Abstract
Antiplatelet therapy plays a fundamental role in reducing atherothrombotic events by several pathways. The present work reviews available evidence on antiplatelet therapy both for primary prevention and in the presence of established peripheral, cerebral, or cardiac ischemic disease. Due to the importance of adherence to therapy to achieve optimal effects, special attention is given to the use of fixed-dose oral formulations in the clinical subset of patients in whom double antiplatelet therapy has proven indications.
Introduction
Interaction between platelets and the endothelium, whilst a valuable mechanism under normal conditions to stop bleeding from a wound, is involved in the majority of morbidity and mortality events occurring in Western countries.Citation1–Citation3
In fact platelets, an enucleated bone marrow product, ensure vascular integrity by differencing lesions from normal endothelium. This ability depends on contact of the platelet with several extracellular matrix constituents, including von Willebrand factor, proteoglycans, collagen type IV, nidogen, laminin, and fibulin.Citation3,Citation4
In this context, adenosine diphosphate (ADP) is an important platelet activator, inducing A2 thromboxane synthesis (). For example, clopidogrel reduces platelet aggregation by irreversible binding inactivation of ADP receptors (P2Y12), a requisite for glycoprotein IIb/IIIa receptor activation, whereas aspirin (ASA) inhibits thromboxane A2 production with consequent glycoprotein IIb/IIIa receptor inactivation.Citation6–Citation8
Figure 1 Platelet inhibition mechanisms.
Copyright © 2003, American College of Cardiology. Reproduced with permission from Metha SR, Yousuf S. Short- and long-term oral antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention. J Am Coll Cardiol. 2003;41(4 Suppl S):79S–88S.
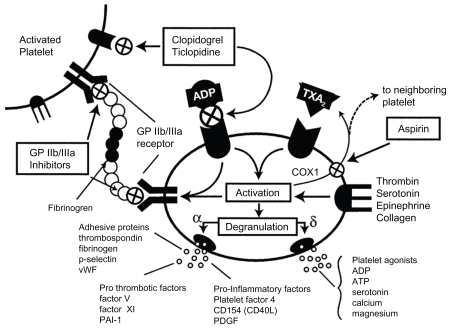
Considering the striking role of platelet and endothelial interaction in the pathogenesis of atherothrombotic events, the possibility of reversible or irreversible modulation of platelet activation represents one of most imperative contemporary medical challenges. Citation9 The present work reviews available evidence for antiplatelet therapy both in primary and secondary prevention. Due to the importance of adherence to therapy for achieving the desired effects, special attention is paid to the use of fixed-dose oral formulations in the clinical subset of patients in whom double antiplatelet therapy has proven indications.
Primary prevention
Single antiplatelet therapy in primary prevention is widely debated.Citation11–Citation13 Several studies focusing on this topic have included different populations with regard to gender prevalence, diabetes mellitus status, and baseline medication use (eg, statin treatment). For these reasons, clear evidence is lacking in high-risk populations. The major clinical trials testing the effects of single antiplatelet therapy in primary prevention are listed in .
Table 1 Major clinical trials testing the effects of single antiplatelet therapy in primary prevention
A randomized clinical trial by Sacco et al tested the effect of ASA 100 mg/day plus vitamin E 300 mg/day on the composite endpoint of cardiovascular death, stroke, or myocardial infarction in patients with at least one cardiovascular risk factor (n = 4495). After a median follow up of 3.7 years, ASA treatment was associated with a nonsignificant reduction in the main endpoint (relative risk [RR] 0.90; 95% confidence interval [CI]: 0.50–1.62) in diabetic patients, accompanied by a similarly nonsignificant increase in cardiovascular deaths (RR 1.23; 95% CI: 0.69–2.19). However, in nondiabetic patients with at least one other cardiovascular risk factor, total cardiovascular events were significantly reduced (RR 0.59; 95% CI: 0.37– 0.94).Citation14
In another randomized trial, Ridker et al assigned nearly 40,000 healthy women to receive ASA 100 mg or placebo and compared outcomes (nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) at 10-year follow-up. At the end of the study, the risk of stroke was reduced by 17% in the ASA versus the placebo group (RR 0.83; 95% CI: 0.69–0.99). While the risk of ischemic stroke was reduced by 24% (RR 0.76; 95% CI: 0.63–0.93), the increase in hemorrhagic risk was not significant (RR 1.24; 95% CI: 0.82–1.87). Fatal or nonfatal myocardial infarction was not affected by ASA use (RR 1.02; 95% CI: 0.84–1.25), as cardiovascular death (RR 0.95; 95% CI: 0.74–1.22). Of note is that gastrointestinal bleeding was more frequent in the ASA arm (RR 1.40; 95% CI: 1.07–1.83). The authors of this trial concluded that ASA treatment seemed to lead to superior outcomes compared with placebo only in women aged over 65 years.Citation15
Fowkes et al selected patients at increased risk, without previous cardiovascular events, by an atherosclerosis indicator, ie, impaired ankle brachial index.Citation16 Over 3000 patients were enrolled and were randomized to receive ASA 100 mg daily or placebo. The primary endpoint was a composite of fatal or nonfatal coronary events, stroke, or revascularization. The secondary endpoint was all-cause mortality. After the eight-year follow-up, no difference emerged concerning the primary endpoint (RR 1.03; 95% CI: 0.84–1.27). Also, for all-cause mortality, the study groups achieved similar results (RR 0.95; 95% CI: 0.77–1.16). However, the risk of major hemorrhage was significantly increased in the ASA group (RR 1.71; 95% CI: 0.99–2.97).
Due to the equivocal results of the aforementioned and other clinical randomized trials, several meta-analyses have been performed and recently published. Berger et al included in their meta-analysis all randomized clinical trials evaluating the role of ASA in primary prevention in the years 1966–2005. Approximately 95,000 patients were considered. In women, a significant 12% reduction in cardiovascular events (RR 0.88; 95% CI: 0.79–0.99) and a 17% reduction in strokes (RR 0.83; 95% CI: 0.70–0.97) was reported for ASA arms in these studies. No significant reduction in myocardial infarction or cardiovascular mortality was detected. In men, ASA therapy was associated with a significant 14% reduction in cardiovascular events (RR 0.86; 95% CI: 0.78–0.94) and a 32% reduction in myocardial infarction (RR 0.68; 95% CI: 0.54–0.86). There was no significant effect on stroke or cardiovascular mortality.Citation17
De Berardis et al performed a meta-analysis of randomized controlled trials to evaluate the effect of low-dose ASA versus placebo in people with diabetes and without cardiovascular disease. The primary endpoint was major cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, and all-cause mortality). Six studies were evaluated, with a total of approximately 10,000 patients. Aspirin was not associated with a statistically significant reduction in risk of major cardiovascular events (RR 0.90; 95% CI: 0.81–1.00), cardiovascular mortality (RR 0.94; 95% CI: 0.72–1.23), or all-cause mortality (RR 0.93; 95% CI: 0.82–1.05). However, the risk of myocardial infarction was significantly reduced in the ASA arm, but only in males (RR 0.57; 95% CI: 0.34–0.94). Overall, bleeding events were not increased (RR 2.50; 95% CI: 0.76–8.21).Citation18
As in the previous study, Younis et al evaluated the role of ASA for primary prevention in diabetic patients. The authors included in their work six randomized clinical trials in 7000 patients. Aspirin use did not play a protective role in overall mortality (RR 0.96; 95% CI: 0.78–1.18), major cardiovascular events (RR 0.90; 95% CI: 0.78–1.05), or myocardial infarction (RR 0.95; 95% CI: 0.76–1.18). Also, according to this study, bleeding risk was not significantly increased in the ASA arm compared with placebo (RR 2.49; 95% CI: 0.70–8.84).Citation19
When including populations with known cardiovascular risk factors, such as hypertension, diabetes, or peripheral vascular disease, the benefits of antiplatelet therapy are offset by the increase in hemorrhagic risk.Citation20–Citation23 McQuaid and Laine performed a systematic review focusing on bleeding risk in cardiovascular prophylaxis using ASA. Aspirin increased the risk of major bleeding (RR 1.71; 95% 1.41–2.08), major gastrointestinal bleeding (RR 2.07; 95% CI: 1.61–2.66), and intracranial bleeding (RR 1.65; 95% CI: 1.06–5.99) versus placebo. Of the studies included in this review, only one reported an increase in gastrointestinal bleeding with ASA versus clopidogrel (RR 1.45; 95% CI: 1.00–2.10). However, the number needed to treat with clopidogrel versus ASA to prevent one gastrointestinal bleeding episode was at least 800 (at a cost of about one million dollars).Citation24
Based on the aforementioned evidence, the current American College of Cardiology/American Heart Association guidelines recommend ASA for primary prevention only in men with diabetes and intermediate cardiovascular risk, and without an increased risk of bleeding.Citation21 On the other hand, the European Society of Cardiology guidelines do not recommend ASA in primary prevention.Citation25,Citation26
Secondary prevention
Single antiplatelet therapy
In patients with previous peripheral, cerebral, or cardiac ischemic events, antiplatelet therapy has clear beneficial effects. Major clinical trials testing the effects of single antiplatelet therapy in secondary prevention are listed in . However, the increased risk of antiplatelet-related bleeding is well known and needs to be kept in mind.
Table 2 Major clinical trials testing the effects of single antiplatelet therapy in secondary prevention
Baigent et al performed a meta-analysis of 16 randomized trials evaluating ASA for secondary prevention in approximately 17,000 patients. The occurrence of serious events decreased in the ASA group (6.7% versus 8.2% per year; P < 0.0001), with reductions of about 20% in total strokes (2.08% versus 2.54% per year; P = 0.002) and in coronary events (4.3% versus 5.3% per year; P < 0.0001). These results were achieved with a nonsignificant increase in hemorrhagic stroke (0.04% versus 0.03%; P = 0.05).Citation11
In search of ways to reduce the gastrointestinal side effects of ASA, Garcia et al identified over 2000 patients who suffered from gastrointestinal bleeding and compared these patients with randomly selected controls. The RR of gastrointestinal bleeding was 1.9 (95% CI: 1.6–2.3), without significant differences between standard and enteric-coated ASA.Citation27,Citation28
Besides ASA, other antiplatelet drugs have been proven to be effective in secondary prevention. In CAPRIE (Clopidogrel versus Aspirin in Patients at risk of Ischaemic Events), clopidogrel therapy was compared with ASA in a randomized manner in 19,000 patients with previous cardiovascular events. At the end of two years of follow-up, the authors reported a significant risk reduction of the primary endpoint (ischemic stroke, myocardial infarction, or vascular death) in the clopidogrel group (8.7%; 95% CI: 0.3–16.5) compared with ASA, with a similar safety profile between the two drugs (any stroke, myocardial infarction, or death from any cause being 7.0%; 95% CI: 0.9–14.2).Citation29
Furthermore, several studies evaluating ASA monotherapy versus thienopyridines have been performed. In the STAMI (Ticlopidine Versus Aspirin After Myocardial Infarction) trial, ticlopidine was compared with ASA following thrombolysis. The primary endpoint was the composite of fatal and nonfatal myocardial infarction, fatal and nonfatal stroke, angina with objective evidence of myocardial ischemia, and vascular death. At the end of the study, the primary endpoint occurred in 8% of patients, without differences between the groups.Citation30
TASS (the Ticlopidine Aspirin Stroke Study) evaluated the effects of ticlopidine versus ASA in patients with recent ischemic cerebral events. The three-year event rate for nonfatal stroke or death from any cause was 17% and 19% in the ticlopidine and ASA arms, respectively (P = 0.048).Citation31
In the future, newer compounds, including ticagrelor and prasugrel, will provide supplementary data on the effectiveness of antiplatelet therapy in the secondary prevention setting.Citation32
Double antiplatelet therapy
In high atherothrombotic risk conditions, given the superior antithrombotic effect derived from multiple platelet pathway inhibition, double antiplatelet therapy has been proposed to further reduce cardiovascular events ().Citation33–Citation35
Table 3 Major clinical trials testing the effects of double antiplatelet therapy
In the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial, patients with known cardiovascular disease or with multiple atherothrombotic risk factors were randomized to ASA alone versus ASA plus clopidogrel. Although the trial was intended to include a primary prevention population, the presence of diabetes mellitus and/or its complications in the vast majority of participants does not permit the results to be considered generalizable to a true primary prevention context.
The primary endpoint was a composite of vascular death, myocardial infarction, or stroke. At the end of the 28-month follow-up, the primary endpoint occurred in 6.8% of patients on clopidogrel plus ASA versus 7.3% in the placebo plus ASA arm (P = 0.22), but the death rate from cardiovascular causes was higher in the clopidogrel plus ASA group (3.9% versus 2.%; P = 0.01). Death rate for any cause was similar between the groups at 0.99 (95% CI: 0.86–1.14).Citation36
The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial was the first to evaluate double antiplatelet therapy following an acute coronary syndrome. In this study, over 200 patients with non-ST elevation acute coronary syndrome were randomized to pretreatment plus long-term therapy with ASA plus clopidogrel versus ASA plus placebo. The primary endpoint (the composite of cardiovascular death, myocardial infarction, or urgent target vessel revascularization within 30 days of percutaneous coronary intervention) occurred less frequently in the active treatment group. Long-term clopidogrel plus ASA treatment was associated with lower rates of cardiovascular death, myocardial infarction, or any revascularization (P = 0.03), and of cardiovascular death or myocardial infarction (P = 0.047).Citation37
The long-term effectiveness of dual antiplatelet therapy was later confirmed by the CREDO (Clopidogrel for the Reduction of Events During Observation) trial. In this study, over 2000 patients who were candidates for elective percutaneous coronary intervention were randomized to ASA plus clopidogrel therapy for 12 months versus ASA plus clopidogrel for 28 days. From day 29, placebo patients were treated with ASA only. The primary endpoint (a composite of death, myocardial infarction, or stroke) at one-year follow-up was reduced in the clopidogrel plus ASA group (RR 26.9%; P = 0.02). On other hand, the bleeding risk was not significantly increased in the clopidogrel plus ASA group (8.8% versus 6.7%; P = 0.07).Citation38
In the CLARITY-TIMI (Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis In Myocardial Infarction) trial, patients with ST segment elevation myocardial infarction undergoing fibrinolytic treatment were randomized to clopidogrel (including a loading dose) or placebo. The primary outcome (composite of cardiovascular death, recurrent myocardial infarction, or stroke from percutaneous coronary intervention 30 days after randomization) decreased significantly in the clopidogrel arm (3.6% versus 6.2%; P = 0.008). The clopidogrel group also reported a reduction in myocardial infarction and stroke in patients undergoing a percutaneous coronary procedure (4.0% versus 6.2%; P = 0.03). These results were obtained in the absence of an increase in the risk of bleeding in the clopidogrel arm (2.0% versus 1.9% in the two groups, respectively; P = 0.99).Citation39
Guided by the extreme need of evidence-based data on this topic, a large meta-analysis, including nearly 80,000 patients from the major clinical trials, eg, CURE, CREDO, CLARITY, CHARISMA, and COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) confirmed a lower incidence of all-cause mortality in the ASA plus clopidogrel group compared with the ASA alone group (6.3% versus 6.7%; P = 0.026). Myocardial infarction occurred in 2.7% of the patients on clopidogrel plus ASA compared with 3.3% (P < 0.0001) on ASA alone, and occurrence of stroke was 1.2% and 1.4%, respectively, in the two groups (P = 0.002). Major bleeding was more frequently reported in the double antiplatelet therapy group (1.6% versus 1.3%, respectively; P < 0.0001), however without a significant difference in terms of fatal bleeding (0.28% and 0.27%, respectively; P = 0.79).Citation40
Following the first experience of single antiplatelet agents, the new third-generation thienopyridines, including ticagrelor and prasugrel, have been also suggested for combination treatment. The rapid onset of action and lower probability of nonresponsiveness accompanied by the similar safety profile of prasugrel, for example, compared with clopidogrel, represents a promising step forward in antiplatelet therapy.Citation41
The TRITON-TIMI (Clopidogrel in Acute Coronary Syndrome Subjects Who Are to Undergo Percutaneous Coronary Intervention-Thrombolysis in Myocardial Infarction)Citation38 trial validated the use of prasugrel. This trial randomized 13,000 patients with acute coronary syndromes and planned coronary percutaneous intervention to receive clopidogrel plus ASA versus prasugrel plus ASA. The primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The primary endpoint occurred in 9.9% patients on prasugrel plus ASA versus 12.1% on clopidogrel plus ASA (P < 0.001). Furthermore, the occurrence of myocardial infarction was significantly reduced in the prasugrel arm (9.7% versus 7.4%; P < 0.001). On the other hand, major bleeding occurred in 1.8% of patients in the clopidogrel plus ASA group versus 2.4% of those in the prasugrel plus ASA group (P = 0.03). Life-threatening bleeding was also significantly increased in the prasugrel group (1.4% versus 0.9%; P = 0.01).Citation42
More recently, ticagrelor, a newer antiplatelet agent, has been tested in patients with ischemic heart disease. Compared with clopidogrel, this reversible antiplatelet drug has shown a reduction of cardiovascular death, myocardial infarction, and stroke (9% versus 10.7%; P = 0.0025) with no increase in major bleeding rates (11.5% versus 11.6%; P = 0.8).Citation43
In addition to the aforementioned experience, double antiplatelet therapy has been tested in other clinical settings, including transitory ischemic attacks and stroke. In the randomized MATCH (Management of Atherothrombosis With Clopidogrel in High-Risk Patients) trial, ASA plus clopidogrel was compared with clopidogrel alone in nearly 8000 high-risk patients with a recent ischemic stroke or transient ischemic attack and at least one additional vascular risk factor. The primary endpoint was a composite of ischemic stroke, myocardial infarction, vascular death, or rehospitalization for acute ischemia. At the end of the study, the endpoint occurred in 15.7% in the clopidogrel plus ASA arm versus 16.7% in the clopidogrel alone arm (P = 0.24). However, life-threatening bleeding episodes were higher in the group receiving ASA and clopidogrel versus clopidogrel alone (2.6% versus 1.3%; P < 0.001). Based on these results, the authors concluded that, in this specific clinical setting, adding ASA to clopidogrel is not recommended.Citation44
Fixed-dose antiplatelet combinations
Keeping in mind the consequences induced by partial or complete suspension of double antiplatelet therapy, patient compliance plays a fundamental role. Collet et al designed the nationwide REGINA survey to evaluate how the interruption of dual oral antiplatelet therapy is managed in “real world” practice. Over 2000 physicians were randomly selected to participate in a computer-assisted telephone interview. Knowledge about drug-eluting stents and dual oral antiplatelet therapy was appraised by multiple-choice questions. Based on data from the 93% of the responding practitioners who completed the interview, unjustified interruption of dual oral antiplatelet therapy was quite frequent (22%). Low-molecular-weight heparin was the substituted therapy in over two-thirds of cases (without available evidence) and was associated with longer periods of dual oral antiplatelet therapy interruption.Citation45
This evidence highlights the actual problem of therapy adherence, and the severe consequences (eg, stent thrombosis following percutaneous coronary intervention) related to lack of patient compliance with the prescribed treatment. Besides the impact on the individual’s health status, lack of adherence to medical prescriptions costs about $100 billion per year in the US.Citation46
Treatment adherence is defined as “the extent to which patients take medications as prescribed by their health care providers”. “Compliance” indicates passive patient obedience to doctor prescription, and “adherence” involves a major comprehension level by the patient. Although poor adherence depends on several factors, including provider–patient interaction and drug cost, the number of administrations per day, is indeed a non-negligible element.Citation47–Citation51
In this context, in patients for whom double antiplatelet therapy has proven indications, fixed-dose formulations have a promising role. In patients at high cardiovascular risk, who are often on multiple drug treatment, combining administration of two antiplatelet agents in a single pill, thereby reducing the number of administrations per day represents an excellent option to improve treatment adherence.Citation52,Citation53
The first fixed-dose combination available was Aggrenox® (Boehringer Ingelheim, Ingelheim am Rhein, Germany) containing ASA 25 mg and extended-release dipyridamole 200 mg. Serebruany et al evaluated the results of a combination of extended-release dipyridamole (ER-DP) and low-dose ASA compared with clopidogrel with or without ASA in diabetic patients. The study enrolled 60 patients aged over 40 years, with a diagnosis of type 2 diabetes and a history of transient ischemic attacks. Patients were allocated to receive ER-DP + ASA 200/25 mg twice daily, clopidogrel 75 mg/day, or a daily combination of clopidogrel 75 mg and ASA 81 mg.Citation53 The primary endpoint was the change in platelet receptor expression at 30-day follow-up. ER-DP and ASA therapy was associated with a significant reduction in the glycoprotein IIb/IIIa receptor, clopidogrel monotherapy was associated with ADP-induced platelet aggregation, and the ASA and clopidogrel combination was associated with inhibition of collagen-induced platelet aggregation. These interesting data indeed clarified the molecular action of the best known antiplatelet therapy, but did not add information concerning clinical outcomes.Citation54,Citation55
Knowledge regarding the association between clopidogrel and ASA is to date elementary. From a pharmacokinetic point of view, clopidogrel is rapidly absorbed, reaching peak plasma levels at approximately one hour following dosing. However, the active formulation is formed by oxidation to 2-oxo-clopidogrel and subsequent hydrolysis, regulated primarily by cytochrome P450. The active metabolite binds irreversibly to platelet receptors. Clopidogrel metabolites are then excreted 50% in the urine and 50% in the feces, with a half-life for the main circulating metabolite of about eight hours. On the other hand, ASA is rapidly hydrolyzed in plasma to salicylic acid, with a half-life of 20 minutes, and peak salicylic acid concentrations occur one hour after administration. Salicylic acid is then conjugated primarily in the liver to form salicyluric acid. The half-life of these molecules is about two hours and metabolites are excreted in the urine.
Based on these presumptions, two fixed-dose combination tablets containing ASA and clopidogrel have recently been formulated, ie, DuoPlavin®/DuoCover®, a clopidogrel 75 mg and acetylsalicylic acid 100 mg or 75 mg combination (sanofi-aventis, Paris, France; Bristol-Myers Squibb, New York, NY) and CLOPIVAS-AP®, a fixed-dose combination of clopidogrel 75 mg and ASA 75 mg or 100 mg (Cipla, Mumbai, India).
To date, these formulations are under careful investigation, and the European Medicines Agency has recently requested scientific data concerning bioequivalence and pharmacokinetics before allowing full product marketing. In the event of favorable results, a single tablet with two fixed-dose antiplatelet drugs may be used in a wide spectrum of patients moving towards superior patient adherence, relevant especially in high-risk cardiovascular patients (eg, following coronary intervention), without an increase in side effects or bleeding risk.
Conclusion
Antiplatelet therapy plays a fundamental role in reducing atherothrombotic events in patients at risk via several pathways. The possibility of reversible or irreversible modulation of platelet activation represents one of most important contemporary medical challenges. When fixed-dose formulation tablets become available, adherence with double platelet therapy may increase, with substantial benefits not only for patients, but also for public health expenditure.
Disclosure
The authors report no conflicts of interest in this work.
References
- WareJAHeistadDDSeminars in medicine of the Beth Israel Hospital, Boston. Platelet-endothelium interactionsN Engl J Med199332896286358429855
- KwaanHCSamamaMMThe significance of endothelial heterogeneity in thrombosis and hemostasisSemin Thromb Hemost201036328630020490979
- FusterVSteinBAmbroseJAAtherosclerotic plaque rupture and thrombosis. Evolving conceptsCirculation1990823 SupplII47II592203564
- SaelmanEUNieuwenhuisHKHeseKMPlatelet adhesion to collagen types I through VIII under conditions of stasis and flow is mediated by GPIa/IIa (α2β1-integrin)Blood1994835124412508118028
- SavageBGinsbergMHRuggeriZMInfluence of fibrillar collagen structure on the mechanisms of platelet thrombus formation under flowBlood19999482704271510515874
- SternDMKaiserENawrothPRegulation of the coagulation system by vascular endothelial cellsHaemostasis19881846202214
- JinJDanielJLKunapuliSPMolecular basis for ADP-induced platelet activation: II. The P2YI receptor mediates ADP-induced intracellular calcium mobilization and shape change in plateletsJ Biol Chem19982734203020349442040
- RuggeriZMPlatelets in atherothrombosisNat Med20028111227123412411949
- AngiolilloDJGuzmanLABassTACurrent antiplatelet therapies: Benefits and limitationsAm Heart J20081562 SupplS3S918657680
- MehtaSRYousufSShort- and long-term oral antiplatelet therapy in acute coronary syndromes and percutaneous coronary interventionJ Am Coll Cardiol2003414 Suppl S79S88S12644345
- BaigentCBlackwellLCollinsRAntithrombotic Trialists’ (ATT) CollaborationAspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trialsLancet200937396781849186019482214
- WolffTMillerTKoSAspirin for the primary prevention of cardiovascular events: An update of the evidence for the US Preventive Services Task ForceAnn Intern Med2009150640541019293073
- US Preventive Services Task ForceAspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statementAnn Intern Med2009150639640419293072
- SaccoMPellegriniFRoncaglioniMCPrimary prevention of cardiovascular events with low-dose ASA and vitamin E in type 2 diabetic patients: Results of the Primary Prevention Project (PPP) trialDiabetes Care200326123264327214633812
- RidkerPMCookNRLeeIMA randomized trial of low-dose ASA in the primary prevention of cardiovascular disease in womenN Engl J Med2005352131293130415753114
- FowkesFGRPriceJFStewartMCWAspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index, a randomized controlled trialJAMA2010303984184820197530
- BergerJSRoncaglioniMCAvanziniFPangrazziITognoniGBrownDLAspirin for the primary prevention of cardiovascular events in women and men: A sex-specific meta-analysis of randomized controlled trialsJAMA2006295330631316418466
- De BerardisGSaccoMStrippoliGFAspirin for primary prevention of cardiovascular events in people with diabetes: Meta-analysis of randomised controlled trialsBMJ2009339b453119897665
- YounisNWilliamsSAmmoriBSoranHRole of ASA in the primary prevention of cardiovascular disease in diabetes mellitus: A meta-analysisExpert Opin Pharmacother20101191459146620429671
- LipGYHFelmedenDCAntiplatelet agents and anticoagulants for hypertensionCochrane Database Syst Rev20043CD00318615266473
- BelchJMacCuishACampbellIThe prevention of progression of arterial disease and diabetes (POPADAD) trial: Factorial randomised placebo controlled trial of ASA and antioxidants in patients with diabetes and asymptomatic peripheral arterial diseaseBMJ2008337a184018927173
- OgawaHNakayamaMMorimotoTLow-dose ASA for primary prevention of atherosclerotic events in patients with type 2 diabetesJAMA2008300182134214118997198
- MohlerERIIICombination antiplatelet therapy in patients with peripheral arterial disease: Is the best therapy ASA, clopidogrel, or both?Cath Cardiovasc Interv200974Suppl 1S1S6
- McQuaidKRLaineLSystematic review and meta-analysis of adverse events of low-dose ASA and clopidogrel in randomized controlled trialsAm J Med2006119862463816887404
- PignoneMAlbertsMJColwellJAAspirin for primary prevention of cardiovascular events in people with diabetesJ Am Coll Cardiol201055252878288620579547
- NicolucciADe BerardisGSaccoMTognoniGAHA/ADA vs ESC/EASD recommendations on ASA as a primary prevention strategy in people with diabetes: How the same data generate divergent conclusionsEur Heart J200728161925192717604291
- BarnettHBurrillPIheanachoIDon’t use ASA for primary prevention of cardiovascular diseaseBMJ2010340c180520410163
- Garcia RodriguezLAHernandez-DiazSde AbajoFJAssociation between ASA and upper gastrointestinal complications: Systematic review of epidemiologic studiesBr J Clin Pharmacol200152556357111736865
- CAPRIE Steering CommitteeA randomised, blinded, trial of clopidogrel versus ASA in patients at risk of ischaemic events (CAPRIE)Lancet19963489038132913398918275
- ScrutinioDCimminielloCMarubiniEPitzalisMVDi BiaseMRizzonPTiclopidine versus ASA after myocardial infarction (STAMI) trialJ Am Coll Cardiol20013751259126511300432
- HassWKEastonJDAdamsHPJrA randomized trial comparing ticlopidine hydrochloride with ASA for the prevention of stroke in high-risk patients: Ticlopidine Aspirin Stroke Study GroupN Engl J Med198932185015072761587
- Biondi ZoccaiGLotrionteMGaitaFAlternatives to clopidogrel for acute coronary syndromes: Prasugrel or ticagrelor?World J Cardiol20102613113421160730 CravenLLMiss Valley Med J195678521321513358612
- HarkerLAMarzecUMKellyABClopidogrel inhibition of stent, graft, and vascular thrombogenesis with antithrombotic enhancement by ASA in nonhuman primatesCirculation19989822246124699832493
- LeonMBBaimDSPopmaJJA clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting: Stent Anticoagulation Restenosis Study InvestigatorsN Engl J Med199833923166516719834303
- BhattDLFoxKAHackeWClopidogrel and ASA versus ASA alone for the prevention of atherothrombotic eventsN Engl J Med2006354161706171716531616
- BergerPBBhattDLFusterVBleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: Results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trialCirculation2010121232575258320516378
- MehtaSRYusufSPetersRJfor the Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) InvestigatorsEffects of pretreatment with clopidogrel and ASA followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE studyLancet2001358928152753311520521
- SteinhublSRBergerPBMannJTFryETDeLagoAWilmerCfor the CREDO Investigators, Clopidogrel for the Reduction of Events During ObservationEarly and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trialJAMA2002288192411242012435254
- SabatineMSCannonCPGibsonCMfor the CLARITY-TIMI 28 InvestigatorsAddition of clopidogrel to ASA and fibrinolytic therapy for myocardial infarction with ST-segment elevationN Engl J Med2005352121179118915758000
- HeltonTJBavryAAKumbhaniDJDuggalSRoukozHBhattDLIncremental effect of clopidogrel on important outcomes in patients with cardiovascular disease: A meta-analysis of randomized trialsAm J Cardiovasc Drugs20077428929717696569
- GurbelPATantryUSPrasugrel, a third generation thienopyridine and potent platelet inhibitorCurr Opin Investig Drugs200893324336
- WiviottSDBraunwaldEMcCabeCHPrasugrel versus clopidogrel in patients with acute coronary syndromesN Engl J Med2007357202001201517982182
- CannonCPHarringtonRAJamesFComparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): A randomised double-blind studyLancet2010375971128329320079528
- DienerHCBogousslavskyJBrassLMfor the MATCH InvestigatorsAspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trialLancet2004364943133133715276392
- ColletJPAoutMAlantarAReal-life management of dual antiplatelet therapy interruption: The REGINA surveyArch Cardiovasc Dis20091021069771019913771
- SenstBLAchusimLEGenestRPPractical approach to determining costs and frequency of adverse drug events in a health care networkAm J Health Syst Pharm200158121126113211449856
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- SteinerJFEarnestMAThe language of medication-takingAnn Intern Med20001321192693010836931
- BaileyWCRichardsJMJrBrooksCMSoongSJWindsorRAManzellaBAA randomized trial to improve self-management practices of adults with asthmaArch Intern Med19901508166416682200380
- MacIntyreCRGoebelKBrownGVSkullSStarrMFullinfawROA randomised controlled clinical trial of the efficacy of family based direct observation of anti-tuberculosis treatment in an urban, developed-country settingInt J Tuberc Lung Dis20037984885412971668
- YusufSPaisPAfzalRThe Indian Polycap Study (TIPS)Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): A phase II, double-blind, randomised trialLancet200937396721341135119339045
- HaynesRBAcklooESahotaNMcDonaldHPYaoXInterventions for enhancing medication adherenceCochrane Database Syst Rev20082CD00001118425859
- GreenbergRNOverview of patient compliance with medication dosing: A literature reviewClin Ther1984655925996383611
- LenzTlHillemanDEAggrenox: A fixed-dose combination of ASA and dipyridamoleAnn Pharmacother200034111283129011098344
- SerebruanyVLMalininAPokovAAntiplatelet profiles of the fixed dose combination of extended release dipyridamole and low-dose ASA compared with clopidogrel with or without ASA in patients with type 2 diabetes and history of transient ischemic attack: A randomized, single bind, 30-day trialClin Ther200830224925918343263