Abstract
Matrix metalloproteinases (MMPs) are zinc- and calcium-dependent endoproteinases that have the ability to break down extracellular matrix. The large range of MMPs’ functions widens their spectrum of potential role as activators or inhibitors in tissue remodeling, cardiovascular diseases, and obesity. In particular, MMP-1, -2, and -9 may be associated with exercise and obesity. Thus, the current study reviewed the effects of different types of exercise (resistance and aerobic) on MMP-1, -2, and -9. Previous studies report that the response of MMP-2 and -9 to resistance exercise is dependent upon the length of exercise training, since long-term resistance exercise training increased both MMP-2 and -9, whereas acute bout of resistance exercise decreased these MMPs. Aerobic exercise produces an inconsistent result on MMPs, although some studies showed a decrease in MMP-1. Obesity is related to a relatively lower level of MMP-9, indicating that an exercise-induced increase in MMP-9 may positively influence obesity. A comprehensive understanding of the relationship between exercise, obesity, and MMPs does not exist yet. Future studies examining the acute and chronic responses of these MMPs using different subject models may provide a better understanding of the molecular mechanisms that are associated with exercise, obesity, and cardiovascular disease.
Introduction
The property of matrix metalloproteinases
Matrix metalloproteinases (MMPs) were first observed in 1962 by Jerome Gross and Charles Lapiere in tadpole tissue that exhibited collagenolytic activity.Citation1 Eisen et alCitation2 were able to isolate human MMPs 6 years after its first discovery. MMPs are zinc-and calcium-dependent endoproteinases that play a crucial role in the remodeling of extracellular matrix (ECM) by breaking down its protein components.Citation3 MMPs can be categorized, on the basis of substrate specificity and homology, into the following six family groups: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs (; ). All MMPs share common domain structures that degrade various ECM and nonmatrix.Citation4,Citation5 Specific propeptide and catalytic domains exist (ie, MMP-7 and -26) along with a hemopexin-like, four-bladed, β-propeller domain located on the C-terminus, which is connected to a linker or hinge region (MMP-1, -3, -8, -11, -12, -13, -18, -19, -20, -21, -27, and -28).Citation6 These are the domains and regions that are involved in substrate recognition and inhibitor binding.Citation7–Citation9 MMP-2 and -9 also have a fibronectin-like domain of three type II repeats.Citation6
Figure 1 General structure of MMP groups with SP, propeptide, catalytic, and hemopexin domains.
Abbreviations: CS, cysteine switch; FN, fibronectin; GPI, glycosylphosphatidylinositol; MMP, matrix metalloproteinase; MT-MMP, membrane type-matrix metalloproteinase; SP, signal peptide; TM, transmembrane.
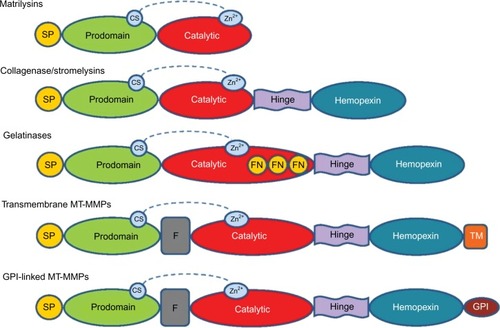
Table 1 Classification of MMPs
Activation of MMPs
There are three general activation mechanisms of MMPs, including the pro-MMP cleavage, phosphorylation, and oxidative stressors. The cleavage of pro-MMP is known as “cysteine switch” that exists in all known MMPs.Citation10 The cleavage targets an intramolecular complex between a single cysteine residue in the prodomain and the zinc ion in the catalytic domain (). This cleavage of MMPs is a required activation before any other forms of catalytic activation may occur. MMPs can be activated by phosphorylation. One study examined ubiquitous MMP-2 in human connective tissue and found that five of 29 potential phosphorylation sites are targets for protein kinase C to alter MMP-2 activity. Enzymatic property changes were confirmed through zymograph, gelatin dequenching assays, and analysis of kinetic parameters.Citation11 MMPs can also be activated by oxidative stressors such as homocysteine (Hcy), nitric oxide (NO), and hydrogen sulfide (H2S). Hcy is a metabolite of the amino acids cysteine and methionine that activates MMPs by the extracellular signal-regulated kinase pathway.Citation12,Citation13 In mice, high levels of Hcy showed increased aortic MMP-2 and -9 along with increased aortic blood pressure, resistance, pulse rate, wall thickness, and extracellular collagen accumulation.Citation14 On the other hand, H2S, a metabolite of Hcy, has been clinically used to treat atherosclerosis, since it deactivates MMP-9 and activates MMP-2 in heart tissue. Thus, H2S may promote angiogenesis and inhibit antiangiogenic factors.Citation15 H2S is an important factor for an anaerobic pathway conversion process of Hcy, and plays a major role in vasodilation and antioxidant health by normalizing the levels of redox stress and MMPs, as seen in vascular remodeling of damaged carotid artery.Citation16 Injected H2S into the carotid artery showed increased levels of MMP-9 and decreased levels of MMP-2.Citation17 One study reported that H2S-knockout mice showed more outgrowth in the vessels, as compared to donor H2S that increased the levels of neointima formation in the carotid artery. This result supports evidence on how H2S can be seen as a clinical route to treat atherosclerosis.Citation17 Although NO plays an important role in MMP regulation, its exact mechanism has not been completely understood. Moreover, the results of previous studies investigating the MMP regulation by NO or NO-dependent pathways are equivocal. One study reported that NO inhibited MMP-9 expression in activated astrocytes,Citation18 while another study showed that NO inhibitor rather decreased MMP-9 in rat cardiac allografts.Citation19 The three MMP regulators mentioned above can give more insight on how MMP levels can be regulated in ways that can benefit the human body against diseases. However, more knowledge of the exact mechanisms related to each regulator is necessary for a full understanding of the MMP processes.
Inhibition of MMPs
Two types of MMP inhibitors exist: endogenous and exogenous inhibitors. Tissue inhibitors of metalloproteinase (TIMPs) are endogenous inhibitors that can be secreted (TIMP-1, TIMP-2, TIMP-4) or bound to ECM components (TIMP-3).Citation20 They inactivate MMPs by forming bonds with catalytic zinc in 1:1 ratios within the MMP structure.Citation21 They do so by creating noncovalent interactions between the N-terminal domain of the TIMP and the active site of MMPs. Recent studies have shown TIMPs’ therapeutic potential in cardiovascular diseases (CVDs). The MMP-inhibitory effects of TIMP-1 involve binding of C-terminal domain to pro-MMP-2 and pro-MMP-9. TIMP-1 may specifically help ECM manipulation in ischemia in such a way that it may be a surrogate marker for increased ECM turnover.Citation22 TIMP-2 reverses ECM remodeling in human cardiac tissue in a dose-dependent manner.Citation23 TIMP-3 prevented degradation of cardiac tissue matrix after a myocardial infarction by reducing MMPs in vascular smooth muscle cells.Citation24 Changes in the ECM of atrial fibroblast in rheumatic heart disease have been associated with TIMP-4 expression.Citation25
Exogenous inhibitors include hydroxamic acid derivative and thiirane gelatinase inhibitor SB-3CT on several MMPs.Citation26–Citation29 Batimastat (BB-94), a hydroxamic acid derivative, has been recently found to treat aneurysms on using nanoparticle technology to directly administer anti-MMP target to abdominal aorta aneurysm.Citation30 In rat aorta cell culture, administration of BB-94 decreased 90% of MMP-9 activity and 10% of MMP-2 activity, while showing no effect on TIMP-2 activity. The effect of change in MMP activity was tested by injecting BB-94 directly into the abdominal aorta. The blank control showed a 269% increase in the aneurysm, while the treatment group showed only a 40% increase in expansion. Another derivative, marimastat, has shown success as a therapeutic drug in repressing non-small-cell lung cancer in a Phase I trial.Citation31 Oral administration of marimastat was added to the accepted treatment with carboplatin and paclitaxel in order to test whether or not marimastat would affect the kinetics of the treatment. TIMPs and hydroxamic acid derivatives play significant roles in general physiology and pathology, and thus can develop as a therapeutic target in the future.
The roles of MMPs in CVD and obesity
Breakdown of ECM by MMPs includes physiological processes such as embryonic development, reproduction, and tissue remodeling, as well as disease processes. MMPs are involved in the remodeling process of cell membrane and in cell behaviors like proliferation, migration, and apoptosis.Citation32 CVD is the number one leading cause of death in the world and includes any disease associated with the heart and blood vessels, such as myocardial infarction, heart failure, atherosclerosis, stroke, aortic aneurysms, and so on.Citation33 MMPs are expressed and activated in many different types of CVDs including atherosclerosis, myocardial infarction, and cardiac dysfunction.Citation34,Citation35 In particular, MMP-2 and -9 degrade a major element in the basement membrane, collagen IV, which helps in cellular arrangement of skeletal muscle.Citation36 MMPs also play a role in disease diagnoses. For instance, MMP-2 and -9 are found to be independent predictors for kidney disease progression and its associated mortality.Citation37 Obesity is strongly associated with CVDs and occurs when pre-existing, fully differentiated adipocytes are enlarged by excess energy input and accumulate to a point where the pathological expansion becomes a concern.Citation38 Higher levels of MMPs are associated with obesity and CVDs. For instance, MMP-1, -2, -3, -7, -9, -10, -11, and -12 are found at higher levels in atherosclerotic arteries.Citation39 Among these MMPs, the current review focused on MMP-1, -2, and -9 due to their strong association with obesity and CVDs.
MMP-1
MMP-1 may be involved in plaque burden, although plaque morphology was not tested.Citation40 A strong correlation between MMP-1 mRNA and lupus erythematic atherosclerosis has been reported.Citation41 Elevated MMP-1 was also associated with myocardial infarction and angiographic coronary artery disease, although the mechanistic pathway was not examined.Citation42 One study promoted inflammation and atherosclerosis using C-reactive protein to examine the effects of MMP-1 in human mammary arteries and carotid arteries. Both MMP-1 and its mRNA expression had increased significantly, suggesting an association with inflammation and plaque vulnerability.Citation43 Higher levels of MMP-1 have also been observed in several types of human carotid atherosclerosis, and also, histological associations with plaque instability have been found.Citation44 However, controversial results were obtained on the association of MMP-1 with obesity, since the expression of MMP-1 may be different between obese and nonobese people. According to a recent study, MMP-1 may stimulate tissue remodeling during adipose tissue expansion in obesity. Certain MMP-1 alleles showed increased frequency with high body mass index, potentially suggesting a defensive role.Citation45 In contrast, MMP-1 was reported to have a strong association with nonobese individuals.Citation46 In spite of the contradictory effect of MMP-1 on obesity, the majority of evidence leans toward MMP-1 increasing with obesity.
MMP-2
MMP-2 has been reported to promote atherosclerosis. Elevated MMP-2 may negatively affect vascular permeability and play an important role in the progression of heart failure.Citation47 One study reported that MMP-2 knockout mice showed decrease in atherogenesis.Citation48 Increased duration of ischemia and delayed functional recovery have been linked to elevated MMP-2 levels,Citation49 while decreased MMP-2 levels provided protection from cardiac dysfunction.Citation50 The role of MMP-2 in voluntary exercise and its effects on infarct size have been investigated in rats.Citation51 A 6-week voluntary wheel-running exercise, where the rats self-selected the time, duration, and intensity in a nonstressful environment, was used as the exercise protocol. Induced ischemia by left anterior descending coronary artery occlusion ex vivo and angina provoked by epinephrine plus phentolamine protocols were used on the rats. Serum MMP-2, coronary effluent MMP-2 activity, and infarct size all showed a significant decrease after exercise. These results show that MMP-2 may be soon viewed as a cardioprotective molecule for myocardial infarctions and perfusions.
MMP-9
It has been reported that MMP-9 may be involved in elastase action related to aortic stiffening and development of isolated systolic hypertension in healthy and younger individuals.Citation52 However, MMP-9 may attenuate atherosclerotic development and prevent plaque development, since MMP-9 knockout mice showed plaque development.Citation53 Both MMP-2 and -9 have been linked to increased inflammation under high coronary risk events and high plaque instability.Citation54,Citation55 An early study showed that genetically obese rats had high levels of MMP-2 and low levels of MMP-9,Citation56 suggesting that MMP-2 may be involved in adipose ECM degradation. In addition, high-fat diet-induced obese (HFDIO) mice had lower MMP-9 mRNA, and an antigrowth myostatin (MSTN), which is known to be suppressed in HFDIO condition, showed resistance to HFDIO.Citation57 This indicates that decreased levels of MMP-2 and increased MMP-9 levels can prevent or regulate obesity development.
The effects of exercise on MMPs
It is evident that exercise favorably affects CVDs and obesity. According to the recent studies examining the effects of exercise on the regulation of MMPs, the responses of MMPs to exercise may be more dependent upon the mode and length of exercise performed in a variety of subject models (). Thus, understanding the relationship between MMPs and exercise is particularly important in obese population, since obesity is strongly associated with CVDs and other types of metabolic diseases.Citation58 In the current review, we examined how different types of exercise (resistance and aerobic) influence MMP-1, -2, and -9.
Table 2 Effects of different types of exercise on MMPs
Resistance training and MMPs
Based on the previously published studies, the responses of MMPs to resistance training are more likely related to duration of exercise training. Resistance training lasting from 5 to 12 weeks may increase MMP-2 and -9 in both animal and human subjects,Citation59–Citation62 whereas acute bout of resistance training may decrease these MMPs.Citation63 In an animal study, HFDIO rats that performed resistance training had significantly increased MMP-2 levels in bicep and gastrocnemius muscles. A comparable difference in MMP-2 levels between obese and nonobese rats indicated that it was related to the levels of obesity and also suggested that this exercise-induced increase in MMP-2 in rats may prevent obesity. In this regard, MMP-2 could potentially be a negative regulator of obesity.Citation62 In another study, rats fed with high-fat diet performed a 12-week strength training program that consisted of vertical ladder exercise three times a week with weights attached to their tails. Following 12 weeks of training, rats showed increase in muscle MMP-2, suggesting that high levels of MMP-2 may be inversely related to obesity.Citation59 One study examined the effects of oxidative stress on MMP-2 and -9 in skeletal muscle using the exercise protocol composed of leg presses for 45 minutes, four times a week for 5 weeks. The levels of MMP-2 increased by day 10, and MMP-2 mRNA in myofibril increased with training as well. The responses of MMP-9 and its mRNA activity at day 10 were relatively lower as compared to those of MMP-2, but held the same level of activity after the first exercise.Citation60 One study examining the relationship between diabetes and MMPs reported that patients with type 2 diabetes who performed a rowing exercise at 65%–70% of VO2 (volume of oxygen intake) peak significantly increased MMP-2 mRNA in skeletal muscle, suggesting that exercise-induced changes in MMP-2 may benefit type 2 diabetes.Citation61 In contrast, obese elderly women who performed acute eccentric resistance training (ten repetitions at 110% of ten-repetition maximum) had decreased levels of both MMP-2 and -9 in the plasma after 48 hours of the training session. The authors concluded that this exercise-induced reduction in MMP-2 and -9 may be a positive sign in the transient defense of inflammatory MMPs associated with obesity and atherosclerosis.Citation63
Aerobic exercise and MMPs
The impact of aerobic exercise training on MMPs may be related to duration of exercise. In general, the long-term aerobic exercise training lasting up to 12 weeks may decrease both MMP-2 and -9,Citation51,Citation64,Citation65 while these MMPs increase following acute bouts of exercise.Citation66–Citation68 Moreover, only limited information regarding the effects of aerobic exercise on MMP-1 is currently available. MMP-1 in serum and heart tissue decreased following aerobic exercise training (up to 12 weeks) in female subjects with metabolic syndromeCitation69 and in aged mice.Citation64 MMP-9 and its mRNA activity in skeletal muscle significantly increased after a 65-minute aerobic cycling exercise, while MMP-2 mRNA did not change.Citation68 In an animal study, apoE−/− mice were given a Western diet to develop plaque while participating in a 30-minute treadmill exercise program (5 days/week) for 10 weeks. Exercise intervention did not attenuate aortic plaque, but MMP-2 and -9 significantly decreased.Citation65 Another study examining the effects of voluntary wheel-running exercise for 6 weeks reported a reduction in serum MMP-2 as part of a cardio-protective mechanism against cardiac injury.Citation51 Aged rats that performed a 45-minute aerobic exercise on a treadmill for up to 12 weeks (5 days/week) showed decrease in both MMP-1 and -2 in the heart tissue, while MMP-9 was not altered.Citation64 To examine the influence of exercise with various durations and resting periods, Wistar rats were separated into several groups that performed either 1, 3, or 6 days of exercise training (three sessions per day) with an 1-, 3-, or 6-hour resting period between each session. No significant difference was seen in either protein MMP-2 or -9 in the calcaneal tendon in any of the groups, except the group that performed the 6-day with 3-hour rest exercise protocol which showed an increase in MMP-2.Citation66 One study examined the responses of MMP-2 and -9 levels of hippocampi samples in rats following treadmill exercise. The rats exercised at moderate intensity on the treadmill for 30 minutes for 7 days, and hippocampi samples were analyzed by gel zymography to examine the changes in the proteolytic activity of MMP-2 and -9 at 0, 6, 12, and 24 hours postexercise. Results showed that MMP-2 did not change significantly, whereas the 12-hour samples exhibited a significant increase in MMP-9.Citation67
Conclusion
The previous studies we reviewed show that the response of MMPs following resistance exercise is more related to the length of resistance exercise training. In general, long-term resistance exercise training may increase both MMP-2 and -9, while acute bouts of resistance exercise may decrease these MMPs. Furthermore, aerobic exercise training leads to an inconsistent result in MMP-2 and -9, although some studies showed a reduction in MMP-1. Also, a relatively lower level of MMP-9 has been observed in obese subjects, indicating that an exercise-induced increase in MMP-9 may play a positive role in obesity.
Elevated MMP-1 has been shown to be strongly associated with CVDs and obesity. Thus, a treatment targeting on lowering MMP-1 may benefit the most in patients with CVDs or obesity. In this regard, an exercise intervention should include a long-term aerobic training as it can reduce the MMP-1 levels and prevent obesity. Reducing MMP-2 would improve prognosis of CVDs, and long-term aerobic training would help reduce MMP-2 levels as well. However, the potential drawback of long-term aerobic training would be decreased level of MMP-9, which may hinder some beneficial effects of exercise on obesity and plaque development. Therefore, it is important to balance aerobic exercise with long-term resistance training, which may increase MMP-9, thereby offering the most positive effects of exercise training on improvement in MMPs. Future studies examining the effects of different types of aerobic and resistance exercise training or a combination of these two forms of training would give more insight on how exercise influences MMPs. Also, exercise intensity may play a role in changes in MMPs, although it has not been fully examined. Therefore, findings on how different types, intensities, and duration of exercises and when to measure MMPs will open a new spectrum of molecular mechanism that is mostly unknown. Finding which MMPs are associated with obesity, exercise, and CVDs is the first step toward understanding the mechanism of this potentially powerful group of proteins. More knowledge of MMPs could lead to more effective treatment or approach to weight loss or new drugs that safely help patients with obesity, CVDs, or other types of diseases. Not all major MMPs have been fully tested with different types and duration of exercise regimens. This calls for research on different MMPs in exercise to get a better understanding on how the human body can naturally manipulate MMP metabolism to benefit the body as a whole.
Disclosure
The authors report no conflicts of interest in this work.
References
- GrossJLapiereCMCollagenolytic activity in amphibian tissues: a tissue culture assayProc Natl Acad Sci U S A1962481014102213902219
- EisenAZJeffreyJJGrossJHuman skin collagenase. Isolation and mechanism of attack on the collagen moleculeBiochim Biophys Acta196815136376454967132
- RenaudSLeppertDMatrix metalloproteinases in neuromuscular diseaseMuscle Nerve200736111317410592
- NagaseHWoessnerJFJrMatrix metalloproteinasesJ Biol Chem199927431214912149410419448
- PuenteXSSanchezLMOverallCMLopez-OtinCHuman and mouse proteases: a comparative genomic approachNat Rev Genet20034754455812838346
- SekhonBMatrix metalloproteinase – an overviewRes Rep Biol20101120
- ChaudharyAKSinghMBhartiACAsotraKSundaramSMehrotraRGenetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neckJ Biomed Sci2010171020152059
- KadoglouNPLiapisCDMatrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysmsCurr Med Res Opin200420441943215119978
- ChoCBunchDOFaureJEFertilization defects in sperm from mice lacking fertilin betaScience19982815384185718599743500
- Van WartHEBirkedal-HansenHThe cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene familyProc Natl Acad Sci U S A19908714557855822164689
- SariahmetogluMCrawfordBDLeonHRegulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylationFASEB J200721102486249517435175
- MoshalKSSenUTyagiNRegulation of homocysteine-induced MMP-9 by ERK1/2 pathwayAm J Physiol Cell Physiol20062903C88389116251475
- BescondAAugierTChareyreCGarconDHornebeckWCharpiotPInfluence of homocysteine on matrix metalloproteinase-2: activation and activityBiochem Biophys Res Commun1999263249850310491321
- OvechkinAVTyagiNSenU3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic miceAm J Physiol Lung Cell Mol Physiol20062915L90591116815886
- GivvimaniSMunjalCGargoumRHydrogen sulfide mitigates transition from compensatory hypertrophy to heart failureJ Appl Physiol (1985)201111041093110021233344
- VacekTPGillespieWTyagiNVacekJCTyagiSCHydrogen sulfide protects against vascular remodeling from endothelial damageAmino Acids20103951161116920352463
- YangGLiHTangGIncreased neointimal formation in cystathionine gammalyase deficient mice: role of hydrogen sulfide in alpha5beta1-integrin and matrix metalloproteinase-2 expression in smooth muscle cellsJ Mol Cell Cardiol201252367768822200376
- ShinCYLeeWJChoiJWDown-regulation of matrix metallo-proteinase-9 expression by nitric oxide in lipopolysaccharide-stimulated rat primary astrocytesNitric Oxide200716442543217452115
- EgiKConradNEKwanJSchulzeCSchulzRWildhirtSMInhibition of inducible nitric oxide synthase and superoxide production reduces matrix metalloproteinase-9 activity and restores coronary vasomotor function in rat cardiac allograftsEur J Cardiothorac Surg200426226226915296881
- Melendez-ZajglaJDel PozoLCeballosGMaldonadoVTissue inhibitor of metalloproteinases-4. The road less traveledMol Cancer200878519025595
- BrinckerhoffCEMatrisianLMMatrix metalloproteinases: a tail of a frog that became a princeNat Rev Mol Cell Biol20023320721411994741
- DinhWFuthRScheffoldTIncreased serum levels of tissue inhibitor of metalloproteinase-1 in patients with acute myocardial infarctionInt Heart J200950442143119609047
- NguJMTengGMeijndertHCHuman cardiac fibroblast extracellular matrix remodeling: dual effects of tissue inhibitor of metal-loproteinase-2Cardiovasc Pathol201423633534325060386
- JiaZBTianHKangKExpression of the tissue inhibitor of metalloproteinase-3 by transplanted VSMCs modifies heart structure and function after myocardial infarctionTranspl Immunol201430414915824727088
- SunYHuangZYWangZHTGF-beta1 and TIMP-4 regulate atrial fibrosis in atrial fibrillation secondary to rheumatic heart diseaseMol Cell Biochem20154061–213113825971370
- KrugerASoeltlRSopovIHydroxamate-type matrix metallo-proteinase inhibitor batimastat promotes liver metastasisCancer Res20016141272127511245418
- KohnoTHochigaiHYamashitaETsukiharaTKanaokaMCrystal structures of the catalytic domain of human stromelysin-1 (MMP-3) and collagenase-3 (MMP-13) with a hydroxamic acid inhibitor SM-25453Biochem Biophys Res Commun2006344131532216603129
- SkarjaGABrownALHoRKMayMHSeftonMVThe effect of a hydroxamic acid-containing polymer on active matrix metalloproteinasesBiomaterials200930101890189719147221
- GuZCuiJBrownSA highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemiaJ Neurosci200525276401640816000631
- NosoudiNNahar-GohadPSinhaAPrevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metallo-proteinase activity with batimastat-loaded nanoparticlesCirc Res201511711e808926443597
- GoffinJRAndersonICSupkoJGPhase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancerClin Cancer Res20051193417342415867243
- MurphyGNagaseHLocalizing matrix metalloproteinase activities in the pericellular environmentFEBS J2011278121521087456
- MozaffarianDBenjaminEJGoASAmerican Heart Association Statistics Committee and Stroke Statistics SubcommitteeHeart disease and stroke statistics – 2015 update: a report from the American Heart AssociationCirculation20151314e2932225520374
- GalisZSSukhovaGKLarkMWLibbyPIncreased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaquesJ Clin Invest1994946249325037989608
- ThorpEBContrasting inflammation resolution during atherosclerosis and post myocardial infarction at the level of monocyte/macrophage phagocytic clearanceFront Immunol201233922566922
- HoppsECaimiGMatrix metalloproteinases in metabolic syndromeEur J Intern Med20122329910422284236
- HsuTWKuoKLHungSCHuangPHChenJWTarngDCProgression of kidney disease in non-diabetic patients with coronary artery disease: predictive role of circulating matrix metalloproteinase-2, -3, and -9PLoS One201387e7013223922934
- SpaldingKLArnerEWestermarkPODynamics of fat cell turnover in humansNature2008453719678378718454136
- BackMKetelhuthDFAgewallSMatrix metalloproteinases in atherothrombosisProg Cardiovasc Dis201052541042820226959
- LehrkeMGreifMBroedlUCMMP-1 serum levels predict coronary atherosclerosis in humansCardiovasc Diabetol200985019751510
- ZhangHYBaoSMShouWLExpression of matrix metallo-proteinase-1 mRNA in peripheral blood mononuclear cells of systemic lupus erythematosus patients and its relationship with atherosclerosisChin Med J (Engl)2009122212593259719951575
- HorneBDCampNJCarlquistJFMultiple-polymorphism associations of 7 matrix metalloproteinase and tissue inhibitor metallo-proteinase genes with myocardial infarction and angiographic coronary artery diseaseAm Heart J2007154475175817893005
- MonteroIOrbeJVaroNC-reactive protein induces matrix metalloproteinase-1 and -10 in human endothelial cells: implications for clinical and subclinical atherosclerosisJ Am Coll Cardiol20064771369137816580524
- NikkariSTO’BrienKDFergusonMInterstitial collagenase (MMP-1) expression in human carotid atherosclerosisCirculation1995926139313987664418
- NhoYKHaEYuKIMatrix metalloproteinase-1 promoter is associated with body mass index in Korean population with aged greater or equal to 50 yearsClin Chim Acta20083961–2141718602909
- HuangHLWuSHsuLAGenetic variants associated with circulating MMP1 levels near matrix metalloproteinase genes on chromosome 11q21-22 in Taiwanese: interaction with obesityBMC Med Genet2013143023497408
- GielenSSchulerGAdamsVCardiovascular effects of exercise training: molecular mechanismsCirculation2010122121221123820855669
- KuzuyaMNakamuraKSasakiTChengXWItoharaSIguchiAEffect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient miceArterioscler Thromb Vasc Biol20062651120112516556856
- KandasamyADChowAKAliMASchulzRMatrix metallopro-teinase-2 and myocardial oxidative stress injury: beyond the matrixCardiovasc Res201085341342319656780
- Fert-BoberJLeonHSawickaJInhibiting matrix metallopro-teinase-2 reduces protein release into coronary effluent from isolated rat hearts during ischemia-reperfusionBasic Res Cardiol2008103543144318512095
- PosaASzaboRKupaiKCardioprotective effects of voluntary exercise in a rat model: role of matrix metalloproteinase-2Oxid Med Cell Longev2015201587680525874025
- YasminMcEnieryCMWallaceSMatrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffnessArterioscler Thromb Vasc Biol200525237215556929
- JohnsonJLGeorgeSJNewbyACJacksonCLDivergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteriesProc Natl Acad Sci U S A200510243155751558016221765
- Nascimento DdaCDurigan RdeCTibanaRADuriganJLNavaltaJWPrestesJThe response of matrix metalloproteinase-9 and -2 to exerciseSports Med201545226927825252612
- HeoSHChoCHKimHOPlaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9J Clin Neurol201172697621779294
- ChaveyCMariBMonthouelMNMatrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiationJ Biol Chem200327814118881189612529376
- BigaPRFroehlichJMGreenleeKJGaltNJMeyerBMChristensenDJGelatinases impart susceptibility to high-fat diet-induced obesity in miceJ Nutr Biochem20132481462146823465590
- LavieCJMilaniRVVenturaHOObesity and cardiovascular disease: risk factor, paradox, and impact of weight lossJ Am Coll Cardiol200953211925193219460605
- LeiteRDDurigan RdeCde Souza LinoADResistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet ratsMetabolism201362101477148423790633
- RullmanENorrbomJStrombergAEndurance exercise activates matrix metalloproteinases in human skeletal muscleJ Appl Physiol (1985)2009106380481219131480
- Scheede-BergdahlCBergdahlASchjerlingPQvortrupKKoskinenSODelaFExercise-induced regulation of matrix metalloproteinases in the skeletal muscle of subjects with type 2 diabetesDiab Vasc Dis Res201411532433424903024
- SouzaMVLeiteRDSouza LinoADResistance training improves body composition and increases matrix metalloproteinase 2 activity in biceps and gastrocnemius muscles of diet-induced obese ratsClinics (Sao Paulo)201469426527024714835
- NascimentoDDNavaltaJWDuriganJLAcute eccentric resistance exercise decreases matrix metalloproteinase activity in obese elderly womenClin Physiol Funct Imaging201636039145
- KwakHBKimJHJoshiKYehAMartinezDALawlerJMExercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heartFASEB J20112531106111721148111
- ShonSMParkJHNahrendorfMExercise attenuates matrix metalloproteinase activity in preexisting atherosclerotic plaqueAtherosclerosis20112161677321334624
- De AroAAFerrucciDLBorgesFPStach-MachadoDRMacedoDVPimentelERExhaustive exercise with different rest periods changes the collagen content and MMP-2 activation on the calcaneal tendonAnat Rec (Hoboken)2014297228128824376193
- NishijimaTKawakamiMKitaIA bout of treadmill exercise increases matrix metalloproteinase-9 activity in the rat hippocampusNeurosci Lett201559414414925841788
- RullmanERundqvistHWagsaterDA single bout of exercise activates matrix metalloproteinase in human skeletal muscleJ Appl Physiol (1985)200710262346235117255365
- DonleyDAFournierSBRegerBLAerobic exercise training reduces arterial stiffness in metabolic syndromeJ Appl Physiol (1985)2014116111396140424744384
