Abstract
Due to safety concerns in recent years, much effort has been devoted to improving the outcomes associated with drug-eluting stents (DESs). This review summarizes the current status of methodological and technical achievements reported in second-generation DES. Novel stents are described based on the component (the platform, the polymer, and the drug) that has undergone the most significant changes compared to earlier generation DES. An overview of the currently available evidence on the use of novel coronary devices in patients undergoing coronary revascularization is also reviewed.
Introduction
The introduction of first-generation drug-eluting stents (DESs) in the setting of percutaneous coronary intervention (PCI) has led to a significant decrease in the need for repeat revascularization, a common limitation associated with the use of bare-metal stents (BMS). In-stent restenosis, the result of a maladaptive neointimal tissue proliferation, is dramatically reduced by the long-lasting inhibitory effect exerted by the local elution of antiproliferative agents.Citation1–Citation3
First-generation DES commonly consist of three elements: an antiproliferative drug, a durable polymer that serves for drug loading and modification of release kinetics, and the stent platform (). The first generation of DES employs a cobalt–chromium alloy, a durable polymer, and elutes sirolimus (Cypher; Cordis, Warren, NJ) or paclitaxel (TAXUS™; Boston Scientific, Natick, MA). Sirolimus- and paclitaxel-eluting stents seem to provide similar rates of revascularization, although several studies report a more profound inhibition of neointimal hyperplasia by sirolimus.Citation4–Citation6 While other first-generation DES have been produced,Citation7 the second-generation DES now include the zotarolimus- (Endeavor; Medtronic, Minneapolis, MN) and the everolimus- (Xience V; Abbott Vascular, Redwood City, CA) eluting stents.Citation8,Citation9
Despite the higher efficacy compared to BMS, concerns remain regarding the long-term safety of DES, including localized hypersensitivity and late stent thrombosis.Citation10–Citation12 Although the exact mechanisms of these pathological reactions have not yet been fully elucidated, they seem to be most likely associated with the presence of durable polymers, representing a trigger for inflammation and subsequent impaired re-endothelialization.Citation13 In previous years, several efforts have been devoted by researchers to address these limitations of DES. In particular, attention has been focused on possible advancements of platform, carrier, and pharmacological technologies, and many innovations have been provided aiming at improving biocompatibility and long-term outcomes of patients undergoing PCI.
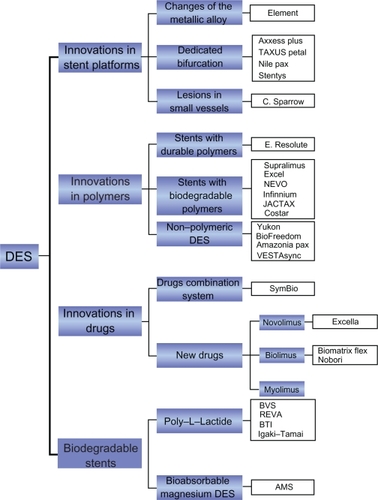
This review summarizes the current status of methodological and technical achievements reported in second-generation DES, giving an account of the most recent clinical data and the latest improvements in treatment of de novo coronary lesions. For the purpose of the following discussion, novel stents will be described based on the component (the platform, the polymer, and the drug) that has undergone the most significant changes compared to earlier generation DES (). We searched Medline to identify studies which assessed second-generation DES, using search terms such as ‘novel stents’, ‘second-generation drug eluting stent’, ‘stent polymer’, and ‘biodegradable stents’. In addition, we identified relevant abstracts and presentations at the annual meetings of the American Heart Association, the American College of Cardiology, the European Society of Cardiology, the European Association of Percutaneous Coronary Intervention, Transcatheter Cardiovascular Therapeutics, and Transcatheter Cardiovascular Therapeutics Asia Pacific from July to September 2010. Expert slide presentations were consulted online from tctmd.com to complete data from abstracts. Internet-based sources of information on the results for clinical trials in cardiology (see http://www.theheart.org and http://www.tctmd.com) were also searched.
Innovations in stent platforms
Recent innovations in stent platforms include changes of the metallic alloy (ie, shifting from cobalt–chromium or stainless steel to platinum–chromium platforms) or development of dedicated stent platforms for the treatment of specific subsets of lesions (ie, bifurcations and small-vessel lesions).
Changes of the metallic alloy
Element™ Stent Platform
The Element Stent Platform (Boston Scientific) is made up of a platinum–chromium alloy. The platinum–chromium platform has a new stent architecture with a thin strut thickness (81 μm), which features high radiopacity, high radial strength, and conformability. The Element Stent Series includes an everolimus-eluting (PROMUS Element™; Boston Scientific) and a paclitaxel-eluting stent (TAXUS™ Element; Boston Scientific). Both stents have received the Conformité Européenne (CE) Mark.
The safety and effectiveness of the PROMUS Element stent for the treatment of de novo atherosclerotic coronary lesions are the object of the ongoing prospective, randomized, multicenter PLATINUM (Clinical Trial to Assess the PROMUS Element Stent System for Treatment of De Novo Coronary Artery Lesions) trial (Clinicaltrials.gov identifier NCT00823212). In the prospective, randomized PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) Workhorse trial (NCT00484315), the TAXUS Element has proven to be noninferior to the first-generation TAXUS Express with regard to the incidence of 12-month target lesion failure (TLF) (5.6% vs 6.1% for TAXUS Element and TAXUS Express, respectively, P = 0.78) for the treatment of de novo atherosclerotic lesions of up to 28 mm in length in native coronary arteries of 2.75–4.0 mm diameter. In addition, there were no differences in terms of in-stent late loss between the two stents (0.34 ± 0.55 vs 0.26 ± 0.52 mm, P = 0.33) at 9-month follow-up.Citation14 Conversely, the TAXUS PERSEUS Small Vessel trial (NCT00489541) is a prospective, multicenter, single-arm superiority trial, currently ongoing, which aims to assess the safety and efficacy of the TAXUS Element stent for the treatment of de novo atherosclerotic lesions ≤20 mm in length in native coronary arteries of ≥2.25 to <2.75 mm diameter.
Dedicated bifurcation stents
The dedicated stents have been developed with the goal of addressing some of the most common issues associated with PCI of a bifurcation lesion, including difficulties in maintaining side-branch access throughout the procedure, main vessel stent struts jailing the side-branch ostium, distortion of main vessel stent after side-branch dilatation, and failure to cover and scaffold the side-branch ostium.Citation15 First-generation bifurcation-dedicated BMS include the Multi-Link Frontier™ (Guidant Corp, Santa Clara, CA),Citation16 the SLK-View™ (Advanced Stent Technologies, Pleasanton, CA),Citation17 the Petal™ (Advanced Stent Technologies),Citation18 the Sideguard™ (Cappella Inc, Galway, Ireland),Citation19,Citation20 the Twin-Rail™ (Invatec Srl, Roncadelle, Italy),Citation21 the Nile Croco™ (Minvasys, Genevilliers, France),Citation22 the Tryton™ (Tryton Medical Inc, Durham, NC),Citation23 the Sidekick™ (Y-med Inc, San Diego, CA),Citation24 and the Antares SAS™ (TriReme Medical Inc, Pleasanton, CA).Citation25 These stents lack a drug-eluting coating and are associated with a high rate of restenosis ranging between 28% and 54%.Citation26 A new generation of bifurcation-dedicated DES is currently under clinical investigation and will be discussed in the following paragraphs.
Axxess Plus™
The Axxess (Devax Inc, Irvine, CA) stent is a self-expanding nickel–titanium alloy stent featuring a conical configuration designed to conform with the bifurcation anatomy, which provides easy access to the distal branches for easy placement of subsequent stents (). The stent is coated with a resorbable polymer which elutes Biolimus A9. The Axxess Plus registry reported low in-stent late loss (0.09 ±0.56 mm) and restenosis (4.8%) with the Axxess stent at 6 months follow-up.Citation27 The DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) study (n = 302) showed encouragingly low major adverse cardiac events (MACEs) (7.7%) and target lesion revascularization (TLR) rates (4.3%) at 9 months follow-up.Citation28 The randomized COBRA (COmplex BifuRcation Lesions: A Comparison Between the AXXESS Device and Culotte Stenting: An Optical Coherence Tomography) study (NCT00895791) will enroll 40 patients and assess vessel healing at 9 months by optical coherence tomography (OCT) in bifurcation lesions treated using the Axxess stent or the culotte technique. This stent has received the CE Mark in July 2010.
TAXUS Petal™ stent
The TAXUS Petal (Boston Scientific) dedicated stent is the improved version of the AST Petal stent, acquired by Boston Scientific in 2004. Its platinum–chromium platform is stronger and more radiopaque than a stainless steel platform with four radiopaque markers for good visualization on angiography and includes a main branch section with a side-branch opening to provide mechanical scaffolding and drug delivery to the side-branch ostium. The TAXUS Petal stent is coated with the same poly(styrene-b-isobutylene-b-styrene) polymer as the TAXUS Express and the TAXUS Libertè stents and releases paclitaxel in the same manner. The first-in-man study of the TAXUS Petal stent reported a composite rate of all death, myocardial infarction (MI), or target vessel revascu-larization (TVR) of 3.7% and 14.8% at 30-day and 12-month follow-up, respectively, 6-month in-segment late loss of 0.47 ± 0.45 and 0.41 ± 0.57 mm for the proximal and distal main branch, respectively, and 0.18 ± 0.39 mm for the side branch.Citation29
Nile Pax™
The Nile Pax (Minvasys) dedicated stent is a chromium–cobalt stent, with a 73-μm strut thickness and a modular design, developed using the Pax technology, previously utilized in the Amazonia Pax stent. Similar to this latter stent, the Nile Pax is characterized by a polymer-free paclitaxel abluminal coating to avoid long-term lack of endothelization and to deliver the drug exclusively onto the arterial wall. Paclitaxel is applied using a microdrop spray crystallization process. The stent has a dedicated bifurcation delivery system made of specific balloon catheters.Citation30 The safety and efficacy of this novel stent is currently evaluated in the prospective, nonrandomized, single-arm multicenter BIPAX (dedicated bifurcation with the PAX technology) trial on 102 patients. Preliminary results have demonstrated good results for the treatment of de novo bifurcation lesions including high acute success (99%), neither cardiac death nor stent thrombosis at 30 days clinical follow-up and absence of thrombotic events after hospital discharge.Citation31
STENTYS™
The STENTYS (Stentys S.A.S., Paris, France) dedicated stent is a provisional, self-expanding nitinol stent developed both as a DES and as a BMS. The main peculiarity of this stent is the possibility of creating a side-branch access anywhere in the stent after implantation in the main vessel. The side-branch opening is created by inflating the angioplasty balloon into the mesh, thus, disconnecting the interconnections. The drug-eluting form elutes paclitaxel (dose: 0.8 μg/mm2) which is loaded in a blend of a durable polymer of polysulfone and polyvinyl-pyrrolidone. In the first-in-man trial of 40 patients, the stent showed a 95.5% procedural success rate, which is defined as technical and angiographic success in the absence of any MACE at hospital discharge. Disconnection of the strut to create the side-branch opening was successfully achieved in 95% of patients. After stent implantation in the main branch, stenting the side branch was necessary in one-third of the cases.Citation32 In the prospective, nonrandomized, multicenter APPOSITION I study (n = 25), the in-stent late loss was 0.71 ± 0.70 mm and the rate of angiographic rest-enosis (>50%) was 25% at 6 months; no cases of death, stent thrombosis, or MI were noted at the same follow-up.Citation33 In the randomized, multicenter APPOSITION II study (NCT01008085), 80 patients with ST-elevation myocardial infarction (STEMI) were randomized to the STENTYS stent versus a conventional balloon-expandable stent and followed up with OCT for 3 days. Preliminary data reported a significant reduction in stent strut malapposition with the STENTYS stent (0.51% vs 5.33%, P <0.001).Citation34 This stent has received the CE Mark in May 2010.
Lesions in small vessels
A small vessel diameter is still an independent predictor of angiographic and clinical restenosis. DES have been employed for the treatment of lesions located in the small vessels, but their impact on clinical and angiographic outcomes is not yet well established. It can be expected that DES implantation could bring a reduction in restenosis and the need for repeat revascularization, as shown in several studies, but large, randomized studies are necessary to elucidate the safety and efficacy of both traditional and next-generation DES in this scenario.Citation35,Citation36
Cardiomind Sparrow™
The Cardiomind Sparrow (Biosensors, Morges, Switzerland) stent is a self-expanding nitinol stent loaded into a 0.014″-guidewire platform, which is developed to treat lesions in small vessels. It has a closed cell design and an ultrathin strut (0.0024″). The stent is deployed through a proprietary mechanism, which enables the electrolysis of mechanical latches at each end of the device. This process is initiated and controlled by electrical energy delivered from a handheld battery.Citation37
Two versions of the Cardiomind Sparrow stent have been developed. The first version was a BMS assessed in the prospective, multicenter, feasibility CARE I study that enrolled 22 patients and reported a rate of MACE of 9.5% at 24-month follow-up and an in-stent late lumen loss of 0.73 ±0.57 mm at 6-month follow-up.Citation38 The second version is a DES with a strut thickness of 67 μm (compared to 140 μm of Cypher stent and 132 μm of TAXUS stent) in which the anti-proliferative agent sirolimus is combined in the SynBiosys™ polylactic acid (PLA) biodegradable copolymer matrix. The dose of the drug is ∼6 μg/mm. This second-generation stent was compared to the BMS version tested in the CARE I study and traditional BMS in the prospective, multicenter, multinational three-arm randomized CARE-II study. The trial showed an in-stent late lumen loss of 0.29 ± 0.45 mm in the sirolimus-eluting stent arm versus 0.86 ± 0.54 of bare metal Cardiomind and 0.99 ± 0.45 mm of BMS at 8-month follow-up; the rates of binary restenosis were 6.7%, 45.2%, and 44.0%, respectively, and the rates of cumulative MACE were 6.25%, 8.6%, and 16.7% at the same follow-up.Citation39,Citation40
Conclusions
The innovations in stent platform discussed in this section anticipate promising results, in particular for the treatment of challenging lesions, such as long lesions, bifurcations lesions, and those located in small vessels, but larger studies with adequate follow-up are needed to evaluate the safety of novel metal alloys and modified stent designs.
Innovations in polymers
Stents with durable polymers
Endeavour Resolute™
The Endeavour Resolute (Medtronic CardioVascular Inc, Santa Rosa, CA) is a next-generation zotarolimus-eluting stent developed by Medtronic to improve the clinical outcomes associated with the first-generation Endeavor stent. It is comprised of a Driver cobalt–chromium stent platform, similar to the Endeavor stent, the antiproliferative agent zotarolimus, and, instead of the Endeavor stent’s phosphorylcholine polymer, a new biocompatible polymer called BioLinx™.Citation41 This polymer is a blend of three different polymers, a hydrophobic C10 polymer, a hydrophilic C19 polymer, and a hydrophilic polyvinyl-pyrrolidinone, designed to provide a robust coating and to enhance polymer biocompatibility, reducing the risk of delayed healing and late stent thrombosis. The polymer also extends the duration of drug exposure in the vessel, such that ∼50% of the zotarolimus is released within the first week, with the remaining drug released beyond 31 days.Citation42
The stent’s clinical effectiveness and safety have been evaluated in 139 patients enrolled in the prospective, multi-center, nonrandomized, single-arm, controlled first-in-man RESOLUTE trial, which showed a late lumen loss of 0.22 ± 0.27 mm and an in-stent binary restenosis of 1.0% at 9 months angiographic follow-up.Citation41 The cumulative rates of MACE, TLR, and target vessel failure (TVF) reported at 2-year follow-up were 10.1%, 1.4%, and 7.9%, respectively.Citation43
Recently, the Endeavour Resolute stent has been compared to the Xience™ V everolimus-eluting stent in the prospective, multicenter, randomized, two-arm, international, noninferiority RESOLUTE-III all-comers trial (NCT00617084), in which 2300 patients were enrolled. The results of this study showed the Resolute to be noninferior to Xience V for the primary endpoint of composite TLF (cardiac death, target vessel MI, or clinically driven TLR) at 12 months follow-up (Resolute 8.2% vs Xience V 8.3%, Pnoninferiority < 0.001).Citation44
The Endeavour Resolute stent has received the CE Mark in 2007.
Excella™ stent
This stent is described in the Innovations in drugs – New drugs section.
Stents with biodegradable polymers
Sirolimus-based
Supralimus™
The Supralimus stent (Matrix; Sahajanand Medical Technologies, Surat, Gujarat, India) is a stainless steel stent with a two-layer biodegradable polymer coating. The base layer is a mix of poly-l-lactic acid (PLLA), poly-lactide-co-glycolide, and polyvinyl-pyrrolidone, which releases 50% of sirolimus within the first week and the remaining 50% in the next 41 days. The surface polyvinyl-pyrrolidone layer has a protective function and degrades completely within 2 h after implantation.
In the prospective, nonrandomized, first-in-man SERIES I study, 100 patients were treated with the Supralimus stent. Data showed a 6% rate of MACE at 9 months follow-up and an event-free survival rate of 93% at 30 months. The rates of 6-month in-stent and in-segment restenosis in a prespecified subgroup of 60 patients were 0% and 1.7%, respectively; the in-stent and in-segment late loss were 0.09 ±0.28 and 0.02 ± 0.37 mm, respectively, at the same angiographic follow-up.Citation45
The safety and efficacy of the Supralimus stent in the treatment of unselected patients with acute coronary syndrome undergoing PCI have been evaluated in the prospective, multicenter E-SERIES registry which showed acceptable rates of MACE, TLR, and stent definite and probable thrombosis of 10.0%, 2.7%, and 0.6%, respectively, at 12 months follow-up.Citation46
Further data will be produced by the prospective, multicenter, randomized, noninferiority SERIES III trial (NCT00917163), which is currently ongoing. This study includes a head-to-head comparison with the Xience V stent for the primary endpoint of in-stent luminal late loss at 9 months after stent implantation.
Excel™
The Excel stent (JW Medical Co Ltd, Shandong Province, Weihai, China) is composed of the S-Stent’s (Biosensor International) stainless steel laser-cut platform, which confers a high flexibility, a very thin coating (10–15 μm) of sirolimus, and a PLA biodegradable polymer, which can be expected to have a complete degradation within 6–9 months. The Excel stent has been investigated in the CREATE (Multi-Center Registry Trial of EXCEL Biodegradable Polymer Drug-Eluting Stent, NCT00331578) registry in 2077 patients. The predefined primary endpoint was the incidence of MACE (cardiac death, nonfatal MI, and TLR) at 12 months follow-up after stent implantation. The trial reported MACE rates of 2.7% and 3.1% at 12- and 18-month follow-up, respectively, and an angiographic in-stent late lumen loss of 0.21 ± 0.39 mm.Citation47 In this study, 80.5% of patients discontinued clopidogrel treatment within 6 months, given the use of a biodegradable polymer. The rate of stent thrombosis proved to be 0.87% at 18 months of follow-up.
NEVO™
The NEVO stent (Cordis) is made up of a cobalt–chromium alloy platform, with open cell design to improve vessel conformability. It is the first DES applying the Cordis RES Technology™, which consists of hundreds of reservoirs embedded in the struts, each acting as a depot loaded with a drug–polymer mix. The active ingredient in the drug–polymer composition is sirolimus, with similar drug dose and release kinetics as Cypher. The polymer loaded into reservoirs is a poly-lactic acid-co-glycolic acid (PLGA). Polymer’s degradation time can be controlled by altering the lactide/glycolide ratio, and specifically for NEVO stent, PLGA completes bioabsorption in a very short duration of 90 days. This technology offers several advantages, first a higher drug/polymer ratio and thus both higher drug dose and lower polymer mass than conventional DES. Moreover, reservoirs coat a small percentage of stent surface, which is therefore for the most part bare metal, in contrast to other DESs that are totally polymer coated. This feature conceivably increases stent vessel biocompatibility.Citation48
In the randomized, multicenter, single-blind NEVO RES-I (Comparison of the Conor Sirolimus-eluting Coronary Stent to the TAXUS Liberté Paclitaxel-eluting Coronary Stent in the Treatment of Coronary Artery Lesions, NCT00606333) trial, the NEVO stent was compared to the TAXUS Liberté paclitaxel-eluting coronary stent in 394 patients. NEVO was associated with significantly lower in-stent late lumen loss (0.13 vs 0.36 mm, P < 0.001) at 6-month quantitative coronary angiography (QCA) follow-up.Citation49 In addition, NEVO showed lower rates of death (0.5% vs 2.2%), MI (2.0% vs 3.2%), MACE (6.1% vs 10.8%), and TLR (3.6% vs 5.9%) than TAXUS at 12 months follow-up. No cases of stent thrombosis occurred at the same follow-up in the NEVO group in contrast with two late thromboses (one possible and one probable) in the TAXUS group.Citation50 Further studies have been designed to investigate the NEVO stent, such as the ongoing prospective, multicenter NEVO RES-II study (NCT00714883). The NEVO II trial (NCT01202058) will be a randomized, noninferiority trial comparing the NEVO stent to the Xience everolimus-eluting coronary stent with the aim of assessing clinical outcomes during 5 years of follow-up. The prospective CYNERGY (Cynergy: the CYPHER-NEVO Registry) study (NCT01106378) is recruiting participants to assess the noninferiority of NEVO, once commercially available, to CYPHER in patients with acute STEMI, diabetes mellitus, or multivessel disease.
Biolimus-based
The BioMatrix Flex stent and the Nobori stent, both coated with a biodegradable polymer, are described in the Innovations in drug – New drugs section.
Paclitaxel-based
Infinnium™
The biodegradable polymers, which coat the stainless steel balloon-expandable platform of the Infinnium stent (Matrix; Sahajanand Medical Technologies) are poly-l-lactide, poly-dl-lactide-co-glycolide, poly-l-lactide-co-caprolactone, and polyvinyl-pyrrolidone. These polymers are stratified in composition with the antiproliferative agent paclitaxel, each layer with a different drug release kinetics. Infinnium stent’s safety and efficacy have been assessed in 103 patients enrolled in the multicenter, prospective, nonrandomized SIMPLE II study, which aimed at investigating the incidence of MACE (primary endpoint) at 30 days and in-stent binary restenosis by QCA at 6 months follow-up. Results showed rates of MACE at 30 days, 6 months, and 9 months to be 2.9%, 4.9%, and 9.7%, respectively. Data from QCA indicated in-stent and in-segment binary restenosis rates of 7.3% and 8.3% associated with in-stent and in-segment late loss of 0.38 ± 0.49 and 0.18 ± 0.46 mm, respectively.Citation51
The Infinnium stent has been compared with the above-mentioned Supralimus stent and a BMS control in the randomized, multicenter PAINT trial (NCT00752362), which reported a significant reduction in late loss and TVR at 9 months follow-up for DES (both Infinnium 0.54–0.44 mm and Supralimus 0.32–0.43 mm) compared with BMS (0.90–0.45 mm).Citation52 This stent has received the CE Mark.
JACTAX™
The JACTAX™ (Boston Scientific) Liberté is made up of a premounted stainless steel platform coated with a polymer-drug blend of a low molecular weight biodegradable poly-lactide polymer and paclitaxel. This process, known as Juxtaposed Abluminal Coating technology, applies minimal amount of polymer-drug composition in discrete areas (2750 microdots/16 mm stent) exclusively located onto the abluminal surface, such that the other three sides of the stent remain BMS surface. Polymer thickness is ≤1 μm, approximately 15 times thinner than that of the TAXUS Liberté stent polymer. The drug paclitaxel is fully released in 60 days, while the polymer degrades within 4 months.
The prospective, single-center, randomized OCTDESI (Optical Coherence Tomography Drug Eluting Stent Investigation) trial (NCT00776204) was designed to evaluate the long-term proportion of strut coverage and the vessel wall response with the JACTAX stent at 6 months, with either low drug dose (LD) or high drug dose (HD), compared to the TAXUS Libertè stent. Data showed that the primary endpoint of the percentage of uncovered stent struts per patient at 6 months follow-up measured by OCT was similar for the three devices (5.3% ± 14.7% for TAXUS Liberté, 7.0% ± 12.2% for JACTAX HD, and 4.6% ± 7.3% for JACTAX LD; P = 0.81).Citation53,Citation54 There were no deaths, Q wave MIs, or stent thromboses at 12-month clinical follow-up.
An evaluation of safety and clinical performance of the JACTAX HD stent have been performed in a prospective, multi-center, nonrandomized first-in-man study (NCT00754728), which recruited 103 patients who had underwent PCI with the aforementioned stent compared to an historical control group of patients treated with the TAXUS Liberté stent from the ATLAS trial.Citation55 Results in patients treated with JACTAX HD stent showed that the primary endpoint of MACE (cardiac death, MI, and ischemia-related TVR) occurred at 9 months in 7.8% of patients, a value significantly below the 17% noninferiority limit, thus meeting the prespecified criteria for noninferiority to the TAXUS Liberté stent. Moreover, there was no death, Q wave MI, or stent thrombosis during follow-up. Results of QCA demonstrated an in-stent late loss of 0.33 ± 0.45 mm and an in-stent binary restenosis of 5.2%, which are comparable values to those observed in similar-matched patients from the TAXUS ATLAS trial. Mean net volume obstruction by intravascular ultrasound (IVUS) was 11.4% ± 11.2%.Citation56 The JACTAX LD stent has been assessed in the prospective, multicenter, randomized JACTAX LD Drug Eluting Stent Trial (NCT00754975) and completed in August 2010. This trial randomized 130 patients from the JACTAX LD stent or the TAXUS Libertè. The study results are awaited.
CoStar™
The CoStar stent (Conor MedSystems, Menlo Park, CA) is a cobalt–chromium stent that elutes paclitaxel without the use of a surface polymer using holes located on the surface of the stent, each one acting as a reservoir for a drug-polymer blend. The polymer loaded into the reservoirs is a bioresorb-able PLGA polymer, which degrades mediating drug delivery such that at the end of biodegradation process, only the bare-metal platform remains. The CoStar stent was first evaluated in several study (PISCES and COSTAR I ed EuroStar) and showed satisfactory results.Citation57–Citation59 However, in the multicenter, single-blind, two-arm, randomized, controlled, noninferiority trial COSTAR II study (NCT00165035) (n = 1700), the CoStar stent was not demonstrated to be noninferior in clinical and angiographic performance compared with the paclitaxel-eluting TAXUS stent: the MACE rate was 11.0% for Costar and 6.9% for TAXUS (P < 0.005) at 8-month follow-up, and the in-segment late loss was 0.49 mm for CoStar and 0.18 mm for Taxus (P < 0.0001).Citation60 The CoStar stent has received the CE Mark.
Nonpolymeric stent
YUKON®
A microporous stainless steel platform forms the basis of the completely polymer-free YUKON stent (Translumina, Hechingen, Germany), which has been combined with rapamycin using a technology denominated ISAR (Individualised Drug-Eluting Stent System to Abrogate Restenosis). This technology consists of a two-component system, a mobile stent-coating device for the spraying process of the drug and a disposable stent cartridge holding the premounted stainless steel microporous stent. The ISAR process allows to coat the platform with various drugs directly on-site customizing the drug dose. During the coating process, as soon as the two components are correctly located, the drug is sprayed on the stent surface, and it is subsequently dried by removing the ethanol with pressured air. The entire process takes ∼8 min.Citation61 According to previous studies, ISAR stents are safe and effective with a 2% rapamycin-coating solution.Citation62 The microporous surface has the function of drug reservoir and retards drug release, consequently the polymer serves no function.
In the randomized ISAR-TEST, in which a total of 450 patients were enrolled, the YUKON stent has been compared to the polymer-based, paclitaxel-eluting TAXUS stent for the treatment of de novo coronary lesions, with the aim of assessing the noninferiority of the rapamycin-eluting stent. The results of the study demonstrated that the YUKON stent is noninferior to the TAXUS stent with regards to the incidence of angiographic and clinical restenosis.Citation63 In the prospective, randomized ISAR-TEST-3 (Rapamycin-Eluting Stents With Different Polymer Coating to Reduce Restenosis), the Yukon DES was compared to a rapamycin-eluting stent with biodegradable polymer and to a rapamycin-eluting stent with permanent polymer (Cypher) in 605 patients. The study was completed in 2007 and reported a 6- to 8-month in-stent late loss of 0.47 mm for the YUKON stent compared with 0.17 mm for the rapamycin-eluting stent with biodegradable polymer and 0.23 mm for Cypher, showing low efficacy for the polymer-free YUKON stent.Citation64
The safety of the YUKON stent has also been evaluated in a real-world registry (n = 410), in which a group of patients treated with YUKON stent was compared to a group of those who had undergone PCI with TAXUS stent. This study showed no statistically significant differences in terms of MACE at 6 months follow-up between the two groups of patients; specifically, one MI occurred in the TAXUS stent group and no case in the YUKON stent group (0.2% vs 0%, respectively), and 15 TLR (7.3%) were performed in the YUKON stent group versus 7 in the TAXUS stent group (3.4%).Citation65 A prospective, observational study weighed the difference in in-stent late lumen loss between 6–8 months and 2 years in patients treated with permanent-polymer DES Cypher or TAXUS or with polymer-free rapamycin-eluting stents. Angiographic data obtained after 2-year follow-up indicate that the absence of permanent polymer from DES has a protective function against delayed in-stent late lumen loss (YUKON stent 0.01 ± 0.42 mm, Cypher stent 0.17 ± 0.50 mm, and TAXUS stent 0.13 ± 0.50 mm; P < 0.001).Citation66 This stent has received the CE Mark and is available for sale in Europe.
BioFreedom™
The BioFreedom (Biosensors) stent is a stainless steel polymer-free stent, currently under development and releasing Biolimus A9™, a rapamycin derivate with immunosuppressive and antiproliferative properties which has already been used on the BioMatrix, Nobori, Xtent, and DEVAX stents. In the first cohort of the prospective, multicenter, randomized, single-blinded BioFreedom FIM Clinical Trial (NCT01172119), the stent was compared to the TAXUS Libertè stent in two-drug dosage versions, namely a standard-dose version (SD, 15.6 μg/mm of stent length) and a LD version (7.8 μg/mm of stent length). The 4-month follow-up results reported in the first cohort (n = 74) showed a significant reduction (P < 0.0001) of in-stent late loss in both the BioFreedom SD and LD groups (0.08 and 0.12 mm, respectively), compared to the TAXUS Libertè group (0.37 mm). In addition, no cases of stent thrombosis were noticed in the study during 4 months. The 12-month angiographic follow-up of the second cohort (n = 107) demonstrated the noninferiority of the BioFreedom SD compared to TAXUS stent with regard to in-stent late loss (0.17 vs 0.35 mm, respectively; P = 0.001) and a trend toward superiority (P = 0.11). The rates of MACE at the same follow-up were 6.1% for BioFreedom SD, 11.6% for BioFreedom LD, and 5.5% for TAXUS stent in all patients (first and second cohorts), with no case of stent thrombosis.Citation67 In a recent study, the BioFreedom stent showed equivalent early and superior late reduction of neointimal proliferation compared with the polymer-coated sirolimus-eluting Cypher stent in a porcine model.Citation68
Amazonia Pax®
The Amazonia Pax stent (Minvasys) is made up of a polymer-free chromium–cobalt alloy platform, with open cell design, eluting the antiproliferative agent paclitaxel. The drug layer (thickness: 5 μm) is applied using a microdrop spray crystallization process, exclusively onto the abluminal surface, and is loaded with a paclitaxel dose of 2.5 μg/mm2. The stent is designed to release ∼98% of the drug in 30 days, returning to regular chromium–cobalt after 45 days. Strut and coating thicknesses are 73 and 5 μm, respectively, ensuring a total stent thickness of 78 μm, less than that found on other stents (Cypher stent: 152.6 μm and TAXUS stent: 148 μm). Clinical evaluation of the Amazonia Pax stent is currently ongoing in the prospective, randomized, multicenter-active controlled, single-blinded PAX A trial and in the prospective, nonran-domized PAX B trial. The 4 months follow-up results of the PAX A trial, in which the Amazonia Pax stent was compared with the TAXUS stent, showed in-stent late lumen loss of 0.77 and 0.42 mm (P = 0.20), respectively, and a percentage of stent obstruction by IVUS of 19% and 6% (P = 0.08), respectively, revealing no significant difference between these two stents in angiog raphy and IVUS quantitative parameters.Citation69,Citation70 Minvasys has received the CE Mark for Amazonia Pax stent in 2010.
VESTAsync™
In the VESTAsync-eluting stent (MIV Therapeutics, Atlanta, GA), a polymer-free stainless steel platform is incorporated with a nanothin-microporous hydroxyapatite surface coating impregnated with a low-dose lipid-sirolimus mixture. The hydroxyapatite coating dissolves completely after 9–12 months, while the elution of sirolimus is complete within 3 months. The stent has been evaluated in the prospective, nonrandomized, single-center VESTASYNC I FIM clinical trial in 15 patients. Data have confirmed the sustained efficacy of this polymer-free stent showing a lumen late loss of 0.36 ± 0.23 mm by QCA analysis and an in-stent percentage volume obstruction by IVUS of 4.0 ± 2.2 mm3 at 9 months follow-up. Long-term clinical follow-up reported no MACE (cardiac/noncardiac death, cerebrovascular accident, nonfatal MI, and stent thrombosis) at 12 months follow-up and only a case of TLR at 36 months follow-up.Citation71 In the randomized VESTASYNC II study (n = 120), the VESTAsync sirolimus-eluting stent was compared with a control BMS. The 8-month QCA analysis showed an in-stent late lumen loss of 0.39 ± 0.20 mm for VestaSync stent compared with 0.74 ± 0.52 on the control BMS (P = 0.03).Citation72
Conclusions
The polymer is one of the main DES elements in charge of hypersensitivity and late stent thrombosis after implantation. Therefore, innovations in this component, as showed by the early results of some devices of this category already used in daily practice, are likely to exert a positive influence on PCI outcomes in the near future.
Innovations in drugs
Drugs combination system
SymBio™
A new solution in developing DES consists of loading a stent with more drugs. This technology has been used in the SymBio stent (Conor Medsystems). This stent includes the Conor reservoirs technology, already used in the Costar stent, which provides the capability to load drug in hundreds of small holes, each acting as a drug-polymer reservoir, and to control the delivery time and rate. In the SymBio stent, in contrast to the Costar stent loaded with paclitaxel, two drugs, specifically paclitaxel and pimecrolimus, are loaded in adjacent reservoirs. Pimecrolimus is an anti-inflammatory agent with immunosuppressant properties used for the topical treatment of atopic dermatitis.
Despite the combination of two drugs in a unique stent, in the randomized, multicenter GENESIS (Randomized, MultiCenter Study of the Pimecrolimus-Eluting and Pimecrolimus/Paclitaxel-Eluting Coronary Stent Systems) trial (NCT00322569), which compared the paclitaxel-eluting Costar stent, the pimecrolimus-eluting Corio™ stent, and the dual-combination drugs SymBio stent by Conor Medsystem, data failed to demonstrate either SymBio or Corio angiographic noninferiority when compared with paclitaxel-eluting stent. The in-stent late loss values at 12 months follow-up were 0.58 ± 0.58 mm for the Costar stent, 1.40 ± 0.67 mm for the Corio stent, and 0.96 ± 0.73 mm for the SymBio stent (P < 0.001).Citation73
New drugs
Novolimus
Excella™
The Excella novolimus-eluting stent (Elixir Medical, Sunnyvale, CA) is a next-generation DES, which consists of a cobalt–chromium platform with a strut thickness of 0.0032″ and eight-crown design for optimal scaffolding, a biocompatible methacrylate polymer with a coating thickness of <3 μm (compared with 7.6 μm for everolimus-eluting stents), and a new ‘limus’ family-related drug called novolimus. This drug is a macrocyclic lactone, metabolite of sirolimus, which demonstrates high potency to inhibit neointimal proliferation. This feature results in a drug dose of 5 μg/mm, lower than that found in other stents like zotarolimus- or everolimus-eluting stents (10 μg/mm). Both lower drug dose and polymer load seem to confer to the stent significant safety and efficacy.
The results from the first-in-man EXCELLA I study, which recruited 15 patients, showed an in-stent late loss of 0.31 ± 0.25 mm and a neointimal volume of 6.0% ± 4.4% by IVUS at 8 months follow-up.Citation74 Recently, the randomized EXCELLA II (Elixir Medical Clinical Evaluation of the Novolimus-Eluting Coronary Stent System: a Randomized Study With a Single-Arm Registry) study (NCT00792753) demonstrated an angiographic lumen late loss of 0.11 ±0.32 mm and a neointimal volume obstruction of 20.9% ± 11.3%.Citation75
Biolimus
BioMatrix™ Flex
The BioMatrix Flex biolimus-eluting stent (Biosensors) incorporates a stainless steel stent platform with a strut thickness of 112 μm and an abluminal PLA biodegradable polymer. The PLA polymer completely degrades into carbon dioxide and water within 6–9 months. In the randomized LEADERS (Trial Limus Eluted from A Durable Versus ERodable Stent Coating) trial (NCT00389220) (n = 1707), the BioMatrix stent has been compared to the Cypher sirolimus-eluting stent with a noninferiority study design. At 12 months, the BioMatrix stent was noninferior to Cypher stent for MACE (10.6% and 12.0%, respectively; P = 0.37).Citation76 The BioMatrix stent noninferiority to Cypher stent has been ratified at 2 years follow-up with similar outcomes.Citation77 Recently, the safety and efficacy of the BioMatrix stent have also been demonstrated in a single-center trial. This study reported an in-stent late loss of 0.24 ± 0.39 mm for the BioMatrix group and 0.71 ± 0.47 mm for the control group (S-Stent, P < 0.001).Citation78 The BioMatrix stent has received the CE Mark.
Nobori™
The Nobori stent (Terumo Corporation, Tokyo, Japan) elutes Biolimus A9 and consists of the stainless steel S-stent and a biodegradable PLA polymer, similar to that of the BioMatrix stent, except for the delivery system and the stent coating process. For the nonrandomized, multicenter Nobori Core study, 107 patients were recruited to demonstrate the similarity between the Nobori stent and Cypher stent. It reported an in-stent late loss of 0.10 ± 0.26 and 0.13 ± 0.44 mm (P = 0.66), respectively, at 9 months angiographic follow-up, thus confirming the predefined hypothesis of similarity between the two stents.Citation79 The randomized Nobori 1 trial (n = 243) demonstrated the noninferiority of the Nobori stent versus TAXUS Libertè stent for the primary endpoint of angiographic in-stent late loss at 9 months postprocedure (Nobori 0.11 ± 0.30 mm and TAXUS Libertè 0.32 ± 0.50 mm; P = 0.001). In addition, in both stents, the rate of MACE was low, and no cases of stent thrombosis occurred at the same follow-up.Citation80 The Nobori stent has the potential to reduce thrombotic events, promoting a complete endothelial regeneration.Citation81 Further and more extensive evaluations of this stent are awaited in the coming years. In particular, for a multicenter, randomized study (NCT01186120), ∼500 patients are being recruited to compare the Nobori stent with the PROMUS Element stent for long coronary lesions. A randomized trial (NCT01097434), currently enrolling participants (estimated enrollment, 45 patients), will evaluate the superiority of the Nobori stent versus the Xience V stent with regard to the absolute percentage of uncovered stent strut segments. The prospective, randomized ISAR-TEST 6 trial (NCT01068106) will compare the efficacy and the safety of the Nobori stent with that of the Xience V stent. The Nobori stent has received the CE Mark.
Myolimus
Myolimus™ Eluting Coronary system
Myolimus is a macrocyclic lactone, sirolimus analogous, used in the Myolimus™ Eluting Coronary system (Elixir Medical) in composition with three proprietary polymer technologies: a durable polymer, a biodegradable polymer developed to biodegrade within 6 months, and a biodegradable polymer which biodegrades within 9 months. The results of the single-arm, multicenter, first-in-man study on the Elixir Myolimus-eluting stent with a durable polymer show an in-stent late loss of 0.15 ± 0.11 mm at angiographic analysis and a neointimal volume of 1.4% ± 1.2% at IVUS at 6 months follow-up.Citation82
Clinical data obtained from a single-arm, multicenter, first-in-man study on the Myolimus-eluting stent with bioab-sorbable polymers indicate a late lumen loss of 0.13 ± 0.27 mm at angiographic analysis and a neointimal volume of 5.4% ± 8.4% at IVUS at 12 months of follow-up.Citation83
Conclusions
The combination of new drugs, as the biolimus, with other innovative DES components, as biodegradable polymers, has already provided good results, and it is an established fact in the landscape of interventional cardiology.
Biodegradable stents
In spite of so many improvements in the development of DES, there are still many concerns regarding the neointimal proliferation and the risk of late stent thrombosis by using this technology. These complications have been attributed to the presence of uncovered metal struts in direct contact with blood and to the possibility of incomplete vessel healing. So far, DES have been modified in their components, namely drug, polymer, and platform, by the several aforementioned solutions with the aim of averting the complications of coronary angioplasty.
In contrast to older devices, biodegradable stents represent a new concept of coronary stent. The need for vessel scaffolding is transitory, so a permanent stent is unnecessary after the vessel has healed. Biodegradable stents completely degrade after implantation and, causing the disappearance of any foreign material exposed to blood if endothelialization is delayed or incomplete, they may hypothetically reduce the risk for late stent thrombosis. Moreover, biodegradable stents may have other potential advantages, such as promoting reconstitution of local vascular compliance, avoiding covering of side-branch, facilitating the use of lesion imaging with multislice computed tomography, angiography, or cardiac magnetic resonance, and allowing further surgical or percutaneous treatments to the same lesion.Citation84,Citation85 Biodegradable stents were first implanted in 1980s with good results, but none has received either the CE Mark or United States Food and Drug Administration approval. Further ameliorations and large-scale randomized trials with long-term follow-up are necessary to enhance and evaluate the safety and efficacy of this technology.
Poly-l-lactide stents
Bioresorbable Vascular Scaffold™
The Bioresorbable everolimus-eluting Vascular Scaffold (BVS; Abbott Vascular) is made up of a backbone of PLLA coated with a thin layer of poly-d,l-lactide (PDLLA) and elutes everolimus. PLLA is a fully biodegradable semicrystalline polymer, which forms a conglomeration of crystalline and amorphous phases when it solidifies. The structure and the state of this polymer change in relation to temperature and deformation history. PDLLA is a biodegradable polymer coating PLLA, which forms, similar to the PLLA, an amorphous phase when it solidifies at room and physiological temperatures. The PDLLA allows controlled release of the drug such that ∼80% of everolimus is eluted within 28 days. The absorption process of PLLA and PDLLA occurs via hydrolysis, producing lactic acid, and subsequently degrades via the Krebs cycle, and small particles <2 μm in diameter are phagocytosed by macrophages. With regard to preclinical trials, it seems that complete absorption of the stent could occur within 2–3 years after implantation ().
Figure 4 Bioabsorption process of the BVS stent and relative degradation times.
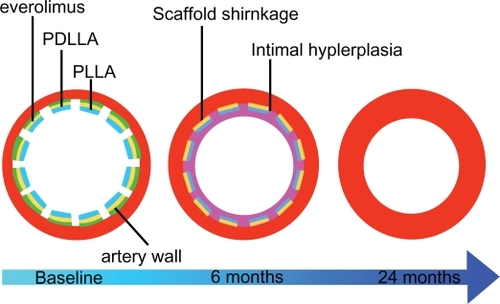
Two versions of the stent have been developed. The design of the BVS stent Revision 1.0 consists of circumferential out-of-phase zigzag hoops of PLLA with a strut thickness of 150 μm, linked either directly or by straight bridges, with two radiopaque markers at each end which enable a good visualization on angiography. It has been evaluated in 30 patients in the cohort A of the prospective, open-labeled, first-in-man ABSORB (Bioabsorbable Vascular Solutions First-in-Man Clinical Investigation: a Clinical Evaluation of the Bioabsorbable Vascular Solutions Everolimus-Eluting Coronary Stent System in the Treatment of Patients With Single de Novo Native Coronary Artery Lesions) study (NCT00300131).Citation86 The in-stent late loss reported at 2 years angiographic follow-up did not significantly differ from that found at 6 months follow-up (0.48 ± 0.28 mm vs 0.43 ± 0.37 mm; P = 0.233). The multi-imaging approach (IVUS-virtual histology and OCT) at the same follow-up showed that the stent was incorporated into the vessel wall and that several implanted stents were no longer discernible by OCT.Citation87 The rate of MACE at 6 months follow-up was 3.3%, and no new MACE events occurred between 6 months and 3 years. No stent thrombosis or TLR occurred up to 3 years follow-up. A significant restoration of vasomotion was reported at the same follow-up, but the study showed some limitations. In particular, the duration of radial support may be insufficient to resist the coronary remodeling after PCI, and the scaffold shrinkage could cause quite high in-stent late loss value reported at 6 months follow-up.Citation88
The BVS Revision 1.1 consists of the same polymer, but different process refinements allow the stent to increase its radial force, provide radial support for a longer time, and, consequently, avoid the slightly higher, but nonsignificant, recoil shown by QCA.Citation89–Citation91 This stent version has a different strut distribution, reducing the maximum circular unsupported cross-sectional area, in contrast to BVS Revision 1.0.Citation92 It is currently under evaluation in the Cohort B of the non-randomized ABSORB trial (NCT00856856) in 80 patients. Data reported an in-scaffold late loss of 0.19 mm at 6 months angiographic follow-up and a MACE rate of 4.4% (TVF 4.4%) at 9 months clinical follow-up (one non-Q wave MI and one ischemia driven-TLR); no scaffold thrombosis occurred over the follow-up.Citation93
REVA™
The scaffolding of the REVA bioabsorbable stent (REVA Medical, San Diego, CA) consists of a biodegradable tyrosine polycarbonate polymer, which metabolizes to amino acids, ethanol, and carbon dioxide. The rate of re-absorption depends on molecular weight and can be modified for different utilizations, for example, specific use in vulnerable plaques or diabetic lesions. This polymer is also radiopaque because of binding iodine molecules directly into the polymer backbone. The expansion mechanism of the stent is based on the ‘slide and lock design’. This technology enables the stent to expand without significant deformation, providing high-radial strength compared to stainless stent with negligible recoil (<1%).Citation94 The first-generation stent, which did not elute any drug, has been assessed in the prospective, multicenter, single-arm, safety, first-in-man RESORB (REVA Endovascular Study of a Bioresorbable Coronary Stent) study (27 patients enrolled), which showed a higher than anticipated rate of TLR at 4–6 months follow-up, most likely caused by reduction in stent diameter, even though without evidence of shrinkage or negative vessel remodeling.Citation95,Citation96 The next-generation stent, called the ReZolve™ stent, uses a ‘slide and lock’ mechanism, but it has been optimized using a coating of sirolimus, a more robust material, and a spiral design to enhance dimensional stability and to maintain mechanical integrity under high loading both initially and over time. This stent is undergoing clinical evaluation.Citation97
BTI™
A bioabsorbable polymer of salicylic acid, the active ingredient of aspirin with anti-inflammatory and antiplatelet properties, constitutes the basis for the BTI stent (Bioabsorbable Therapeutics Inc, Menlo Park, CA). The stent has two layers composed of salicylic-acid derivates: the core provides mechanical scaffolding, while the surface layer contains sirolimus that is eluted during the biodegradation process together with a dose of salicylic acid of ∼10 μg.Citation98 The blend of sirolimus and salicylic acid ensures both anti-proliferative and anti-inflammatory actions. The complete absorption of the stent is expected within 6–12 months. Following successful preclinical trials on porcine coronary arteries, the multicenter first-in-man WHISPER trial demonstrated acceptable safety and the absence of acute or chronic recoil, but on the other hand, data reported an insufficient neointimal suppression presumably due to the low dose and very rapid elution of sirolimus. As a result, the first-generation stent has been modified by increasing the drug dose and slowing down the elution time. Today, this second-generation stent is under testing in a preclinical trial on porcine coronaries.Citation99,Citation100
IGAKI-TAMAI™
The bioabsorbable IGAKI-TAMAI stent (Kyoto Medical Planning Co Ltd, Kyoto, Japan) is made up of a monofilament of PLLA polymer (thickness: 0.17) with a zigzag design. It has two radiopaque gold markers at each end to facilitate visualization on angiography. This PLLA stent is both thermal self-expanding and balloon expandable; it gets its original size in 0.2 sec when heated to 70°C. The stent expansion in the implant site by balloon occurs within 30 sec and is performed by balloon inflation of a heated dye at 80°C. After the inflation process, the self-dilatation continues progressively until the definitive equilibrium size. The IGAKI-TAMAI stent was the first-biodegradable stent implanted in a human. The first version of the stent lacked an antiproliferative drug. In the first-in-man study, it was evaluated on 15 patients treated with 25 stents in 19 lesions. Data reported at 6 months indicate a late loss of 0.48 ± 0.32 and no ST or MACE at clinical follow-up, but the rates of angiographic restenosis and of TLR per lesion were both 10.5%. IVUS revealed an expansion of the stent over the follow-up and the presence of stent strut at 6 months; in addition, the mean stent cross-sectional area was similar at 3 and 6 months (8.18 and 8.13 mm2; P = 0.30).Citation101
In another study, in which 50 elective patients with 63 lesions treated with 84 IGAKI-TAMAI stents were enrolled, a 10-year follow-up (the longest available follow-up of a biodegradable coronary stent) reported rates of cardiac and noncardiac death-free survival of 97.8% and 87.0%, respectively, a rate of MACE-free survival of 48.4%, and a rate of TLR of 23.8%.Citation102,Citation103 Data showed a low complication rate, but at present, due to concerns about the use of heat to induce self-expansion, no further human coronary implants have been carried out, and today, the stent is only available in Europe for peripheral use. A version of the stent with a coating of paclitaxel has been deployed in porcine coronary arteries; the drug coating was shown to effectively inhibit proliferation and coronary stenosis after vascular intervention in a long-term follow-up.Citation104
Bioabsorbable magnesium stent
One of the problems with the bioabsorbable stent is the above-mentioned lower radial strength when compared to stainless steel traditional devices. Biodegradable metallic stents have been developed with the aim of exceeding these limitations; in particular, they provide a combination of the advantages of a polymeric biodegradable stent, such as the complete degradation of the implants and a fast recovery of vasomotion, with the benefits of stainless steel stents, including an effective radial force.
The absorbable-metal stent (AMS; Biotronik, Berlin, Germany) is a balloon-expandable stent, laser cut from a tube of bioabsorbable alloy which is composed mainly of magnesium (>90%). This technology for tube production provides a reduced wall thickness. The expected degradation time is <56 days.Citation105 Three generations of stent have been designed. The first-generation stent (AMS-1), which lacked a drug coating, was evaluated in 63 patients in the prospective, nonrandomized, multicenter PROGRESS-AMS trial, showing no deaths, nonfatal MIs, or stent thromboses at 4 and 12 months follow-up. On the other hand, the rate of TLR was 39.7% and 45% at 4 and 12 months follow-up, respectively, and the in-stent late loss was 1.08 ± 0.49 mm at 4 months angiographic follow-up.Citation106
A second-generation stent, called AMS-2, was developed to improve the results of the AMS-1. It is characterized by a slower degradation, a thinner strut thickness, and a modern design. In preclinical studies, the AMS-2 demonstrated encouraging results with regard to neointimal proliferation and to prolonged stent integrity. After the AMS-2, a further version of the stent, called AMS-3, has been developed. This version of the stent, which is the first drug-eluting absorbable magnesium stent, has already been reported in animal trials and showed significant improvement in minimum lumen diameter at 14 and 28 days compared to AMS-1 and late loss indices comparable to conventional DES.Citation107 Currently, the nonrandomized first-in-man trial BIOSOLVE-I (BIOTRONIKS-Safety and Clinical Performance Of the First Drug-Eluting Generation Absorbable Metal Stent In Patients With de Novo Lesions in NatiVE Coronary Arteries. NCT01168830), which will assess the safety of the AMS-3, is recruiting participants.
Conclusions
Recent approaches in development of next-generation DES have been reported in this review. In past years, a lot of effort has been devoted to improving the safety and efficacy of new coronary stents. Some of the above are already available for daily practice, while others need further studies to validate their use on humans. Larger trials and longer follow-up are necessary to assess the effectiveness of these novel devices, but, given that DES will continue to have a prominent part in PCI in the near future, the aims so far reached suggest the achievement of important results.
Acknowledgements
Corrado Tamburino has received speaker’s honoraria from Abbott Vascular, Medtronic, and Celonova Bioscience.
Disclosure
Davide Capodanno and Fabio Dipasqua report no conflicts of interest.
References
- HoffmannRMintzGSDussaillantGRPatterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound studyCirculation1996946124712548822976
- SousaJECostaMAAbizaidACSustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angio-graphic and intravascular ultrasound follow-upCirculation2001104172007201111673337
- HtayTLiuMWDrug-eluting stent: a review and updateVasc Health Risk Manag20051426327617315599
- MoriceMCColomboAMeierBREALITY Trial InvestigatorsSirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trialJAMA2006295889590416493102
- MillauerNJüniPHofmannASirolimus versus paclitaxel coronary stents in clinical practiceCatheter Cardiovasc Interv201177151220506333
- AbizaidASirolimus-eluting coronary stents: a reviewVasc Health Risk Manag20073219120117580729
- UyanCArincHGunduzHAkdemirRImmediate and six months clinical and angiographic results of intracoronary paclitaxel-coated stent implantation – the Meo:DrugStar-1 studyVasc Health Risk Manag20084117317618629363
- KirchnerRMAbbottJDUpdate on the everolimus-eluting coronary stent system: results and implications from the SPIRIT clinical trial programVasc Health Risk Manag200951089109720057901
- SheibanIVillataGBollatiMSillanoDLotrionteMBiondi-ZoccaiGNext-generation drug-eluting stents in coronary artery disease: focus on everolimus-eluting stent (Xience V)Vasc Health Risk Manag200841313818629361
- VirmaniRGuagliumiGFarbALocalized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious?Circulation2004109670170514744976
- CamenzindEStegPGWijnsWStent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concernCirculation20071151114401455 discussion 1455.17344324
- TogniMWindeckerSCocchiaRSirolimus-eluting stents associated with paradoxic coronary vasoconstrictionJ Am Coll Cardiol200546223123616022947
- JonerMFinnAVFarbAPathology of drug-eluting stents in humans: delayed healing and late thrombotic riskJ Am Coll Cardiol200648119320216814667
- KereiakesDJCannonLAFeldmanRLClinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trialJ Am Coll Cardiol201056426427120493653
- ChieffoAAranzullaTCColomboADrug eluting stents: focus on Cypher sirolimus-eluting coronary stents in the treatment of patients with bifurcation lesionsVasc Health Risk Manag20073444145117969375
- LefèvreTOrmistonJGuagliumiGThe Frontier stent registry: safety and feasibility of a novel dedicated stent for the treatment of bifurcation coronary artery lesionsJ Am Coll Cardiol200546459259816098421
- IkenoFKimYHLunaJAcute and long-term outcomes of the novel side access (SLK-View) stent for bifurcation coronary lesions: a multicenter non-randomized feasibility studyCatheter Cardiovasc Interv200667219820616404749
- OrmistonJWebsterMEl-JackSMcNabDPlaumannSSThe AST petal dedicated bifurcation stent: first-in-human experienceCatheter Cardiovasc Interv200770333534017722036
- DoiHMaeharaAMintzGSDaniLLeonMBGrubeESerial intravascular ultrasound analysis of bifurcation lesions treated using the novel self-expanding sideguard side branch stentAm J Cardiol200910491216122119840565
- GrubeEBifurcation and left main dedicated stentsProceedings of TCT2009 Sep 21–25San Francisco
- AlbieroRInvatec twin-rail stent: design features and clinical updatesProceedings of TCT2008 Oct 12–17Washington
- Van GeunsRJThe Minvasys Nile paclitaxel-eluting sidebranch access stent: results from the BiPAX studyProceedings of TCT2009 Sep 21–25San Francisco
- OnumaYMüllerRRamcharitarSTryton I, First-In-Man (FIM) study: six month clinical and angiographic outcome, analysis with new quantitative coronary angiography dedicated for bifurcation lesionsEuroIntervention20083554655219608479
- IschingerTANew devices for complex vascular interventions. The Y-Med catheter technologyProceedings of Euro PCR2006 May 16–19Paris, France
- HermillerJBThe TriReme Medical Antares sidebranch access stent: design specifications and clinical trial resultsProceedings of TCT2009 Sep 21–25San Francisco
- SheibanIOmedéPBiondi-ZoccaiGMorettiCSciutoFTreviGPUpdate on dedicated bifurcation stentsJ Interv Cardiol200922215015519379474
- GrubeEBuellesfeldLNeumannFJSix-month clinical and angiographic results of a dedicated drug-eluting stent for the treatment of coronary bifurcation narrowingsAm J Cardiol200799121691169717560877
- VerheyeSAgostoniPDuboisCL9-month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) studyJ Am Coll Cardiol200953121031103919298915
- OrmistonJALefèvreTGrubeEAlloccoDJDawkinsKDFirst human use of the TAXUS Petal paclitaxel-eluting bifurcation stentEuroIntervention201061465320542797
- Van GeunsRJThe Minvasys Nile paclitaxel-eluting sidebranch access stent: design specifications and clinical trial resultsProceedings of TCT2009 Sep 21–25San Francisco
- CostaRABIPAX bifurcation study: first resultsProceedings of Euro PCR2010 May 25–28Paris, France
- VerheyeSGrubeERamcharitarSFirst-in-man (FIM) study of the Stentys bifurcation stent – 30 days resultsEuroIntervention20094556657119378675
- VerheyeSLatest results of the STENTYS clinical programmeProceedings of Euro PCR2010 May 25–28Paris, France
- Van GeunsRJAPPOSITION II study resultsProceedings of TCT2010 Sep 21–25Washington
- RathoreSSmall coronary vessel angioplasty: outcomes and technical considerationsVasc Health Risk Manag2010691592221057576
- TanimotoSDaemenJSerruysPWUpdate on stents: recent studies on the TAXUS stent system in small vesselsVasc Health Risk Manag20073448149017969378
- AbizaidACde Ribamar CostaJJuniorWhitbournRJChangJCThe CardioMind coronary stent delivery system: stent delivery on a 0.014″ guidewire platformEuroIntervention20073115415719737701
- WhitbournRJThe CARDIOMIND stent-on-a-wire self-expanding micro-stent: results from CARE I (two year follow-up) and introduction to CARE II (sirolimus-eluting micro-stent)Proceedings of TCT2008 Oct 12–17Washington
- BotelhoRThe Cardiomind Sparrow DES program (CARE II): a bioabsorbable polymer sirolimus-eluting ‘micro-stent’Proceedings of TCT2009 Sep 21–25San Francisco
- AbizaidACCARE II 8 month follow-up results with the CardioMind 0.014″ Sparrow sirolimus-eluting nitinol stent systemJ Am Coll Cardiol201056B53
- MeredithITWorthleySWhitbournRRESOLUTE InvestigatorsClinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trialJACC Cardiovasc Interv200921097798519850258
- UdipiKMelderRJChenMThe next generation Endeavor Resolute Stent: role of the BioLinx Polymer SystemEuroIntervention20073113713919737697
- MeredithITWorthleySGWhitbournRLong-term clinical outcomes with the next-generation Resolute Stent System: a report of the two-year follow-up from the RESOLUTE clinical trialEuroIntervention20105669269720142220
- SilberSRESOLUTE: new insights from resolute all-comersProceedings of TCT2010 Sep 21–25Washington
- DaniSKukrejaNParikhPBiodegradable-polymer-based, sirolimus-eluting Supralimus stent: 6-month angiographic and 30-month clinical follow-up results from the series I prospective studyEuroIntervention200841596319112780
- CostaJSupralimus bioabsorbable-polymer sirolimus-eluting stent technology in patients with acute coronary syndrome undergoing percutaneous coronary intervention: initial results of the prospective, international, multicenter, E-SERIES registryProceedings of TCT2009 Sep 21–25San Francisco
- HanYJingQXuBCREATE (Multi-Center Registry of Excel Biodegradable Polymer Drug-Eluting Stents) InvestigatorsSafety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in ‘real-world’ practice: 18-month clinical and 9-month angiographic outcomesJACC Cardiovasc Interv20092430330919463441
- FaloticoRParkerTGrishaberRPriceSCohenSARogersCNEVO™: a new generation of sirolimus-eluting coronary stentEuroIntervention20095Suppl FF88F93
- SpauldingCOrmistonJAAbizaidAon behalf of the NEVO RES-I Investigators6 Months results of the NEVO RES-ELUTION I (RES-I) trial. A randomized, multi-center, single-blind comparison of the NEVO™ sirolimus-eluting coronary stent versus the TAXUS™ Liberté paclitaxel-eluting coronary stent system in de novo native coronary artery lesionsProceedings of Euro PCR2009 May 19–22Barcelona, Spain
- MauriLon behalf of NEVO RES-I InvestigatorsNEVO RES-elution trial – 12-month results and pivotal trial overviewProceedings of TCT2010 Sep 21–25Washington
- VranckxPSerruysPWGambhirSBiodegradable-polymer-based, paclitaxel-eluting infinnium stent: 9-month clinical and angio-graphic follow-up results from the SIMPLE II prospective multi-centre registry studyEuroIntervention20062331031719755306
- LemosPAMoulinBPerinMAPAINT Trial InvestigatorsRandomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-year results of the PAINT trialCatheter Cardiovasc Interv200974566567319670303
- GuagliumiGValsecchiOAprileAJACTAX paclitaxel-eluting stent program. Ultra-thin abluminal PLA polymer. Focus on the randomized OCTDESI strut coverage evaluationProceedings of TCT2009 Sep 21–25San Francisco
- GuagliumiGSirbuVMusumeciGStrut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: Optical Coherence Tomography Drug-Eluting Stent Investigation (OCTDESI)Circ Cardiovasc Interv20103436737520647562
- TurcoMAOrmistonJAPopmaJJPolymer-based, paclitaxel-eluting TAXUS Liberté stent in de novo lesions: the pivotal TAXUS ATLAS trialJ Am Coll Cardiol200749161676168317448368
- GrubeESchoferJHauptmannKEA novel paclitaxel-eluting stent with an ultrathin abluminal biodegradable polymer 9-month outcomes with the JACTAX HD stentJACC Cardiovasc Interv20103443143820398872
- SerruysPWSianosGAbizaidAThe effect of variable dose and release kinetics on neointimal hyperplasia using a novel paclitaxel-eluting stent platform: the Paclitaxel In-Stent Controlled Elution Study (PISCES)J Am Coll Cardiol200546225326016022951
- KaulUGuptaRKMathurACobalt chromium stent with anti-proliferative for restenosis trial in India (COSTAR I)Indian Heart J200759216517219122251
- DawkinsKDVerheyeSSchühlenHThe European cobalt STent with Antiproliferative for Restenosis trial (EuroSTAR): 12 month resultsEuroIntervention200731828819737689
- KrucoffMWKereiakesDJPetersenJLCOSTAR II Investigators GroupA novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease: primary results of the COSTAR (Cobalt Chromium Stent With Antiproliferative for Restenosis) II studyJ Am Coll Cardiol200851161543155218420096
- WesselyRHausleiterJMichaelisCInhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coatingArterioscler Thromb Vasc Biol200525474875315681298
- HausleiterJKastratiAWesselyRInvestigators of the Individualizable Drug-Eluting Stent System to Abrogate Restenosis ProjectPrevention of restenosis by a novel drug-eluting stent system with a dose-adjustable, polymer-free, on-site stent coatingEur Heart J200526151475148115975990
- MehilliJKastratiAWesselyRIntracoronary Stenting and Angiographic Restenosis–Test Equivalence Between 2 Drug-Eluting Stents (ISAR-TEST) Trial InvestigatorsRandomized trial of a non-polymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen lossCirculation2006113227327916391155
- FitzgeraldPCommentary: ISAR test 3Proceedings of TCT2008 Oct 12–17Washington
- RuefJStorgerHSchwarzFHaaseJComparison of a polymer-free rapamycin-eluting stent (YUKON) with a polymer-based paclitaxel-eluting stent (TAXUS) in real-world coronary artery lesionsCatheter Cardiovasc Interv200871333333918288747
- ByrneRAIijimaRMehilliJDurability of antirestenotic efficacy in drug-eluting stents with and without permanent polymerJACC Cardiovasc Interv20092429129919463439
- GrubeEBIOFREEDOM: a prospective randomized trial of polymer-free biolimus a9-eluting stents and paclitaxel-eluting stents in patients with coronary artery diseaseProceedings of TCT2009 Sep 21–25San Francisco
- TadaNVirmaniRGrantGPolymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine modelCirc Cardiovasc Interv20103217418320407114
- FajadetJThe Minvasys Amazonia PAX and Nile PAX polymer free paclitaxel eluting stent programProceedings of TCT2009 Sep 21–25San Francisco
- AbizaidAPAX A trial: 4-months follow-up resultsProceedings of Euro PCR2010 May 25–28Paris, France
- CostaJRJrAbizaidACostaR1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trialJACC Cardiovasc Interv20092542242719463465
- AbizaidAVESTAsync I update, and First Report of the VESTAsync II randomized trial with a hydroxyapatite polymer-free sirolimus-eluting stentProceedings of TCT2009 Sep 21–25San Francisco
- VerheyeSAgostoniPDawkinsKDThe GENESIS (Randomized, Multicenter Study of the Pimecrolimus-Eluting and Pimecrolimus/Paclitaxel-Eluting Coronary Stent System in Patients with De Novo Lesions of the Native Coronary Arteries) trialJACC Cardiovasc Interv20092320521419463427
- CostaJRJrAbizaidAFeresFEXCELLA First-in-Man (FIM) study: safety and efficacy of novolimus-eluting stent in de novo coronary lesionsEuroIntervention200841535819112779
- SerruysPWGargSAbizaidAA randomised comparison of novolimus-eluting and zotarolimus-eluting coronary stents: 9-month follow-up results of the EXCELLA II studyEuroIntervention20106219520520562069
- GargSSarnoGSerruysPWThe twelve-month outcomes of a biolimus eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymerEuroIntervention20106223323920562074
- KlaussVLEADERS: two-year follow-up from a prospective randomized trial of biolimus A9-eluting stents with a bioabsorbable polymer vs. sirolimus-eluting stents with a durable polymerProceedings of TCT2009 Sep 21–25San Francisco
- EstevesVAbizaidACentemeroMPFive-year clinical results of the biolimus A9 eluting stent with biodegradable polymerProceedings of Euro PCR2010 May 25–28Paris, France
- OstojicMSagicDBeleslinBFirst clinical comparison of Nobori-Biolimus A9 eluting stents with Cypher-Sirolimus eluting stents: Nobori Core nine months angiographic and one year clinical outcomesEuroIntervention20083557457919608483
- ChevalierBSilberSParkSJNOBORI 1 Clinical InvestigatorsRandomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial – Phase 2Circ Cardiovasc Interv20092318819520031715
- HamilosMIOstojicMBeleslinBNOBORI CORE InvestigatorsDifferential effects of drug-eluting stents on local endothelium-dependent coronary vasomotionJ Am Coll Cardiol200851222123212918510958
- RutschWWitzenbichlerBSchuhlenHMulticentre first-in-man study with the lowest known limus dose on the Elixir medical myolimus™ eluting coronary stent system with a durable polymer: 12-month clinical and six month angiographic and IVUS follow-upProceedings of Euro PCR2009 May 19–22Barcelona, Spain
- SchoeferJDudekDWitzenbichlerBMulticenter, first-in-man study on the Elixir Myolimus-eluting coronary stent system with bio-absorbable polymer: 12-month clinical and angiographic/IVUS resultsProceedings of Euro PCR2010 May 25–28Paris, France
- WykrzykowskaJJOnumaYSerruysPWAdvances in stent drug delivery: the future is in bioabsorbable stentsExpert Opin Drug Deliv20096211312619239384
- OrmistonJASerruysPWBioabsorbable coronary stentsCirc Cardiovasc Interv20092325526020031723
- OrmistonJASerruysPWRegarEA bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trialLancet2008371961689990718342684
- SerruysPWOrmistonJAOnumaYA bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methodsLancet2009373966789791019286089
- OrmistonJABVS cohort A: four year results and lessons learnedProceedings of TCT2010 Sep 21–25Washington
- TanimotoSSerruysPWThuesenLComparison of in vivo acute stent recoil between the bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting cobalt chromium coronary stent: insights from the ABSORB and SPIRIT trialsCatheter Cardiovasc Interv200770451552317503509
- García-GarcíaHMGonzaloNPawarRAssessment of the absorption process following bioabsorbable everolimus-eluting stent implantation: temporal changes in strain values and tissue composition using intravascular ultrasound radiofrequency data analysis. A sub-study of the ABSORB clinical trialEuroIntervention20094444344819284065
- SarnoGOnumaYGarcia GarciaHMIVUS radiofrequency analysis in the evaluation of the polymeric struts of the bioabsorbable everolimus-eluting device during the bioabsorption processCatheter Cardiovasc Interv201075691491820091822
- OkamuraTGargSGutiérrez-ChicoJLIn vivo evaluation of stent strut distribution patterns in the bioabsorbable everolimus-eluting device: an OCT ad hoc analysis of the revision 1.0 and revision 1.1 stent design in the ABSORB clinical trialEuroIntervention20105893293820542778
- SerruysPWBVS cohort B: device design, 6-month update, and first report of the 12-month resultsProceedings of TCT2010 Sep 21–25Washington
- KaluzaGLCurrent status of polymeric biodegradable drug eluting stents II: REVA Medical, IncProceedings of TCT2006 Oct 22–27Washington
- GrubeEThe REVA tyrosine-derived polycarbonate bioabsorbable stent: final results from the RESORB first-in-man clinical trial and next generation designsProceedings of TCT2008 Oct 12–17Washington
- PollmanMJEngineering a bioresorbable stent: REVA programme updateEuroIntervention20095Suppl FF54F57
- AbizaidAThe REVA tyrosine-derived polycarbonate bioabsorbable stent: lessons learned and future directionsProceedings of TCT2009 Sep 21–25San Francisco
- JabaraRPendyalaLGevaSChenJChronosNRobinsonKNovel fully bioabsorbale salicylate-based sirolimuseluting stentEuroIntervention20095Suppl FF58F64
- AbizaidAThe BTI salycilate-based polyanhydride ester absorbable sirolimus-eluting stent: update from the Whisper studyProceedings of TCT2008 Oct 12–17Washington
- JabaraRPoly-anhydride based on salicylic acid and adipic acid anhydrideProceedings of Euro PCR2009 May 19–22Barcelona, Spain
- TamaiHIgakiKKyoEInitial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humansCirculation2000102439940410908211
- NishioSLong-term (>10 years) clinical outcomes of first-in-man biodegradable poly-l-lactic acid coronary stentsProceedings of Euro PCR2010 May 25–28Paris, France
- OnumaYGargSOkamuraTTen-year follow-up of the IGAKI–TAMAI stent. A posthumous tribute to the scientific work of Dr. Hideo TamaiEuroIntervention20095Suppl FF109F111
- VogtFSteinARettemeierGLong-term assessment of a novel biodegradable paclitaxel-eluting coronary polylactide stentEur Heart J200425151330134015288161
- HeubleinBRohdeRKaeseVNiemeyerMHartungWHaverichABiocorrosion of magnesium alloys: a new principle in cardiovascular implant technology?Heart200389665165612748224
- ErbelRDi MarioCBartunekJPROGRESS-AMS (Clinical Performance and Angiographic Results of Coronary Stenting with Absorbable Metal Stents) InvestigatorsTemporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trialLancet200736995761869187517544767
- WaksmanRCurrent state of the absorbable metallic (magnesium) stentEuroIntervention20095Suppl FF94F98