Abstract
Purpose
The purpose of this study was to clarify the difference between effects of nitroglycerin (NTG) on the functional stiffness in patients with and without coronary artery disease (CAD) using a newly developed stiffness index, cardio-ankle vascular index (CAVI).
Subjects and methods
The two subject groups in this study were normal controls (n=31) and CAD patients (n=25). The normal controls had no medical history and were not on regular medications. On the other hand, the CAD patients had received various treatments like antihypertensive drugs, hypoglycemic agents, and statins. This study was conducted in CAD patients under medications. After a single sublingual administration of NTG 0.3 mg, CAVI, blood pressure (BP), and heart rate (HR) were measured every 5 minutes for 20 minutes. Comparisons of each parameter before and after taking NTG were evaluated for statistical significance using analysis of variance and post hoc tests. Tukey–Kramer test was used for post hoc comparisons.
Results
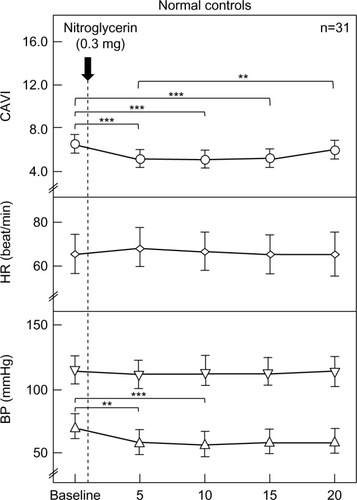
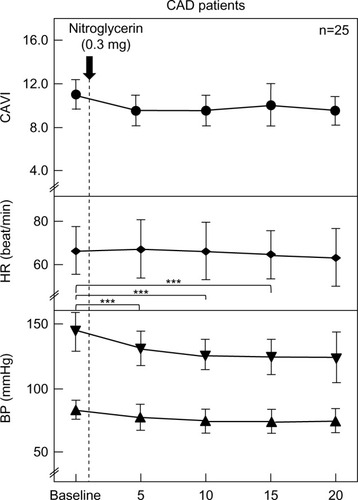
In the normal controls, CAVI significantly decreased from baseline after 5, 10, and 15 minutes (from 6.5±0.9 to 5.2±0.9, 5.5±0.9, and 5.7±0.9, respectively). Systolic BP and HR were not significantly changed. Diastolic BP significantly decreased from baseline after 5 and 10 minutes (from 72±8 to 64±9 and 63±9 mmHg, respectively). On the other hand, CAVI, HR, and diastolic BP were not changed significantly in CAD patients. Systolic BP was significantly decreased from baseline after 5, 10, and 15 minutes (from 147±16 to 131±14, 129±12, and 129±13 mmHg, respectively). In the comparison of the two groups, ΔCAVI was not significantly different between the normal controls and CAD patients (−1.4±0.7 vs −1.4±0.9, −1.1±0.7 vs −1.4±1.0, −0.8±0.7 vs −1.2±1.0, and −0.5±0.7 vs −1.1±1.0 at 5, 10, 15, and 20 minutes, respectively). ΔHR was not significantly different between the two groups. ΔSystolic BP in the CAD patients was significantly higher than in the normal controls at 5, 10, 15, and 20 minutes (normal controls vs CAD; −3±7 vs −10±11, −3±5 vs −10±11, −3±6 vs −13±10, and −1±6 vs −11±10 mmHg, respectively). ΔDiastolic BP in the normal controls was significantly higher than in the CAD patients at 5 and 10 minutes (normal controls vs CAD; −8±6 vs −4±4 and −9±4 vs −6±5 mmHg, respectively).
Conclusion
After NTG administration, the stiffness of the arteries from the origin of the aorta to the ankle as measured by CAVI decreased in both the normal controls and CAD patients, indicating that the response of arterial smooth muscle cells to nitric oxide is preserved even in CAD patients under medication.
Introduction
Arterial stiffness affects the homeostasis of a living body, in which the vasodilation effect of nitric oxide (NO) plays a vital role. The salutary effects of nitroglycerin (NTG) in patients with angina pectoris are thought to be the result of the widespread action of the drug as a smooth muscle vasodilator.Citation1 This vasodilation effect operates in the coronary artery and in systemic veins to induce pooling. It has been suggested that NTG may increase distensibility of peripheral muscular arteries and reduce systemic resistance; this action would explain the manner by which NTG reduces myocardial oxygen need and relieves the symptoms.Citation1–Citation4 However, the effects of NTG on the arterial stiffness of the arteries from the origin of the aorta to the ankle have not been quantitatively evaluated. As a method for quantitative assessment of arterial stiffness, pulse wave velocity (PWV) has been used for the last several decades and it was thought to be a kind of surrogate marker of arteriosclerosis;Citation5–Citation9 however, PWV depends inherently on blood pressure (BP) changes at the time of measurement.Citation10 Therefore, there had been difficulties in interpreting the data dealing with various therapies or conditions associated with BP changes.Citation11–Citation16
In 2006, the cardio-ankle vascular index (CAVI) was developed as an arterial stiffness index, which was derived from the stiffness parameter beta theory with the application of the Bramwell–Hill equation.Citation5,Citation6,Citation17 It is essentially independent from BP at the time of measurement.Citation18,Citation19 CAVI increases with aging,Citation20 and it is enhanced in arteriosclerotic patients with coronary artery disease (CAD),Citation21–Citation23 cerebral infarction,Citation24,Citation25 and chronic kidney diseases.Citation26 CAVI is also enhanced in patients with coronary risk factors such as hypertension, diabetes mellitus, smoking, and sleep apnea syndrome; it can be decreased by the treatment of these risks.Citation27–Citation35 Thus, CAVI has become established as a marker of arteriosclerosis. The stiffness shown by CAVI is thought to comprise organic stiffness and functional stiffness.Citation36 Organic stiffness is mainly concerned with collagen, elastin, and hyaluronic acid, whereas functional stiffness is mainly smooth muscle cell contraction.Citation37 Functional stiffness has been demonstrated using the α1-adrenoceptor blocker doxazosin, which dilates peripheral arteries and decreases BP. When doxazosin was administered to men, BP as well as CAVI decreased, indicating that CAVI was reflecting the degree of smooth muscle contraction of the arteries.Citation19 Chiba et al reported that NTG decreased CAVI in rabbits.Citation38 They inserted a catheter in healthy rabbits’ arteries directly and developed the system that measured CAVI every 1 minute. Intravenous infusion of NTG significantly decreased CAVI, which returned to the predrug control values immediately after stopping the infusion.
The purpose of this study was to clarify the difference between effects of NTG on the functional stiffness in patients with and without CAD using the newly developed stiffness index CAVI.
Methods
Subjects
The normal controls were 31 healthy volunteers (males 21; females 10) aged 24–50 years (mean ± standard deviation [SD], 31.1±1.3 years), who came to our institute responding to our public announcement. They had no medical history and were not on regular medications. The CAD subjects were 25 patients (males 20; females 5) aged 60–84 years (mean ± SD, 73.0±5.9 years). The CAD patients comprised 19 with angina pectoris and six with old myocardial infarction; 17 patients underwent percutaneous coronary intervention, and eight patients had coronary artery bypass grafting. Blood sampling was performed after fasting for 12 hours. The details of baseline clinical characteristics are shown in .
Table 1 Characteristics of the subjects
Each participant gave written informed consent after receiving a detailed description of the procedures in accordance with the Declaration of Helsinki; the study was reviewed and approved by the Ethics Committee of Toho University.
Experimental methods and design
The CAD patients continued taking their daily medications during the course of the study. The study was conducted in a quiet and temperature-controlled room (25°C–26°C). The subjects were studied at approximately the same time of day after a standardized light meal and were asked to avoid caffeine or tobacco on the day of the study. After resting for 10 minutes in supine position on a bed, CAVI was measured at baseline. Next, a tablet of NTG (0.3 mg) was administered sublingually and CAVI was measured every 5 minutes for 20 minutes using a Vasela 1500 (Fukuda Denshi Co., Ltd, Tokyo, Japan). Simultaneously, BP and heart rate (HR) were measured using Vasela 1500.
Statistical analysis
Data were expressed as mean ± SD. Comparisons of each parameter before and after taking NTG were evaluated for statistical significance by analysis of variance and post hoc tests. Tukey–Kramer test was used for post hoc comparisons. To analyze the difference between the two groups, Student’s unpaired t-test was used. P-value <0.05 was considered sig nificant. All statistical analysis was double-sided. Statistical analysis was performed using SPSS software package (SPSS Inc., Chicago, IL, USA).
Results
The clinical background of the normal controls and CAD patients is listed in . Age, body mass index, systolic BP, diastolic BP, and CAVI were significantly higher in the CAD patients than in the normal controls (P<0.001). In addition, the CAD patients had higher glycated hemoglobin and triglyceride concentration, and lower high-density lipoprotein cholesterol level (P<0.001). In these patients, 88% received treatment with statins; thus, low-density lipoprotein cholesterol level was not significantly different between the two groups. Creatinine was significantly higher in the CAD patients (P<0.05). The CAD patients underwent polysomnography for sleep apnea syndrome screening as risk assessment; 48% had sleep apnea syndrome with apnea hypopnea index ≥20, and 44% were receiving treatment with continuous positive airway pressure.
The changes in CAVI, HR, and systolic/diastolic BP in the normal controls due to the administration NTG 0.3 mg are shown in . CAVI significantly decreased from baseline after 5, 10, and 15 minutes (from 6.5±0.9 to 5.2±0.9, 5.5±0.9, and 5.7±0.9, respectively). Systolic BP and HR were not significantly changed. Diastolic BP significantly decreased from baseline after 5 and 10 minutes (from 72±8 to 64±9 and 63±9 mmHg, respectively).
Figure 1 Changes in CAVI, HR, and BP after sublingual administration of nitroglycerin in normal controls (n=31).
Abbreviations: BP, blood pressure; CAVI, cardio-ankle vascular index, HR, heart rate.
The changes in each parameter in the CAD patients due to the administration of NTG 0.3 mg are shown in . CAVI, HR, and diastolic BP were not changed significantly. Systolic BP was significantly decreased from baseline after 5, 10, and 15 minutes (from 147±16 to 131±14, 129±12, and 129±13 mmHg, respectively).
Figure 2 Changes in CAVI, HR, and BP after sublingual administration of nitroglycerin 0.3 mg in CAD patents (n=25).
Abbreviations: BP, blood pressure; CAD, coronary artery disease; CAVI, cardio- ankle vascular index; HR, heart rate.
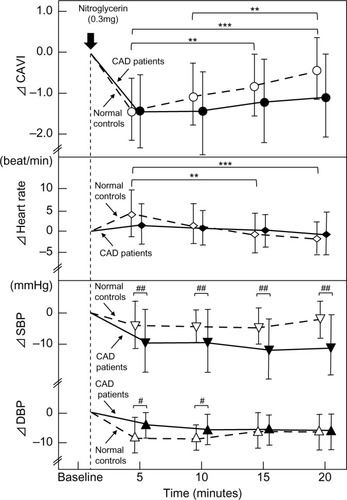
The comparison of the difference (Δ) in each parameter between the two groups is shown in . In the comparison of the two groups, ΔCAVI was not significantly different between the normal controls and CAD patients (−1.4±0.7 vs −1.4±0.9, −1.1±0.7 vs −1.4±1.0, −0.8±0.7 vs −1.2±1.0, and −0.5±0.7 vs −1.1±1.0 at 5, 10, 15, and 20 minutes, respectively). ΔHR was not significantly different between the two groups. ΔSystolic BP in the CAD patients was significantly higher than in the normal controls at 5, 10, 15, and 20 minutes (normal controls vs CAD; −3±7 vs −10±11, −3±5 vs −10±11, −3±6 vs −13±10, and −1±6 vs −11±10, respectively). ΔDiastolic BP in the normal controls was significantly higher than in the normal controls at 5 and 10 minutes (normal controls vs CAD: −8±6 vs −4±4 and −9±4 vs −6±5, respectively).
Figure 3 Comparison of ΔCAVI, Δheart rate, and Δblood pressure between normal controls and CAD patients during NTG administration.
Abbreviations: CAD, coronary artery disease; CAVI, cardio-ankle vascular index; DBP, diastolic blood pressure; NTG, nitroglycerin; SBP, systolic blood pressure; SD, standard deviation.
Discussion
To date, several reports have discussed the effect of NTG on arterial stiffness using PWV.Citation39,Citation40 However, as shown in the current study, BP decreased after NTG administration. Thus, even when PWV decreased, it cannot be concluded that NTG actually decreased arterial stiffness because PWV decreases when BP decreases.Citation19 On the other hand, CAVI is independent of BP at the time of measurement.Citation18,Citation19 Taken together, the effects of the drugs that affect BP on proper arterial stiffness can be evaluated using CAVI. In this study, CAVI decreased in both the healthy young adults and CAD patients after NTG administration as shown in and , indicating that NTG decreased proper arterial stiffness of the artery from the origin of the aorta to the ankle. This is the first report to demonstrate the effect of NTG on proper arterial stiffness of the artery in vivo.
As to the mechanism of NTG, several hypotheses have been raised in the last 30 years. Generally, NTG dilates the coronary arteryCitation4 and increases coronary blood flow.Citation41 However, Ogilvie reported that NTG has been demonstrated to possess unique vascular actions that dilate systemic venous vessels more potently than arterial vessels, leading to a reduction in cardiac output caused by the decreased venous return.Citation42 The decreased CAVI in our study might indicate that NTG dilated the arterial tree composing the aorta, the so-called elastic artery, and the femoral and tibial arteries, the so-called muscular arteries. This dilation might facilitate decreasing afterload to the heart. Thus, NTG might decrease both the preload and afterload to the heart, and also relieve the stress to the heart muscles. It is also suggested that CAVI may be a useful index to at least partially express the afterload. This leads to the question of which artery might best respond to NTG, the aorta as an elastic artery or the femoral and tibial arteries as muscular arteries. Further precise studies are now under investigation.
In the comparison between the healthy volunteers and CAD patients, after NTG administration, ΔCAVI was not significantly different between the two groups.
Previously, there were some reports showing that vasodilator response to NO is maintained in CAD patients. Testa et al reported that vascular responses to NTG in patients with abnormal and normal findings on coronary angiograms were similar using blood flow velocity in the superficial femoral artery.Citation43 Thanyasiri et al reported as to whether femoral vascular reactivity and/or fibrinolytic capacity are impaired in acute coronary syndrome patients over and above any dysfunction associated with stable CAD.Citation44 They found that there was a significant impairment in large arterial endothelium-dependent dilatation in response to acetylcholine in both stable and acute coronary syndrome patients compared with control patients, whereas there was no significant difference in the response to NTG between the groups. These results indicate that NTG administration decreased the stiffness of the arteries from the origin of the aorta to the ankle in both the healthy volunteers and CAD patients to the same extent, which means that the response of arterial smooth muscle cells to NO was preserved even in CAD patients.
As shown in , the decrease in systolic BP after the administration of NTG in the CAD patients was higher than that in the normal control group, while the decrease in diastolic BP in CAD patients was smaller than that in the normal control group. Arterial stiffness has a close relationship with diastolic BP. Moreover, arterial wall stiffness is a major determinant of left ventricular afterload.Citation45 In CAD patients, arteriosclerotic states become worse with atherosclerotic changes compared to healthy volunteers. Diastolic BP might be difficult to decrease after the administration of NTG.
During the progression of arteriosclerosis, one of the major vascular qualitative changes is the calcification of arteries. Smooth muscle cells are even present in an advanced arteriosclerotic artery. Those smooth muscle cells wererelaxed or became contractual functionally. It is difficult to distinguish this organic state and functional state strictly. But, we might be able to guess the organic stiffness as the baseline of CAVI, and functional stiffness as the rapid changed CAVI by NTG.
Study limitations
The CAD patients were examined under the controlled state of their various coronary risk factors. The fact that we cannot remove the effects of their medications is the limitation of our study.
Conclusion
After NTG administration, the stiffness of the arteries from the origin of the aorta to the ankle as measured by CAVI decreased in both the healthy volunteers and CAD patients at nearly the same extent, suggesting that the response of the arterial smooth muscle cells to NO is preserved even in CAD patients under medications.
Acknowledgments
The Department of Vascular Function was funded by Fukuda Denshi Co., Ltd.
Disclosure
Yamamoto T. is employed by Fukuda Denshi Co., Ltd., and was involved in the development of Vasera measuring CAVI. Shirai K. is a visiting professor of the Department of Vascular Function in Toho University, is supported by Fukuda Denshi Co., Ltd., but has no patent and no financial profit. The authors report no further conflicts of interest in this work.
References
- SmulyanHMookherjeeSWarnerRAThe effect of nitroglycerin on forearm arterial distensibilityCirculation198673126412693084126
- IgnarroLJLipptonHEdwardsJCMechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprus-side and nitric oxide: evidence for the involvement of S-nitroso-thiols as active intermediatesJ Pharmacol Exp Ther19812187397496115052
- McGregorMNitrates and myocardial ischemiaCirculation1982666896926749328
- FeldmanRLPepineCJContiCRMagnitude of dilatation of large and small coronary arteries of nitroglycerinCirculation19816423243336788401
- BramwellJCHillAVThe velocity of the pulse wave in manProc R Soc Lond B192293652298306
- BramwellJCHillAVVelocity of transmission of the pulse and elasticity of arteriesLancet19221891892
- HiraiTSasayamaSKawasakiTYagiSStiffness of systemic arteries in patients with myocardial infarctionCirculation198980178862610739
- AsmarRBenetosATopouchianJAssessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studiesHypertension19952634854907649586
- BlacherJAsmarRDjaneSLondonGMSafarMEAortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patientsHypertension19993351111111710334796
- AvolioAPChenSGWangRPZhangCLLiMFO’RourkeMFEffects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban communityCirculation198368150586851054
- SchulzETsilimingasNRinzeRFunctional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatmentCirculation2002105101170117511889009
- FokHJiangBClappBChowienczykPRegulation of vascular tone and pulse wave velocity in human muscular conduit arteries: selective effects of nitric oxide donors to dilate muscular arteries relative to resistance vesselsHypertension20126051220122523045465
- StewartADMillasseauSCKearneyMTRitterJMChowienczykPJEffects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humansHypertension200342591591812975386
- KinlaySCreagerMAFukumotoMEndothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivoHypertension20013851049105311711496
- KellyRPGibbsHHO’RourkeMFNitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral arteryEur Heart J19901121381442107077
- ImaizumiTTakeshitaAAshiharaTNakamuraMThe effects of sublingually administered nitroglycerin on forearm vascular resistance in patients with heart failure and in normal subjectsCirculation19857247477523928194
- HayashiKHandaHNagasawaSOkumuraAMoritakeKStiffness and elastic behavior of human intracranial and extracranial arteriesJ Biomech19801321751847364778
- ShiraiKUtinoJOtsukaKTakataMA novel blood pressure-independent arterial wall stiffness parameter: cardio-ankle vascular index (CAVI)J Atheroscler Thromb200613210110716733298
- ShiraiKSongMSuzukiJContradictory effects of β1- and α1-adrenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI): CAVI is independent of blood pressureJ Atheroscler Thromb2011181495521071883
- NamekataTSuzukiKIshizukaNShiraiKEstablishing baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional studyBMC Cardiovasc Disord2011115121831311
- NakamuraKTomaruTYamamuraSMiyashitaYShiraiKNoikeHCardio-ankle vascular index is a candidate predictor of coronary atherosclerosisCirc J200872459860418362432
- YingchoncharoenTLimpijankitTJongjirasiriSLaothamatasJYamwongSSritaraPArterial stiffness contributes to coronary artery disease risk prediction beyond the traditional risk score (RAMA-EGAT score)Heart Asia201241778223585778
- IzuharaMShiojiKKadotaSRelationship of cardio- vascular index (CAVI) to carotid and coronary arteriosclerosisCirc J200872111762176718802315
- SuzukiJSakakibaraRTomaruTStroke and cardio-ankle vascular stiffness indexJ Stroke Cerebrovasc Dis201122217117521855368
- ChoiSYParkHESeoHKimMChoSHOhBHArterial stiffness using cardio-ankle vascular index reflects cerebral small vessel disease in healthy young and middle aged subjectsJ Atheroscler Thromb201320217818523131963
- NakamuraKIizukaTTakahashiMAssociation between cardio-ankle vascular index and serum cystatin C levels in patients with cardiovascular risk factorJ Atheroscler Thromb200916437137919672028
- OkuraTWatanabeSKurataMRelationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertensionHypertens Res200730433534017541212
- IbataJSasakiHKakimotoTCardio-ankle vascular index measures arterial wall stiffness independent of blood pressureDiabetes Res Clin Pract200880226527018242761
- NagayamaDSaikiAEndoKImprovement of cardio-ankle vascular index by glimepiride in type 2 diabetic patientsInt J Clin Pract201064131796180120946343
- KubozonoTMiyataMUeyamaKAcute and chronic effects of smoking on arterial stiffnessCirc J201175369870221187657
- NoikeHNakamuraKSugiyamaYChanges in cardio-ankle vascular index in smoking cessationJ Atheroscler Thromb201017551752520215706
- KumagaiTKasaiTKatoMEstablishment of the cardio-ankle vascular index in patients with obstructive sleep apneaChest2009136377978619567490
- KatoMKumagaiTNaitoRChange in cardio-ankle vascular index by long-term continuous positive airway pressure therapy for obstructive sleep apneaJ Cardiol2011581748221620678
- KasaiTInoueKKumagaiTPlasma pentraxin3 and arterial stiffness in men with obstructive sleep apneaAm J Hypertens201124440140721193850
- NagayamaDEndoKOhiraMEffects of body weight reduction on cardio-ankle vascular index (CAVI)Obes Res Clin Pract201372e139e14524331775
- HayashiKYamamotoTTakaharaAShiraiKClinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applicationsJ Hypertens2015331742175726114836
- ZiemanSJMelenovskyVKassDAMechanisms, pathophysiology, and therapy of arterial stiffnessArterioscler Thromb Vasc Biol200525593294315731494
- ChibaTYamanakaMTakagiSCardio-ankle vascular index (CAVI) differentiates pharmacological properties of vasodilators nicardipine and nitroglycerin in anesthetized rabbitsJ Pharmacol Sci2015128418519226238254
- WilkinsonIBQasemAMcEnieryCMWebbDJAvolioAPCockcroftJRNitric oxide regulates local arterial distensibility in vivoCirculation200210521321711790703
- BankAJKaiserDRRajalaSChengAIn vivo human brachial artery elastic mechanics: effects of smooth muscle relaxationCirculation19991001414710393679
- BrachfeldNBozerJGorinRAction of nitroglycerin on the coronary circulation in normal and in mild cardiac subjectsCirculation195919569770413652362
- OgilvieRIEffect of nitroglycerin on peripheral blood flow distribution and venous returnJ Pharmacol Exp Ther19782072372380101653
- TestaMBiasucciLMCacciolaMVariable response of the peripheral circulation to acetylcholine in patients with coronary artery diseaseAm J Cardiol1996772149l538546082
- ThanyasiriPCelermajerDSAdamsMREndothelial dysfunction occurs in peripheral circulation patients with acute and stable coronary artery diseaseAm J Physiol Heart Circ Physiol20052892H513H51716014611
- SchillaciGBattistaFSettimiLAnastasioFPucciGCardio-ankle vascular index and subclinical heart diseaseHypertens Res2015381687325231254
