Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) increases low-density lipoprotein cholesterol (LDL-C) concentrations through interference with normal physiologic hepatic LDL receptor (LDLR) recycling. Inhibiting PCSK9 results in improved LDLR recycling, increased LDLR availability on hepatocyte cell surfaces, and reduced blood LDL-C levels, making PCSK9 inhibition a novel therapeutic strategy for managing hypercholesterolemia. Monoclonal antibodies directed against PCSK9 have been developed for this purpose. A large number of clinical trials have demonstrated that monoclonal antibodies against PCSK9 yield substantial reductions in LDL-C when administered as monotherapy or in combination with statins to patients with nonfamilial and familial forms of hypercholesterolemia. Data from long-term trials demonstrate that the LDL-C-lowering effect of PCSK9 inhibitors is durable. These agents are generally well tolerated, and few patients discontinue treatment due to adverse events. Moreover, PCSK9 inhibitors do not appear to elicit the hepatic and muscle-related side effects associated with statin use. The ultimate value of PCSK9 inhibitors will be measured by their effect on clinical outcomes. Early evidence of a reduction in cardiovascular events after 1 year of treatment was shown in a prospective exploratory analysis of two ongoing long-term open-label extension evolocumab trials. Similarly, cardiovascular events were reduced in another exploratory analysis after >1 year of therapy with alirocumab. For the primary care physician, PCSK9 inhibitors represent a welcome additional option for lowering LDL-C in patients with familial forms of hypercholesterolemia and those with clinical atherosclerotic cardiovascular disease who are on maximally tolerated statin therapy.
Introduction
Elevated low-density lipoprotein cholesterol (LDL-C) is a well-established causal risk factor for atherosclerosis.Citation1–Citation4 Currently, statins are the mainstay therapy for lowering LDL-C. Statins reduce both cardiovascular events and mortality in patients with elevated risk of atherosclerotic cardiovascular disease.Citation5–Citation8 Although statins are the most important LDL-C-lowering drugs, LDL-C lowering beyond that achieved with these agents is desirable and beneficial. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) showed that the addition of ezetimibe to simvastatin 40 mg/d in patients with a recent acute coronary syndrome who had LDL-C levels of 1.3–2.6 mmol/L (50–100 mg/dL) if they were receiving lipid-lowering therapy or 1.3–3.2 mmol/L (50–125 mg/dL) if they were not receiving lipid-lowering therapy resulted in additional lowering of LDL-C by an average of 24%.Citation9 This further reduction in LDL-C led to a 13% relative reduction (absolute: 1.7% reduction) in myocardial infarction (P=0.002) and a 21% relative reduction (absolute: 0.7% reduction) in ischemic stroke (P=0.008). These data support evidence from meta-analyses demonstrating merit in attaining very low levels of LDL-C; these meta-analyses demonstrate additional reductions in cardiovascular events in patients achieving LDL-C levels of <1.8 mmol/L (70 mg/dL) or 1.3 mmol/L (50 mg/dL).Citation10,Citation11 A Cholesterol Treatment Trialists (CTT) meta-analysis demonstrated a 37% relative risk reduction in major vascular events with LDL-C reductions of <1.8 mmol/L (70 mg/dL) in statin-treated patients.Citation11 Post hoc analysis of the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, which evaluated the efficacy of rosuvastatin as primary prevention in patients with an LDL-C of <3.4 mmol/L (<130 mg/dL) but high-sensitivity C-reactive protein ≥2.0 mg/L, found that patients who attained an LDL-C of <1.3 mmol/L (<50 mg/dL) had a 65% reduction in the occurrence of a cardiovascular event or death from cardiovascular causes (P<0.0001) compared with placebo. By comparison, patients who did not attain an LDL-C of <1.3 mmol/L (<50 mg/dL) achieved only a 24% reduction.Citation12 Likewise, post hoc analysis of the Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial, which evaluated the efficacy of moderate (pravastatin, 40 mg/d) or intensive (atorvastatin, 80 mg/d) statin therapy plus gatifloxacin antibiotic therapy in patients with a recent acute coronary syndrome, found that patients who achieved an LDL-C of 1.0–1.5 mmol/L (40–60 mg/dL) or <1.0 mmol/L (<40 mg/dL) had further reductions in cardiovascular events compared with patients who achieved an LDL-C of >2.0–2.6 mmol/L (>80–100 mg/dL).Citation13 Post hoc analysis of the Treating to New Targets study showed a significant reduction in the rate of major cardiovascular events across five quintiles of on-treatment-achieved LDL-C concentrations ranging from ≥2.7 mmol/L (≥106 mg/dL) to <1.6 mmol/L (<64 mg/dL; P<0.0001).Citation14
Although substantial evidence supports reaching lower LDL-C levels, many statin-treated patients fail to reach the desired LDL-C levels. A meta-analysis of eight statin trials found that out of patients receiving high-dose statin therapy, 13% failed to reach the LDL-C level of <2.6 mmol/L (<100 mg/dL) and 40% failed to reach <1.8 mmol/L (<70 mg/dL).Citation10 Results are more worrisome in community practice with an international survey finding that 70% of very-high-risk patients (of whom 80% were receiving a statin) failed to reach the LDL-C level of <1.8 mmol/L (<70 mg/dL).Citation15 Reasons for lack of achieving the desired LDL-C levels are variable and likely include high baseline levels, prescription of inadequate statin doses, poor adherence, and the inability to tolerate an adequate statin dose. Statin-associated muscle symptoms are a frequent cause for patients to not take statins or to only take a low dose. The Prediction of Muscular Risk in Observational conditions (PRIMO) study found that 10% of patients in community practice who were taking high-dose statins reported muscle symptoms, a rate significantly higher than suggested by placebo-controlled clinical trials.Citation16 On average, patients fail to take prescribed statins ~20% of the timeCitation17,Citation18 and approximately half of patients discontinue statin therapy by 5 years.Citation17,Citation19 Poor adherence with resulting elevated LDL-C levels place patients at risk for hypercholesterolemia-associated morbidity and mortality. Overall, these data demonstrate that additional options are needed to assist patients toward achieving the required LDL-C levels. The recent approval of monoclonal antibodies against proprotein convertase subtilisin/kexin type 9 (PCSK9) by the US Food and Drug Administration and the European Medicines Agency, among other regulatory agencies, provides physicians with another LDL-C-lowering option.
PCSK9
PCSK9 is a serine protease involved in cholesterol metabolism that is enzymatically inactive following secretion. PCSK9 is a proprotein convertase belonging to the subtilase subfamily.Citation20 In healthy humans, plasma PCSK9 levels decrease with fasting and increase following meals.Citation21,Citation22 Shortly after the discovery of PCSK9,Citation23 it was shown that a gain-of-function mutation in PCSK9 is associated with familial hypercholesterolemia.Citation20,Citation24 Further research demonstrated that loss-of-function mutations in PCSK9 are associated with reduced LDL-C concentrations and that these lifetime reductions confer substantial protection against coronary artery disease.Citation25–Citation27 PCSK9 gene expression is regulated by the nuclear transcription factor sterol regulatory element-binding protein-2.Citation28 Levels of sterol regulatory element-binding protein-2 are increased by statin therapy, which thus also increases PCSK9 levels. PCSK9 inhibition may thus be an especially useful therapeutic strategy in statin-treated patients.
In adults, PCSK9 is expressed predominantly in the liver, and to a lesser extent in the intestine and kidney.Citation23 Currently, the only known physiologically relevant function of circulating PCSK9 is to regulate LDL receptor (LDLR) in the liver. PCSK9 increases LDL-C concentrations through interference with normal physiologic hepatic LDLR recycling. LDL particles are largely removed from the circulation via the LDLR, which are located on the surface of hepatocytes. The LDLR binds LDL and the complex enters the cell through a clathrin-coated vesicle. Intracellularly, the LDL and LDLR dissociate. LDL is delivered to a lysosome and degraded, while the LDLR is recycled back to the hepatocyte cell surface ().Citation29 PCSK9 interferes with this process by preventing the separation of the LDLR from LDL. PCSK9 binds to the cell-surface LDLR; upon LDL binding and internalization, the PCSK9-bound LDLR fails to separate from the LDL particle. As a result, the LDLR is delivered to the lysosome and degraded along with the LDL, thus bypassing the process of recycling to the hepatocyte cell surface ().Citation30 The diminished LDLR concentration on hepatocyte cell surfaces results in elevated plasma LDL-C due to reduced clearance of LDL. Inhibiting PCSK9 therefore results in improved LDLR recycling, increased availability of LDLR on hepatocyte cell surfaces, increased LDL plasma clearance, and reduced blood LDL-C levels, making PCSK9 inhibition an effective therapeutic strategy for LDL hypercholesterolemia.
Figure 1 LDL Recycling, PCSK9 Function, and Effect of PCSK9 Inhibition
Abbreviations: LDL, low-density lipoprotein; LDLRs, LDL receptors; PCSK9, proprotein convertase subtilisin/kexin type 9.
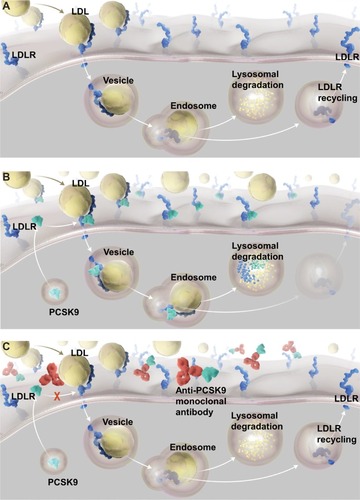
PCSK9 monoclonal antibodies
Currently, most of the data for PCSK9 inhibition come from studies with monoclonal antibodies that are directed against PCSK9 and prevent its interaction with the LDLR ( and ).Citation31,Citation32 Therapeutic monoclonal antibodies play important roles in the management of many inflammatory disorders and cancers because of their ability to bind to a selected target highly specifically, but they have not been widely used in the management of cardiovascular disease as yet. Monoclonal antibodies are target-specific antibodies created through recombinant DNA technology. These proteins have the characteristic Y-shaped protein structure of B-cell-derived antibodies and are designed to bind to a single therapeutic target with high specificity.Citation33 Monoclonal antibodies exert their therapeutic action through a variety of mechanisms, including direct effects associated with the binding of the antibody to the target (target blockade, the mechanism by which current anti-PCSK9 monoclonal antibodies exert their effects)Citation31,Citation32 and indirect effects involving depletion of cells targeted by the monoclonal antibody.Citation33 Monoclonal antibodies are administered parenterally (intravenously, intramuscularly, or subcutaneously).Citation34 Elimination occurs not through the liver or kidneys, but primarily through antigen-specific target-mediated disposition and nonspecific pathways of the reticuloendothelial system.Citation35
Table 1 Anti-PCSK9 monoclonal antibodies marketed or in Phase III development
Monoclonal antibody structures vary according to the proportion of murine components and production methods. After the development of early murine antibodies, investigators sought to replace murine components with human components to reduce the immunogenicity of the antibodies.Citation36,Citation37 Of particular concern is the potential for the development of neutralizing antibodies that can reduce the therapeutic efficacy of monoclonal antibodies.Citation38 Further development initially resulted in chimeric antibodies, which consisted of a human antibody with murine variable regions. Next, humanized antibodies were generated, which contained all human components except for the antigen-binding complementarity-determining regions.Citation36 Finally, fully human monoclonal antibodies were developed using novel platforms.Citation39,Citation40 Evolocumab and alirocumab are fully human anti-PCSK9 monoclonal antibodies,Citation31,Citation41–Citation43 while bococizumab is a humanized monoclonal antibody.Citation32
Clinical trials
A large number of clinical trials have now been conducted with anti-PCSK9 antibodies, demonstrating that these agents lead to substantial reductions in LDL-C when administered as monotherapy or in combination with statins and/or ezetimibe to patients with nonfamilial and familial forms of hypercholesterolemia. Published randomized controlled Phase III trials evaluating evolocumab and alirocumab are summarized in . In patients without homozygous familial hypercholesterolemia (HoFH), evolocumab led to mean reductions in LDL-C of 48%–76% compared with placebo and of 38%–47% compared with ezetimibe after 12 or 52 weeks of treatment.Citation44–Citation48 Alirocumab led to mean reductions of 46%–62% compared with placebo and 24%–32% compared with ezetimibe after 24 weeks of treatment.Citation49–Citation55 Note that reductions are consistently reported within this review as placebo/control corrected rather than as change from baseline.
Table 2 Published PCSK9 monoclonal antibody Phase III trials
Combination with statins
Adding a PCSK9 inhibitor to background statin therapy offers a useful strategy to further lower LDL-C in patients unable to achieve optimal LDL-C levels with statin therapy. The majority of clinical trials conducted with PCSK9 inhibitors have evaluated this combination and have included a diverse patient population treated with widely varying statin dose intensities. In some trials, additional non-statin lipid-lowering therapy was also allowed with ezetimibe, the most commonly used second agent.Citation44,Citation46,Citation48,Citation49,Citation51–Citation53 Mean reductions in LDL-C compared with placebo plus background statin therapy among patients receiving PCSK9 inhibitors in addition to statins, with or without other lipid-lowering therapy, ranged from 48% to 76% at week 12 in trials of evolocumab and from 46% to 62% at week 24 in trials of alirocumab.Citation44,Citation46,Citation47,Citation51–Citation53 Different intensities of baseline therapy do not appear to influence the magnitude of lipid lowering achieved with PCSK9 inhibition. In the Durable Effect of PCSK9 Antibody Compared with Placebo Study (DESCARTES), patients were assigned a statin dose based on their screening LDL-C and National Cholesterol Education Program Adult Treatment Panel III risk level. Mean LDL-C reductions with monthly evolocumab compared with placebo at week 52 were 48% in those receiving atorvastatin 80 mg plus ezetimibe 10 mg, 57% in those receiving atorvastatin 80 mg alone, 62% in those receiving atorvastatin 10 mg, and 56% in those receiving diet alone as their baseline concomitant therapy with evolocumab.Citation44 In the LDL-C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy-2 (LAPLACE-2) trial, in which patients were randomized to a baseline statin dose, mean LDL-C reductions with every 2-week evolocumab compared with placebo at week 12 were 68%–76% in patients receiving high-intensity stains and 68%–71% in those receiving moderate-intensity statins.Citation47
Whether PCSK9 inhibitors can provide additional LDL-C lowering in patients receiving combination therapy with statin and ezetimibe is an important and clinically relevant question in the face of the recently published IMPROVE-IT trial.Citation9 In trials that enrolled patients with heterozygous familial hypercholesterolemia (HeFH), more than half of the patients were receiving ezetimibe in addition to their statin.Citation46,Citation51 These trials demonstrated robust LDL-C lowering among all patients, and in subgroup analysis of the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD-2) trial, evolocumab lowered LDL-C to a similar extent between patients receiving ezetimibe and those who were not.Citation46 Additional data are available from the DECARTES trial. Among 189 patients receiving atorvastatin 80 mg plus ezetimibe 10 mg daily, the addition of monthly evolocumab resulted in an average LDL-C reduction of 48% when compared with placebo.Citation44
Monotherapy
Because statin therapy upregulates PCSK9 expression, it is important to know whether PCSK9 inhibition is less effective in patients who are not receiving statins and thus do not have high PCSK9 levels. The Monoclonal Antibody against PCSK9 to Reduce Elevated LDL-C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels-2 (MENDEL-2) trial evaluated evolocumab monotherapy in patients with screening LDL-C ≥2.6 mmol/L (≥100 mg/dL).Citation45 The LDL-C reduction achieved with evolocumab in this placebo-controlled trial (55%–57% vs placebo) was similar to that seen when evolocumab was added to statins. Further evidence comes from the diet-only arm of DESCARTES, which showed that evolocumab monotherapy led to a 56% LDL-C reduction compared with placebo.Citation44 The MENDEL-2 trial also compared evolocumab monotherapy with ezetimibe, finding that evolocumab reduced LDL-C by 38%–39% compared with ezetimibe.Citation45 Alirocumab was also evaluated as monotherapy in patients with screening LDL-C ≥2.6 mmol/L (≥100 mg/dL) in the Efficacy and Safety of Alirocumab SAR236553 (REGN727) vs Ezetimibe in Patients with Hypercholesterolemia (ODYSSEY MONO) trial. This study utilized ezetimibe as its only comparator and alirocumab reduced LDL-C by 32% vs ezetimibe.Citation54 PCSK9 inhibition is thus an effective lipid-lowering strategy even if LDLRs are not upregulated by statins.
Long-term therapy
Data from long-term trials demonstrate that the LDL-C-lowering effect of PCSK9 inhibitors is durable. Patients who had enrolled in one out of 12 Phase II or III evolocumab trials were eligible to enroll in the Open-label Study of Long-Term Evaluation against LDL Cholesterol (OSLER)-1 and OSLER-2 studies.Citation56 In these studies, 4,465 patients were randomized 2:1 to receive evolocumab plus standard therapy or standard therapy alone. At week 12, evolocumab therapy was associated with a 61% reduction in LDL-C compared with standard therapy alone, similar to the 58% reduction observed at 48 weeks. Further long-term data for evolocumab come from the DESCARTES trial, which found a 58% reduction in LDL-C with monthly evolocumab treatment compared with placebo at week 12 and a 57% reduction at week 52.Citation44 Prolonged therapy with alirocumab was evaluated in the Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM) trial, which included 2,341 patients who received alirocumab for 78 weeks.Citation53 The mean LDL-C reduction at weeks 24 and 78 were 61% and 52%, respectively, compared with placebo in intention-to-treat analysis. When patients who discontinued therapy early were excluded, the mean reduction at 78 weeks was 58%.
Effect on lipoprotein(a)
Lipoprotein(a) (Lp(a)) is an independent risk factor for cardiovascular diseaseCitation57,Citation58 and is not lowered by statins or other currently available lipid-lowering therapy apart from nicotinic acid.Citation59 PCSK9 inhibition results in statistically significant reductions in plasma Lp(a).Citation45,Citation54 When compared with baseline, evolocumab monotherapy reduced Lp(a) by 18%–20% after 12 weeks and alirocumab monotherapy reduced Lp(a) by 17% after 24 weeks.Citation45,Citation54 When added to moderate- or high-intensity dose statin therapy, reductions in Lp(a) with evolocumab ranged from 24% to 39% and the reduction with alirocumab was 28% in two representative trials.Citation47,Citation50 In an analysis of multiple evolocumab trials, reductions in Lp(a) were positively correlated with LDL-C reductions.Citation60 Although the mean percentage reduction in Lp(a) was significantly greater in those patients with baseline Lp(a) of ≤125 nmol/L, the absolute reduction was substantially larger in those with levels of >125 nmol/L.
Effect on high-density lipoprotein cholesterol and triglycerides
Whether PCSK9 inhibitors affect high-density lipoprotein cholesterol (HDL-C) or triglyceride levels is of clinical interest. In the MENDEL-2 trial, evolocumab monotherapy increased HDL-C by 6%–9% compared with placebo (P<0.01).Citation45 Reductions in triglyceride levels were 6% with evolocumab 140 mg every 2 weeks (Q2W; P=0.72) and 18% with evolocumab 420 mg every 4 weeks (P<0.001) compared with placebo. In the ODYESSY MONO trial, alirocumab monotherapy increased HDL-C by 6% compared with ezetimibe (P=0.02).Citation54 The mean reduction in triglycerides was 2% (P=0.74) compared with ezetimibe.
Special populations
Heterozygous familial hypercholesterolemia
Trials evaluating the addition of PCSK9 inhibitors to maximal therapy with statins plus ezetimibe and/or other lipid-lowering therapies have shown substantial mean LDL-C reductions of 51%–59% compared with placebo in patients with HeFH.Citation46,Citation51 Data from the RUTHERFORD-2 trial suggest that the nature of the LDLR mutation and its residual function, if a mutation had previously been functionally characterized, does not influence the magnitude of LDL-C lowering in response to PCSK9 inhibition.Citation46 In a post hoc exploratory subgroup analysis of patients with a single LDLR causative mutation in this trial, mean reductions for evolocumab vs placebo were 55%–61% for patients with an LDLR-negative mutation, 49%–66% in patients with an LDLR-defective mutation, 62%–63% in patients with an unclassified LDLR mutation, and 43%–64% in patients with no mutation identified. Patients with HeFH have one nonmutated LDLR allele, and upregulation of this fully functional allele likely accounts for the majority of the response, making the contribution, if any, by the mutated allele less relevant.
Homozygous familial hypercholesterolemia
HoFH is a rare severe genetic disorder that most commonly results from mutations in both LDLR alleles. LDL-C concentrations are extremely high (typically >12.9 mmol/L [500 mg/dL] in untreated patients) and premature cardiovascular disease is ubiquitous, often leading to early death.Citation61 HoFH responds poorly to conventional cholesterol-lowering medications because of severe impairment in LDLR function, with LDL-C reductions of only ~25% with statins.Citation62,Citation63 Evidence supporting the efficacy of PCSK9 inhibition with evolocumab in patients with HoFH comes from the Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities (TESLA) Part B trial.Citation64 In this study of 49 patients with HoFH and a baseline mean (standard deviation [SD]) LDL-C of 9.0 (3.5) mmol/L (348 [135] mg/dL) who were receiving statins and other lipid-lowering therapy other than apheresis, the addition of monthly evolocumab led to a mean 31% reduction in LDL-C compared with placebo. This percentage reduction, while smaller in magnitude than those observed in other populations, corresponded to a mean absolute LDL-C reduction of 2.4 mmol/L (94 mg/dL) and is considered clinically meaningful in this high-risk population. Prespecified subgroup analysis of this trial suggested that some residual LDLR functionality is required for LDL-C-lowering activity. Patients with defective LDLR mutation status in at least one allele showed statistically significant LDL-C reductions of 24%–47%, while the single patient with negative LDLR mutation status in both alleles experienced a 10% increase from baseline in LDL-C.
Statin intolerant
Patients who are unable to tolerate statins due to muscle-related or other side effects represent a population who may benefit from the alternative mechanism of action offered by PCSK9 inhibitors. Due to the subjective nature of muscle symptoms associated with statin therapy and the high prevalence of muscle symptoms in general, regardless of statin treatment, the true number of patients with statin intolerance is difficult to ascertain.Citation65–Citation68 The PRIMO study found that 10% of patients receiving high-dose statins in clinical practice experienced muscle symptoms, which were associated with activity limitations in more than one-third of these patients.Citation16 Effective options for LDL-C lowering in patients who cannot tolerate statins are limited. The Goal Achievement after Utilizing an anti-PCSK9 antibody in Statin Intolerant Subjects Study 2 (GAUSS-2) trial evaluated evolocumab in 307 patients who had failed two or more statins and could tolerate either no statin or only a low dose of statin.Citation48 The majority of patients had discontinued prior statins due to myalgia. However, 20% of patients had experienced either myositis (defined as muscle symptoms with creatine kinase elevation) or rhabdomyolysis. Notably, 56% of patients were at high risk for coronary heart disease as classified by the National Cholesterol Education Program. Patients were randomized to receive either ezetimibe or evolocumab (either 140 mg Q2W or 420 mg monthly). Both regimens of evolocumab resulted in a mean 38% reduction in LDL-C compared with ezetimibe. Evolocumab was well tolerated in this population of patients who had a strong history of muscle symptoms with statin use. Myalgia was reported in 7%–9% of patients receiving evolocumab and 18% of patients receiving ezetimibe. Data from the ODYSSEY ALTERNATIVE trial indicate that alirocumab is also effective in a population of patients who had failed two statins for muscle symptoms.Citation55 This trial included a single-blind placebo run-in phase (N=361) during which 47 patients who experienced muscle symptoms (13%) were excluded. Additionally, an atorvastatin 20 mg control arm was included along with an ezetimibe control, and all arms were placebo controlled. Results from this trial demonstrated a 30% reduction in LDL-C with alirocumab compared with ezetimibe. Interestingly, muscle-related adverse events were statistically significantly lower in the alirocumab arm compared with the atorvastatin arm (32% vs 46%; P=0.042). While demonstrating the efficacy of PCSK9 inhibition in this stain-intolerant population, the ODYSSEY ALTERNATIVE trial also adds to the body of literature illustrating the complexity and multifaceted nature of statin-associated muscle symptoms with a significant proportion of patients excluded during the placebo run-in while many patients were able to take atorvastatin.
Safety
Evolocumab is generally well tolerated and few patients discontinued this agent due to adverse events. In the placebo-controlled evolocumab monotherapy trial, MENDEL-2, adverse event rates were comparable between evolocumab and placebo (44% in each arm).Citation45 Serious events occurred in 1.3% of patients receiving evolocumab and 0.6% of patients on placebo. Adverse events led to study drug discontinuation in 2.3% of patients receiving evolocumab and 3.9% of patients receiving placebo. Injection-site reactions occurred in 5% of each arm. The alirocumab monotherapy trial, ODYSSEY MONO, was ezetimibe controlled; adverse events occurred in 69% of patients receiving alirocumab and 78% of patients receiving ezetimibe.Citation54 Statin combination trials revealed no increase in adverse events when PCSK9 inhibitors were added to statins.Citation47,Citation52 Longer term studies showed continued tolerability. After 52 weeks of evolocumab treatment, 2% of patients discontinued treatment for an adverse event compared with 1% of patients receiving placebo.Citation44 After 78 weeks of alirocumab treatment, 7% of patients discontinued for an adverse event compared with 6% of patients receiving placebo.Citation53 The most common adverse events occurring in clinical trials of evolocumab and alirocumab are summarized in .Citation42,Citation43
Table 3 Adverse events occurring in ≥3% of monoclonal antibody-treated patients and more frequently than with placebo
A potential concern with long-term administration of biologic agents is the development of neutralizing antidrug antibodies and loss of efficacy over time. Pooled data from placebo- and active-controlled evolocumab trials demonstrate that 0.1% of evolocumab-treated patients developed binding antidrug antibodies with no neutralizing antibody formation; these binding antibodies were not associated with altered pharmacokinetics, safety, or clinical response.Citation43 Pooled data from ten placebo- and active-controlled alirocumab trials demonstrated binding antidrug antibodies in 4.8% of alirocumab-treated patients and 0.6% of control-treated patients.Citation42 These patients experienced a higher rate of injection-site reactions compared with those without antidrug antibodies (10.2% vs 5.9%). Neutralizing antibodies developed in 1.2% of alirocumab-treated patients and no control-treated patients; 0.3% of patients had both neutralizing antibodies and transient or prolonged loss of efficacy.
Diabetes and neurocognitive events are of potential concern with statins.Citation70–Citation72 Whether these same side effects occur with PCSK9 inhibition is of interest and has been evaluated in trials. In DESCARTES, mean (standard error) fasting glucose increased by 0.07 (0.04) mmol/L (1.3 [0.7] mg/dL) from baseline with evolocumab compared with 0.02 (0.05) mmol/L (0.4 [0.9] mg/dL) with placebo. The mean (standard error) glycated hemoglobin increase was 0.02% (0.02) with evolocumab vs 0% (0.03) with placebo.Citation44 In the ODYSSEY LONG TERM trial, with >78 weeks of treatment, new onset diabetes occurred in 1.8% (alirocumab) and 2.0% (placebo) of patients and worsening of diabetes occurred in 12.9% (alirocumab) and 13.6% (placebo) of patients.Citation53 Whether statins are associated with memory loss or other adverse effects on cognition remains controversial.Citation73 Any purported mechanism by which memory loss or neurocognitive deficits could occur remains clinically poorly characterized and mechanistically inadequately studied and defined. Preclinical research suggests statins may be associated with a morphologic change in neurons consisting of areas of swelling referred to as “beads on a string”.Citation74 However, whether these changes result from a direct effect of the statin molecule, its mechanism of action, cholesterol lowering (considering that the majority of cholesterol in the brain is derived from local synthesis),Citation75 or another cause is unknown. In clinical trials of PCSK9 monoclonal antibodies, neurocognitive events were infrequent. In the 1-year controlled period of the OSLER-1 and OSLER-2 trials, neurocognitive events occurred in 0.9% and 0.3% of patients receiving evolocumab plus standard therapy and patients receiving standard therapy alone, respectively.Citation56 A dedicated study of cognition (Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in High cardiovascUlar Risk Subjects [EBBINGHAUS]; NCT02207634), which has enrolled subjects participating in the larger ongoing Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) evolocumab cardiovascular outcomes trial (NCT01764633), is fully enrolled and will provide more detailed, prospectively collected information on the effects of evolocumab on neurocognitive function. In the ODYSSEY LONG TERM trial, neurocognitive disorders occurred in 1.2% and 0.5% of patients with alirocumab and placebo, respectively.Citation53
The magnitude of LDL-C lowering afforded by PCSK9 inhibition, particularly when added to existing lipid-lowering therapy, raises concerns about hypothetical risks associated with the attainment of very low LDL-C concentrations, including hemorrhagic stroke, vitamin E deficiency, and impaired steroid hormone synthesis. An analysis of 1,104 patients who entered the long-term extension trial (OSLER-1) after participation in Phase II studies found no increase in overall adverse events, serious adverse events, creatine kinase elevations, or liver enzyme elevations in patients who achieved LDL-C concentrations of <0.6 mmol/L (<25 mg/dL) or <1.3 mmol/L (<50 mg/dL) compared with those who experienced LDL-C ≥1.3 mmol/L (≥50 mg/dL).Citation69 A numerical increase in headache, insomnia, dizziness, and back pain occurred in patients with lower LDL-C levels. Preliminary data from an analysis of 2,836 patients enrolled in two evolocumab trials, DESCARTES and OSLER-1, demonstrated no increase in rates of adverse events and no cases of hemorrhagic stroke among patients who experienced levels of LDL-C <1.0 mmol/L (<40 mg/dL; n=1005) or <0.6 mmol/L (<25 mg/dL; n=644).Citation76 Because lipoproteins are involved in vitamin E transportCitation77 and cholesterol is required for steroidogenesis,Citation78 levels of these compounds in patients who experienced very low LDL-C levels are of interest. Data from the DESCARTES study were analyzed to answer this question.Citation79,Citation80 In all patients receiving evolocumab, absolute vitamin E levels decreased by a mean of 16%; however, cholesterol-normalized vitamin E levels increased by a mean of 19%. The pattern of vitamin E changes were consistent between patients with very low LDL-C concentrations (defined as <0.4 mmol/L [<15 mg/dL], <0.6 mmol/L [<25 mg/dL], and <1.0 mmol/L [<40 mg/dL]) and higher LDL-C concentrations (≥1.0 mmol/L [≥40 mg/dL]). An analysis of steroid hormones found no correlation between changes in cortisol, testosterone, or estradiol and the change in LDL-C from baseline among evolocumab-treated patients. Overall, these data suggest that the attainment of very low LDL-C concentrations with PCSK9 inhibition poses little risk to patients.
Cardiovascular outcomes
The ultimate value of PCSK9 inhibitors will be measured by their effect on clinical outcomes. Early evidence of cardiovascular benefit with evolocumab was shown in a prospective exploratory analysis of the OSLER-1 and OSLER-2 studies.Citation56 Similarly, post hoc analysis was conducted with alirocumab in the ODYSSEY LONG TERM trial.Citation53 Both analyses showed a reduction in major cardiovascular events with evolocumab and alirocumab added to standard therapy compared with standard therapy alone. These data should be interpreted with caution because event numbers are low, but they are reassuring and corroborate other trials demonstrating that additional lowering of LDL-C beyond that achieved with traditional medications results in improved cardiovascular outcomes.Citation9–Citation12 Large cardiovascular outcome trials are underway with evolocumab (FOURIER; NCT01764633), alirocumab (ODYSSEY OUTCOMES; NCT01663402), and bococizumab (SPIRE-1; NCT01975376 and SPIRE-2; NCT01975389).
Primary care practice considerations
For the primary care physician, PCSK9 inhibitors represent a valuable and efficacious addition to the treatment armamentarium for lowering LDL-C in patients who require further lipid lowering beyond statins or are unable to tolerate statins. The mechanism by which these agents lower LDL-C is well defined and supported by results observed in patients who harbor loss-of-function mutations in PCSK9.Citation25,Citation26 PCSK9 inhibitors lower LDL-C from 46% to 76% compared with placebo,Citation44,Citation46,Citation47,Citation51–Citation53 whereas ezetimibe produces an approximate 16%–18% further reduction,Citation49 making these monoclonal antibodies attractive for patients who require substantial additional LDL-C lowering beyond statin therapy. The use of these agents is warranted in patients with familial forms of hypercholesterolemia, and in patients with clinical atherosclerotic cardiovascular disease on maximally tolerated statins who require additional lowering of LDL-C.
Practical considerations play a role in patient acceptance and adherence to these agents. Evolocumab can be dosed Q2W or monthly. These prolonged dosing intervals lessen the burden on patients and may promote adherence. Data with evolocumab demonstrate that the two dosing frequencies (140 mg Q2W and 420 mg monthly) are clinically equivalent.Citation45–Citation48 Thus far, published alirocumab trials have utilized every 2-week dosing. Results with monthly dosing are available in preliminary form (NCT01926782, NCT02023879) and suggest that monthly dosing of alirocumab may also be possible.Citation81,Citation82 However, monthly dosing is currently only approved for evolocumab.Citation42,Citation43
PCSK9 monoclonal antibodies are self-administered subcutaneously, and willingness to self-inject or the availability of a caregiver for injections is necessary. Approximately three-quarters (74.1%) of eligible patients from evolocumab studies decided to enroll in the OSLER extension studies, with the majority of the remaining 25.9% of subjects who chose not to participate citing either personal reasons or the level of commitment required, indicating good patient acceptance of an injectable therapy.Citation56
These agents are expensive when compared with statins, and funding constraints may limit the number of patients who have access. Evolocumab and alirocumab should be prescribed to carefully selected patients (ie, those patients in whom the absolute risk reduction is largest with further LDL-C reduction) with adequate follow-up to ensure adherence. At present, no comparative data are available to guide clinicians in the choice between anti-PCSK9 monoclonal antibodies.
Conclusion
PCSK9 inhibition with monoclonal antibodies is a novel therapeutic approach for lowering LDL-C. These agents offer substantial LDL-C lowering in patients with familial forms of hypercholesterolemia and those with clinical atherosclerotic cardiovascular disease who are on maximally tolerated statins. Evolocumab and alirocumab are generally well tolerated and the prolonged (Q2W and monthly) dosing schedules may offer the benefit of high patient adherence. Results from cardiovascular outcome trials are eagerly awaited.
Acknowledgments
The authors thank Meera Kodukulla, PhD, CMPP of Amgen, Inc. (Thousand Oaks, CA, USA) and Laura Evans, PharmD, on behalf of Amgen Inc., for preliminary drafting of the manuscript.
Disclosure
Dr Dirk J Blom has received research grants and clinical trial payments from Amgen, Inc. (Thousand Oaks, CA, USA) and Sanofi-Aventis (Bridgewater, NJ, USA), and speaker’s honoraria from Amgen, Inc. Dr Dirk J Blom has served as an advisory board member for Amgen, Inc. and Sanofi-Aventis. Dr Peter P Toth is a member of the speaker’s bureau Amarin Corporation, (Dublin, Ireland), Kowa Pharmaceuticals America, Inc. (Montgomery, AL, USA), Merck & Co., Inc. (Whitehouse Station, NJ, USA), Sanofi-Aventis, and Novartis International AG (Basel, Switzerland), and a consultant Amgen, Inc., Kowa Pharmaceuticals America, Inc., Merck Co., Inc., Sanofi-Aventis, and Novartis International AG. Drs Ricardo Dent and Rita Castro are employees and stockholders of Amgen, Inc. The authors report no other conflicts of interest in this work.
References
- The lipid research clinics coronary primary prevention tria results. I. Reduction in incidence of coronary heart diseaseJAMA198425133513646361299
- The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol loweringJAMA198425133653746361300
- StamlerJWentworthDNeatonJDIs relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the multiple risk factor intervention trial (MRFIT)JAMA198625620282328283773199
- WilsonPWD’AgostinoRBLevyDBelangerAMSilbershatzHKannelWBPrediction of coronary heart disease using risk factor categoriesCirculation19989718183718479603539
- AndersonTJGregoireJHegeleRA2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adultCan J Cardiol201329215116723351925
- Expert DyslipidemiaPGrundySMAn International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemiaJ Clin Lipidol20137656156524314355
- PerkJDe BackerGGohlkeHEuropean guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts)Eur Heart J201233131635170122555213
- StoneNJRobinsonJGLichtensteinAH2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation201412925 suppl 2S1S4524222016
- CanonCPBlazingMAGiuglianoRPEzetimibe added to statin therapy after acute coronary syndromesN Engl J Med2015372252387239726039521
- BoekholdtSMHovinghGKMoraSVery low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trialsJ Am Coll Cardiol201464548549425082583
- Cholesterol Treatment Trialists’ (CTT) Collaboration; BaigentCBlackwellLEmbersonJEfficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trialsLancet201037697531670168121067804
- HsiaJMacFadyenJGMonyakJRidkerPMCardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin)J Am Coll Cardiol201157161666167521492764
- WiviottSDCannonCPMorrowDACan low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudyJ Am Coll Cardiol20054681411141616226163
- LaRosaJCGrundySMKasteleinJJTreating to New Targets (TNT) Steering Committee and InvestigatorsSteering Committee and Investigators. Safety and efficacy of atorvastatin-induced very low-density lipoprotein cholesterol levels in patients with coronary heart disease (a post hoc analysis of the treating to new targets [TNT] study)Am J Cardiol2007100574775217719314
- WatersDDBrotonsCChiangCWLipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goalsCirculation20091201283419546386
- BruckertEHayemGDejagerSYauCBegaudBMild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients – the PRIMO studyCardiovasc Drugs Ther200519640341416453090
- EllisJJEricksonSRStevensonJGBernsteinSJStilesRAFendrickAMSuboptimal statin adherence and discontinuation in primary and secondary prevention populationsJ Gen Intern Med200419663864515209602
- EricksonSRLinYNGeospatial analysis of statin adherence using pharmacy claims data in the state of MichiganJ Manag Care Spec Pharm201420121208121525443514
- AvornJMonetteJLacourAPersistence of use of lipid-lowering medications: a cross-national studyJAMA199827918145814629600480
- AbifadelMVarretMRabesJPMutations in PCSK9 cause autosomal dominant hypercholesterolemiaNat Genet200334215415612730697
- BrowningJDHortonJDFasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humansJ Lipid Res201051113359336320716520
- PerssonLCaoGStahleLCirculating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humansArterioscler Thromb Vasc Biol201030122666267220884874
- SeidahNGBenjannetSWickhamLThe secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiationProc Natl Acad Sci U S A2003100392893312552133
- AbifadelMRabesJPDevillersMMutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and diseaseHum Mutat200930452052919191301
- CohenJPertsemlidisAKotowskiIKGrahamRGarciaCKHobbsHHLow LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9Nat Genet200537216116515654334
- CohenJCBoerwinkleEMosleyTHJrHobbsHHSequence variations in PCSK9, low LDL, and protection against coronary heart diseaseN Engl J Med2006354121264127216554528
- LopezDPCSK9: an enigmatic proteaseBiochim Biophys Acta20081781418419118280815
- DubucGChamberlandAWassefHStatins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemiaArterioscler Thromb Vasc Biol20042481454145915178557
- DunnKWMcGrawTEMaxfieldFRIterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosomeJ Cell Biol19891096 pt 2330333142600137
- ZhangDWLagaceTAGarutiRBinding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradationJ Biol Chem200728225186021861217452316
- ChanJCPiperDECaoQA proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primatesProc Natl Acad Sci U S A2009106249820982519443683
- LiangHChaparro-RiggersJStropPProprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primatesJ Pharmacol Exp Ther2012340222823622019884
- MurphyKTraversPWalportMJaneway’s ImmunobiologySeventh edNew York, NYGarland Science, Taylor & Francis Group2008
- LoboEDHansenRJBalthasarJPAntibody pharmacokinetics and pharmacodynamicsJ Pharm Sci200493112645266815389672
- TabriziMATsengCMRoskosLKElimination mechanisms of therapeutic monoclonal antibodiesDrug Discov Today2006111–2818816478695
- WeinerLMFully human therapeutic monoclonal antibodiesJ Immunother20062911916365595
- YangXDJiaXCCorvalanJRWangPDavisCGDevelopment of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapyCrit Rev Oncol Hematol2001381172311255078
- HwangWYFooteJImmunogenicity of engineered antibodiesMethods200536131015848070
- LonbergNHuman antibodies from transgenic animalsNat Biotechnol20052391117112516151405
- LonbergNFully human antibodies from transgenic mouse and phage display platformsCurr Opin Immunol200820445045918606226
- McKenneyJMKorenMJKereiakesDJHanotinCFerrandACSteinEASafety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapyJ Am Coll Cardiol201259252344235322463922
- PRALUENT (alirocumab) [package insert]Bridgewater, NJSanofi-Aventis U.S. LLC2015
- REPATHA (evolocumab) [package insert]Thousand Oaks, CAAmgen, Inc2015
- BlomDJHalaTBologneseMA 52-week placebo-controlled trial of evolocumab in hyperlipidemiaN Engl J Med2014370191809181924678979
- KorenMJLundqvistPBologneseMAnti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumabJ Am Coll Cardiol201463232531254024691094
- RaalFJSteinEADufourRPCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trialLancet2015385996533134025282519
- RobinsonJGNedergaardBSRogersWJEffect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trialJAMA2014311181870188224825642
- StroesEColquhounDSullivanDAnti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumabJ Am Coll Cardiol201463232541254824694531
- BaysHGaudetDWeissRAlirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trialJ Clin Endocrinol Metab201510083140314826030325
- CannonCPCariouBBlomDEfficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trialEur Heart J201536191186119425687353
- KasteleinJJGinsbergHNLangsletGODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemiaEur Heart J201536432996300326330422
- KereiakesDJRobinsonJGCannonCPEfficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I studyAm Heart J20151696906915e91326027630
- RobinsonJGFarnierMKrempfMEfficacy and safety of alirocumab in reducing lipids and cardiovascular eventsN Engl J Med2015372161489149925773378
- RothEMTaskinenMRGinsbergHNMonotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trialInt J Cardiol20141761556125037695
- MoriartyPMThompsonPDCannonCPEfficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trialJ Clin Lipidol20159675876926687696
- SabatineMSGiuglianoRPWiviottSDEfficacy and safety of evolocumab in reducing lipids and cardiovascular eventsN Engl J Med2015372161500150925773607
- Emerging Risk FactorsCErqouSKaptogeSLipoprotein(a) concentration and the risk of coronary heart disease, stroke, and non-vascular mortalityJAMA2009302441242319622820
- NaveAHLangeKSLeonardsCOLipoprotein (a) as a risk factor for ischemic stroke: a meta-analysisAtherosclerosis2015242249650326298741
- HaffnerSOrchardTSteinESchmidtDLaBellePEffect of simvastatin on Lp(a) concentrationsClin Cardiol19951852612677628131
- RaalFJGiuglianoRPSabatineMSReduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trialsJ Am Coll Cardiol201463131278128824509273
- GoldsteinJLHobbsHHBrownMSFamilial hypercholesterolemiaScriverCRBeaudetALSlyWSValleDThe Metabolic and Molecular Bases of Inherited Disease8th edNew York, NYMcGraw-Hill Inc200128632913
- MaraisADRaalFJSteinEAA dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemiaAtherosclerosis2008197140040617727860
- RaalFJPilcherGJIllingworthDRExpanded-dose simvastatin is effective in homozygous familial hypercholesterolaemiaAtherosclerosis199713522492569430375
- RaalFJHonarpourNBlomDJInhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA part B): a randomised, double-blind, placebo-controlled trialLancet2015385996534135025282520
- ArmitageJThe safety of statins in clinical practiceLancet200737096011781179017559928
- GangaHVSlimHBThompsonPDA systematic review of statin-induced muscle problems in clinical trialsAm Heart J2014168161524952854
- NewmanCBTobertJAStatin intolerance: reconciling clinical trials and clinical experienceJAMA2015313101011101225756433
- RosensonRSBakerSKJacobsonTAKopeckySLParkerBAThe National Lipid Association’s Muscle Safety Expert P. An assessment by the statin muscle safety task force: 2014 updateJ Clin Lipidol201483 supplS58S7124793443
- KorenMJGiuglianoRPRaalFJEfficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the open-label study of long-term evaluation against LDL-C (OSLER) randomized trialCirculation2014129223424324255061
- Rojas-FernandezCHGoldsteinLBLeveyAITaylorBABittnerVThe National Lipid Association’s Safety Task F. An assessment by the statin cognitive safety task force: 2014 updateJ Clin Lipidol201483 supplS5S1624793442
- SattarNPreissDMurrayHMStatins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trialsLancet2010375971673574220167359
- StromBLSchinnarRKarlawishJHennessySTealVBilkerWBStatin therapy and risk of acute memory impairmentJAMA Intern Med201517581399140526054031
- SimicIReinerZAdverse effects of statins – myths and realityCurr Pharm Des20152191220122625312733
- KraftRKahnAMedina-FrancoJLA cell-based fascin bioassay identifies compounds with potential anti-metastasis or cognition-enhancing functionsDis Model Mech20136121723522917928
- BjorkhemIMeaneySBrain cholesterol: long secret life behind a barrierArterioscler Thromb Vasc Biol200424580681514764421
- KorenMJBlomDGiuglianoRPSafety and tolerability of very low LDL-C levels in patients treated with 52 weeks of evolocumab (AMG 145)Circulation201413016865
- HacquebardMCarpentierYAVitamin E: absorption, plasma transport and cell uptakeCurr Opin Clin Nutr Metab Care20058213313815716790
- HuJZhangZShenWJAzharSCellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormonesNutr Metab (Lond)201074720515451
- BlomDJDjedjosCSMonsalvoMLEffects of evolocumab on vitamin E and steroid hormone levels. Results from the 52-week, phase 3, double-blind, randomized, placebo-controlled DESCARTES studyCirc Res201511773174126228031
- QamarABhattDLEffect of low cholesterol on steroid hormones and vitamin E levels: just a theory or real concern?Circ Res2015117866266426405182
- StroesESGGuytonJFarnierMEfficacy and safety of different dosing regimens of alirocumab (starting doses of 75 mg every two weeks and 150 mg every four weeks) versus placebo in patients with hypercholesterolemia not treated using statins: the ODYSSEY CHOICE II studyJ Am Coll Cardiol20156510S1370
- RothEMRaderDJMoriartyPMA randomized phase 3 trial evaluating alirocumab every four weeks dosing as add-on to statin or as monotherapy: ODYSSEY CHOICE IPresented at: ISA 2015, the 17th International Symposium on AtherosclerosisMay 23–26, 2015Amsterdam2016 Available from: http://www.athero.org/isa2015/ClinicalBreak/Roth.pdfAccessed January 26, 2016
