in chemoprevention. It may be that indole-3-carbinol is very specific in terms of which glutathione transferase is induced whereas other components of brassica vegetables, for example, sulforaphane, may have a more global influence on phase II enzymes. Indole-3-carbinol and other isothiocyanates are known to act on cancer cells by mechanisms other than induction of carcinogen-metabolizing enzyme systems.Citation47 We plan to carry out a further trial using sulforaphane, and do additional work using human serum and cancer cell lines.
Conclusion
We have conducted a study of indole-3-carbinol in humans with no deleterious or significant side effects. We found glutathione transferase to be present in all volunteers even those who were GSTM1-negative. Dosing with indole-3-carbinol did not appear to alter serum levels of glutathione transferase. This can be explained by several mechanisms. Indole-3-carbinol is very specific and it does globally induce the production of glutathione transferase, but only one of its isoenzymes. Small increases in isoenzymes may not be detectable when global glutathione transferase levels are being measured in the serum. This may indicate that indole-3-carbinol has a role to play in the treatment of colorectal cancer, either in those who are at increased risk or following diagnosis. Whilst some aspects of indole-3-carbinol metabolism have been described,Citation48 much of its metabolism and actions remain undiscovered.
Acknowledgements
We would like to thank the Newcastle Permanent Building Society which kindly donated a large sum of money to enable us to carry out the pilot study, and also Professor Rodney Scott and his staff at the Hunter Pathology Service for providing technical and logistic support. The results included in this paper were presented to the Nutrition Society at its summer meeting in Coleraine, UK, in July 2007.
Disclosures
The authors have no conflicts of interest to report in this study.
References
- SteinmetzKAPotterJDVegetables, fruit, and cancer. I. EpidemiologyCancer Causes Control1991253253571834240
- MichaudDSpiegelmanDClintonSRimmEWillettWGiovannucciEFruit and vegetable intake and incidence of bladder cancer in a male prospective cohortJ Natl Cancer Inst19999160561310203279
- CohenJKristalAStanfordJFruit and vegetable intakes and prostate cancer riskJ Natl Cancer Inst2000921616810620635
- GiovannucciERimmEBLiuYStampferMJWillettWCA prospective study of tomato products, lycopene, and prostate cancer riskJ Natl Cancer Inst200294539139811880478
- TerryPGiovannucciEMichelsKBFruit, vegetables, dietary fiber, and risk of colorectal cancerJ Natl Cancer Inst200193752553311287446
- BlockGPattersonBSubarAFruit, vegetables, and cancer prevention: A review of the epidemiological evidenceNutr Cancer19921811291408943
- McGrathDRSpigelmanADPutative mechanisms of action for indole-3-carbinol in th prevention of colorectal cancerExpert Opin Ther Targets200812672973818479219
- FenwickGRHeaneyRKMullinWJGlucosinolates and their breakdown products in food and food plantsCrit Rev Food Sci Nutr19831821232016337782
- VerhoevenDTGoldbohmRAvan PoppelGVerhagenHvan den BrandtPAEpidemiological studies on brassica vegetables and cancer riskCancer Epidemiol Biomarkers Prev1996597337488877066
- KohlmeierLSimonsenNMottusKDietary modifiers of carcinogenesisEnviron Health Perspect1995103Suppl 81771848741780
- FrydoonfarHRMcGrathDRSpigelmanADInhibition of proliferation of a colon cancer cell line by indole-3-carbinolColorect Dis200243205207
- FrydoonfarHRMcGrathDRSpigelmanADThe effect of indole-3-carbinol and sulforaphane on a prostate cancer cell lineANZ J Surg200373315415612608980
- KristalALippmanSNutritional prevention of cancer: New directions for an increasingly complex challengeJ Natl Cancer Inst2009101636336519276449
- BellMCCrowley-NowickPBradlowHLPlacebo-controlled trial of indole-3-carbinol in the treatment of CINGynecol Oncol200078212312910926790
- GasperAVAl-JanobiASmithJAGlutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoliAm J Clin Nutr20058261283129116332662
- JosephMAMoysichKBFreudenheimJLCruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer riskNutr Cancer200450220621315623468
- SpitzMRDuphorneCMDetryMADietary intake of isothiocyanates: Evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer riskCancer Epidemiol Biomarkers Prev20009101017102011045782
- KolmRHDanielsonUHZhangYTalalayPMannervikBIsothiocyanates as substrates for human glutathione transferases: Structure-activity studiesBiochem J1995311Pt 24534597487881
- MulderTPRoelofsHMPetersWHWagenmansMJSierCFVerspagetHWGlutathione S-transferases in liver metastases of colorectal cancer. A comparison with normal liver and primary carcinomasCarcinogenesis19941510214921537955047
- EngelLSTaioliEPfeifferRPooled analysis and meta-analysis of glutathione S-transferase M1 and bladder cancer: A HuGE reviewAm J Epidemiol200215629510912117698
- FaheyJWTalalayPAntioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymesFood Chem Toxicol1999379–1097397910541453
- HayesJDFlanaganJUJowseyIRGlutathione transferasesAnnu Rev Pharmacol Toxicol200545518815822171
- VillafaniaAAnwarKAmarSGlutathione-S-transferase as a selective inhibitor of oncogenic ras-p21-induced mitogenic signaling through blockade of activation of jun by jun-N-terminal kinaseAnn Clin Lab Sci2000301576410678584
- YehCCHsiehLLTangRChang-ChiehCRSungFCVegetable/fruit, smoking, glutathione S-transferase polymorphisms and risk for colorectal cancer in TaiwanWorld J Gastroenterol200511101473148015770723
- ClapperMLSzarkaCEPfeifferGRPreclinical and clinical evaluation of broccoli supplements as inducers of glutathione S-transferase activityClin Cancer Res19973125309815533
- GrubbenMJvan den BraakCCNagengastFMPetersWHLow colonic glutathione detoxification capacity in patients at risk for colon cancerEur J Clin Invest200636318819216506964
- PietschECHurleyALScottEEOxathiolene oxides: A novel family of compounds that induce ferritin, glutathione S-transferase, and other proteins of the phase II responseBiochem Pharmacol20036581261126912694867
- HabigWHPabstMJJakobyWBGlutathione S-transferases. The first enzymatic step in mercapturic acid formationJ Biol Chem197424922713071394436300
- LampeJWPetersonSBrassica, biotransformation and cancer risk: Genetic polymorphisms alter the preventive effects of cruciferous vegetablesJ Nutr2002132102991299412368383
- MorseMAThe role of glutathione S-transferase P1-1 in colorectal cancer: Friend or foe?Gastroenterology200112141010101311606516
- EatonDLBammlerTKConcise review of the glutathione S-transferases and their significance to toxicologyToxicol Sci199949215616410416260
- BrooksJDPatonVGVidanesGPotent induction of phase 2 enzymes in human prostate cells by sulforaphaneCancer Epidemiol Biomarkers Prev200110994995411535546
- SpigelmanADNugentKPPennaCFouldsSPhillipsRKGlutathione S-transferase Mu phenotype in patients with familial adenomatous polyposis and in unaffected controlsCancer Detect Prev19941842532587982235
- SzarkaCEPfeifferGRHumSTGlutathione S-transferase activity and glutathione S-transferase mu expression in subjects with risk for colorectal cancerCancer Res19955513278927937796404
- ZhongSWyllieAHBarnesDWolfCRSpurrNKRelationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancerCarcinogenesis1993149182118248403204
- YeZParryJMA meta-analysis of 20 case-control studies of the glutathione S-transferase M1 (GSTM1) status and colorectal cancer riskMed Sci Monit2003910SR83SR9114523342
- HoulstonRSGlutathione S-transferase M1 status and lung cancer risk: A meta-analysisCancer Epidemiol Biomarkers Prev19998867568210744127
- AnsherSSDolanPBuedingEBiochemical effects of dithiolthionesFood Chem Toxicol19862454054153744194
- BradfieldCABjeldanesLFEffect of dietary indole-3-carbinol on intestinal and hepatic monooxygenase, glutathione S-transferase and epoxide hydrolase activities in the ratFood Chem Toxicol198422129779826334634
- GillCIHaldarSPorterSThe effect of cruciferous and leguminous sprouts on genotoxicity, in vitro and in vivoCancer Epidemiol Biomarkers Prev20041371199120515247131
- NijhoffWAGrubbenMJNagengastFMEffects of consumption of brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humansCarcinogenesis1995169212521287554064
- SalbeADBjeldanesLFThe effects of dietary brussels sprouts and Schizandra chinensis on the xenobiotic-metabolizing enzymes of the rat small intestineFood Chem Toxicol198523157653871719
- WarkPAGrubbenMJPetersWHHabitual consumption of fruits and vegetables: Associations with human rectal glutathione S-transferaseCarcinogenesis200425112135214215284178
- WhittyJPBjeldanesLFThe effects of dietary cabbage on xenobiotic-metabolizing enzymes and the binding of aflatoxin B1 to hepatic DNA in ratsFood Chem Toxicol19872585815873114106
- BogaardsJJVerhagenHWillemsMIvan PoppelGvan BladerenPJConsumption of Brussels sprouts results in elevated alpha-class glutathione S-transferase levels in human blood plasmaCarcinogenesi199415510731075
- MooreLEHuangWYChatterjeeNGSTM1, GSTT1, and GSTP1 polymorphisms and risk of advanced colorectal adenomaCancer Epidemiol Biomarkers Prev20051471823182716030123
- MuskSRJohnsonITAllyl isothiocyanate is selectively toxic to transformed cells of the human colorectal tumour line HT29Carcinogenesis19931410207920838222057
- GeXYannaiSRennertGGruenerNFaresFA3,3’-Diindolylmethane induces apoptosis in human cancer cellsBiochem Biophys Res Commun199622811531588912651
![Figure 1 Mean serum GST levels for I3C (I) and placebo (P) – [Units – μg/ml].](/cms/asset/5c122fae-48a7-49c6-b137-0838a3fe74bb/dtcr_a_9568_f0001_c.jpg)