Abstract
Chronic pelvic pain (CPP) affects 24% of premenopausal women, accounts for 20%–30% of UK gynecology outpatient appointments, and has an annual pan-European economic cost of €3.8 billion. Despite extensive investigation, often including laparoscopy, up to 55% of women do not receive a diagnosis and endure persistent symptoms. In these patients, clinical management focuses on symptom control rather than treatment. It is possible that pelvic vein incompetence (PVI) is a cause of CPP, although the quality of studies investigating an association is generally low. PVI may develop during and after pregnancy, as uterine blood flow increases significantly, pushing venous valve leaflets apart, and enabling retrograde venous flow. Analogies with varicose veins of the lower limb are helpful, and symptoms are similar. Women with symptomatic PVI report a dull pelvic ache that is worse on standing and sitting and persists throughout the day. It can be relieved by lying down. Early treatments for PVI included laparoscopic ligation; however, since the advent of endovascular occlusive techniques, treatments have lower risk and lower cost, and can be undertaken without sedation or anesthetic. However, there have been no high-quality randomized controlled trials of interventions and, therefore, the evidence is limited to single-center case series.
Introduction
Epidemiology of chronic pelvic pain
The Royal College of Obstetricians and Gynaecologists define chronic pelvic pain (CPP) as lower abdominal or pelvic pain that persists intermittently or continuously for greater than 6 months.Citation1 It should neither be associated with the menstrual cycle, nor should it occur solely with intercourse or pregnancy.Citation1 The term “chronic pelvic pain” does not represent a disease, but is a specific symptom, experienced by women and occurring secondary to a variety of pathologies. Clearly, men are able to experience lower abdominal or pelvic pain too, but the term has been accepted as pertaining solely to women.
A significant health problem, CPP affects thousands of women. Zondervan et al investigated a UK primary care database containing the medical records of 284,162 women aged 12–70, and found an incidence of 38/1,000 patients per year.Citation2 Equivalent figures for back pain (41/1,000), migraine (21/1,000), and asthma (37/1,000) provide useful comparison.Citation2 Latthe et al explored the global epidemiology of CPP in a well-designed and comprehensive systematic review of 459,972 participants, and found that, where high-quality data was available, there was a high prevalence of CPP (24%).Citation3 The prevalence among women who did not seek medical attention but who have CPP would not have been included in these analyses; therefore, the actual prevalence may be greater.
In addition to physical effects, CPP places a significant economic burden on individuals (where it may impede work capability) and on health budgets (where long-term management may be costly). CPP is associated with 20% of all gynecology outpatient appointments,Citation4 costs U$881.5 million per annum in outpatient care costs in the US,Citation5 with a total economic cost of US$2 billion per annum,Citation6 and £158 million in the UK.Citation7 Extrapolating those costs to the European population confers a cost of €3.8 billion per annum. Accordingly, there is a need to develop robust treatments and management strategies for women with CPP to ensure personal and societal costs can be minimized. Pelvic vein incompetence (PVI) may be a significant cause of CPP; this review considers the investigation and management of PVI in women with CPP.
Literature review methods
The MEDLINE, CINAHL, EMBASE and Cochrane Library databases were interrogated, using the following search terms: “pelvic congestion syndrome”, “pelvic venous incompetence”, “ovarian vein incompetence”, “iliac vein incompetence”, “pelvic varices” and “chronic pelvic pain”.
Terminology
The term “pelvic congestion syndrome” is often used as an umbrella term when CPP and PVI are found in the same patient. However, there is no published and accepted definition of which symptoms are characteristic to the syndrome. As we both PVI and CPP can be defined, but not “pelvic congestion syndrome”, we consider this alleged syndrome should, for now, not be a recognized entity.
Pathophysiology of PVI
The pathophysiology of PVI as a cause of CPP is best understood using the analogy of saphenous vein incompetence and chronic venous insufficiency of the lower limb. Here, venous valves fail and, therefore, retrograde flow down the saphenous vein is enabled. The pressure within the vein increases, and causes the venous diameter to increase, further separating the leaflets of the valves and increasing the capacitance of the system. Over time, the veins become tortuous and prominent, and chronic venous hypertension develops. This confers a “low flow” state on the capillary beds, which causes leukocyte trapping and an inflammatory response. The patient experiences aching and pain in the distribution of the affected vein, which is similar to the type of pain experienced by women with symptomatic PVI.Citation8 In addition, symptoms are reported to diminish as the patient lies supine, emptying the pelvic veins and reducing their diameter.
No clear consensus has emerged to identify the underlying cause of the initial valve failure that enables the “vicious circle” of retrograde flow and diametric expansion of the vein, but it is possible that the circulatory demands of the gravid uterus lead to an expansion in venous diameter, preventing the valvular leaflets from meeting within the lumen. Using Doppler sonography, Palmer et al found that pregnancy is associated with arterial flow speed and volumes up to eight times higher than non-pregnant controls.Citation9 Physiological first principles suggest that this must be associated with a correspondingly higher volume of venous return. Veins do not have the elastic recoil of arteries, and so, postpartum, the venous diameter may be wide enough that the valvular leaflets still fail to meet, despite the reduced demand. However, PVI has been observed in women who have never been pregnant, and is often found incidentally in asymptomatic patients.Citation10,Citation11 Attempts have been made to establish a link between female endocrine hormones,Citation12 endothelial cytokines,Citation13 and the development of PVI, but such studies have not been sufficiently robust to confidently define the underlying molecular pathophysiology. To date, there has been very little research into why women develop PVI, and none of the endocrine or physiological studies have been replicated.
If idiopathic PVI can be considered “primary” PVI, then “secondary” PVI can arise as a result of venous obstructive disorders such as deep venous thrombosis, May–Thurner syndrome (compression of the left common iliac vein by the overlying right common iliac artery), and the “Nutcracker” syndrome (compression of the left renal vein between the abdominal aorta and the superior mesenteric artery). These are well-understood conditions that are treated separately from “primary” PVI and, thus, will not be included in this review.
In summary, to explain the pathophysiology of PVI, there are logical assumptions made in reference to lower limb varicose veins and logical hypotheses that are based on established vascular physiological principles; however, no high-quality studies of the pathophysiology of PVI have been completed.
PVI as a cause of CPP
The potential for PVI to cause CPP is accepted by many practitioners; however, there have been no robust case–control studies to support this view, with most data arising from small, non-powered, non-matched cohorts or case series with heterogeneous outcome measures. Beard et al investigated 45 women with CPP who had negative findings on laparoscopy by undertaking transfundal pelvic venography. They found that women with CPP had significantly greater mean maximum pelvic venous diameter than controls (6.73 mm vs 3.25 mm, p<0.01).Citation14 However, 18 women were excluded from the study due to low-quality venographic images, and the cases and controls were not satisfactorily matched for age or parity.
The same group proceeded to compare symptoms of women with PVI and CPP against women with CPP only. In this study, women with PVI were more likely to experience exacerbating postural factors such as walking, standing, lifting, and bending, and were also more likely to experience associated symptoms such as back pain, vaginal discharge and headache.Citation15 Although suggestive of an association, these relatively small studies do not conclusively confirm it. Furthermore, it is important to note that their definition of PVI was based on venous diameter, the time taken for venous contrast to disperse, and the radiological appearance of the venogram. Doppler technology was not available to assess intravenous flow dynamics that could have confirmed reflux within the affected veins. Reliance on surrogate markers of venous incompetence may increase the risk of Type I or Type II errors.
Transvaginal Doppler ultrasound (TVDU) was used by Halligan et al to confirm diagnoses of PVI made during transfundal venographic imaging. This study found that TVDU failed to discriminate between women found to have PVI on venography, and those who did not.Citation16 However, the study was based on the assumption that transfundal venography was a “gold standard” screening tool for PVI, and that Doppler imaging is sensitive in the supine position. In addition, there were statistically significant differences between endometrial depth and uterine volume between the cases and controls, which may have made TVDU more difficult to interpret. Accordingly, their conclusion that the TVDU-detected rate of PVI was no different between cases of CPP and controls may not be valid.
This study highlights the need for standardized diagnostic criteria for PVI – both for research purposes and clinical assessment. Indeed, Champaneria et al’s systematic review of PVI and its association with CPP concludes that meta-analyses of these and similar studies are precluded by the heterogeneity of their diagnostic methods, outcome measures, and statistical credibility.Citation17 Many such studies used the arbitrary scoring system (or a variation thereof) designed by Beard et al as the basis for their study,Citation14 but recent work has sought to define standardized criteria on the basis of measurable physiological data. Hansrani et al used transvaginal Doppler-detected reflux of >0.5 seconds to represent a positive diagnosis of PVI. This study from our group found that women with PVI were more likely than patients with varicose veins or healthy controls to experience pain throughout the month (70% vs 25% vs 18%, p<0.001), pelvic pain (95% vs 62% vs 65%, p<0.008), and dyspareunia (42% vs 18% vs 15%, p=0.007).Citation8 These data suggest that women with PVI are more likely to experience pelvic pain.
Our group reported a characteristic symptom profile that distinguishes PVI from other causes of CPP. Symptoms are similar to symptoms of lower limb varicose veins, but are experience in the pelvis. The pain is described as “aching”, worsens throughout the day, is exacerbated by standing for prolonged periods, and is relieved by lying.Citation8
To date, there have been no authoritative epidemiological studies of PVI as a cause of CPP. Several retrospective and prospective case series’ have reported that 11.2%–28% of patients with CPP have demonstrable PVI on investigation.Citation18–Citation20 However, these non-comparative studies are relatively low quality, with heterogeneity among the cohorts, and with a variety of imaging techniques employed. Moreover, there is heterogeneity of outcome measures, ranging from retrograde flow in the ovarian vein to the presence of pelvic varices. Similarly, studies examining PVI in women with recurrent varicose veins of the lower limb have found prevalence rates of 20%–76%, in the absence of methodological consistency and without adequate control groups.Citation21,Citation22 There remains a need for a well-powered epidemiological study with age- and parity-matched case–control pairs using clearly defined diagnostic criteria.
Imaging techniques
Diagnostic laparoscopy is mandatory in the investigation of CPP. However, it is important to note that most laparoscopies are conducted with the patient supine, with minimal “on table” cranio-caudal tilting. Accordingly, the congested pelvic veins may empty and become difficult to identify during the procedure. Moreover, laparoscopy requires a positive pressure to be created within the abdominal cavity, which may further impede pelvic venous filling. As such, there is a need for non-laparoscopic imaging techniques to diagnose PVI. The ideal modality would enable a dynamic assessment of flow rate and direction within the pelvic venous system, and should be a sensitive and specific means of identifying pelvic varices, while minimizing radiation and contrast exposure.
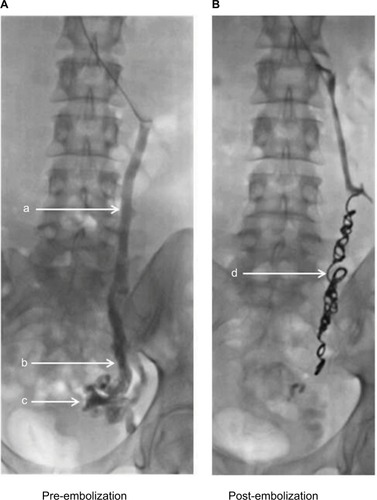
At present, catheter-directed fluoroscopic venography is considered the “gold standard” modality, as it allows the radiologist the opportunity to empty and refill the venous system using a tilting table, and to target individual veins (). As stated previously, the primary challenge for clinicians is to confidently diagnose PVI in the absence of any universally accepted radiological diagnostic criteria. Studies designed to test alternative imaging modalities do so using fluoroscopic or transfundal venography as the reference standard.
Figure 1 Venogram of incompetent left ovarian vein, pre- (A) and post-embolization (B).
The theoretical advantage of Doppler ultrasound techniques is that they are able to assess the direction of venous flow and the venous diameter using a relatively noninvasive technique without the risk of radiation or contrast. As such, they are a useful screening tool for patients being considered for fluoroscopic venography and transvenous occlusion, which (in practice) take place during the same consultation.
Barros et al undertook TVDU in 249 patients and found PVI in 150, with venography confirming PVI in 156 patients. These data demonstrated that TVDU had a sensitivity of 96% (95% confidence interval [CI] 92%–99%) and a specificity of 100% (lower 95% CI 97%).Citation23 This study is particularly useful as all patients found to have PVI on TVDU proceeded to have venography, enabling meaningful comparison. However, it should be noted that the investigators who assessed the venography were not blinded to the results of the TVDU and, therefore, the data are at risk of significant bias. Furthermore, there was no clear definition of what constituted PVI at the time of imaging.
Park et al also used combined transabdominal and transvaginal Doppler ultrasound to assess women with PVI and CPP against healthy controls, and found that mean left ovarian vein diameter was greater in the cases than in the controls (0.79±0.23 vs 0.49±0.15 cm, p<0.001).Citation24 Retrograde flow was identified in 100% of cases and 25% of controls, and all patients had identifiable varices and variable Duplex waveforms during the Valsalva maneuver. This study confirms the potential for TVDU to act as a reliable screening tool for patients suspected to have transvenous occlusion. The authors did not adequately explain why fewer than half the patients went on to have reference standard venography; it should also be noted that the authors did not define whether the Valsalva maneuver was used during the venography.
Magnetic resonance venography (MRV) has also been studied as an imaging modality for PVI. In 1994, Gupta et al reported a case of CPP where MR had been ordered to exclude adenomyosis, but obvious pelvic varices were the primary finding.Citation25 Nascimento et al took this further by reporting a retrospective cases series of 22 healthy women who were noted to have passive reflux from the left renal vein into the left ovarian vein.Citation10 The study by Asciutto et al reported sensitivity and specificity, respectively, of 88% and 67% for identification of ovarian vein reflux, 100% and 38% for the internal iliac veins, and 91% and 42% for the pelvic venous plexus.Citation26 Crucially, the radiologists reporting the subsequent venograms were blinded to the MRV result during reporting. Similarly, Yang et al blinded their radiology reviewers and found sensitivities of 66.7%–75%, and specificities of 100%, although the retrospective design means these data should be considered cautiously.Citation27 MRV is reported to have a high sensitivity to detect pelvic varices in several small case series of patients with CPP, or recurrent varicose veins of the lower limb,Citation28–Citation31 but there remains no authoritative study comparing MRV findings with TVDU to define the most effective screening tool for fluoroscopic venography.
Treatment of PVI
The limited, early research into the treatment of PVI focused on the use of medical interventions, rather than more invasive modalities. Dihydroergotamine,Citation32 medroxyprogesterone,Citation33 etonogestrel implant,Citation34 flavonoidCitation35,Citation36 and goserelinCitation37 have all been investigated and suggested to have some benefit, although outcome measures were variable and the studies were methodologically weak case series with low numbers of participants and no controls. Farquhar reported a randomized controlled trial of medroxyprogesterone (MPA) alone, versus MPA plus psychotherapy, placebo, and placebo plus psychotherapy.Citation38 Visual analog scale (VAS) pain scores were reduced by 50% in the MPA plus psychotherapy group only. The authors did not adequately power the study, and were not able to fully explain why psychotherapy was chosen as one of the treatment arms. In addition, there were significant demographic differences between the four groups, particularly in parity and in the duration of symptoms. To date, there have been no authoritative studies that have shown clear evidence of benefit from medical therapies.
Laparoscopic surgical interventions were briefly “en vogue” before the advent of endovascular techniques in the early 21st century. Grabham described a feasible and safe technique in an extremely limited case series of two patients.Citation39 Gargiulio reported a case series of 23 women with PVI and pelvic pain who underwent laparoscopic ligation who then had complete remission of pain and absence of pelvic varicosities during the 12-month follow-up period; but again, no controls were used, and participant numbers were relatively small.Citation40
The evidence to support the use of endovascular interventions for PVI is dominated by a large number of observational studies, often describing single-institution experience with a variety of occlusive techniques. Again, there is heterogeneity of PVI diagnostic criteria and outcome measures among these methodologically weak studies. The only study at Oxford CEBM Level 2b was authored by Chung and Huh, who randomized 106 CPP patients with venography-confirmed PVI to receive embolization, hysterectomy plus bilateral oophorectomy, or hysterectomy plus unilateral oophorectomy.Citation41 VAS pain scores were reduced at 12 months compared to the control groups. No attempt was made to blind either patients or researchers, and there were no untreated control groups. Again, power calculations were not undertaken. More positively, all patients underwent diagnostic laparoscopy to exclude other causes of CPP prior to randomization, echoing the “real world” experience of such patients.
There have been a significant number of relatively low-quality studies reporting retrospective or prospective case series’ data that cumulatively provide a basis for further investigation.Citation42–Citation56 Most report significantly improved patient outcomes following endovascular embolization (), but there is significant heterogeneity among the diagnostic criteria for PVI and CPP, the outcome measures used, the patient demographics, and the pre-trial clinical assessment received by patients. In particular, many participants had not been reviewed by a gynecologist prior to enrolment, which is contrary to the “real world” practice of most clinicians. Occlusion methods were also variable, with some studies using either metallic coils, sclerotherapy agents, or both (). There was a lack of consistency of imaging among studies, and an over reliance on VAS of pain as the primary outcome measure. From our group, Hansrani et al concluded a systematic review of transvenous occlusion of PVI for CPP by calling for the inclusion of validated quality-of-life and pain questionnaires alongside the VAS.Citation57
Table 1 Summary of studies assessing pain scores after treatment of pelvic vein incompetence
Furthermore, it is important to acknowledge the significant number of studies investigating embolization of pelvic veins to treat recurrent varicose veins of the lower limb. It is now well understood that pelvic venous collaterals contribute to the recurrence of thigh varicose veins after long saphenous vein treatments have been administered. However, in common with studies pertaining to PVI and CPP, the data quality is poor, with most studies scoring Level 4 or 5 on the Oxford CEBM classification.Citation60,Citation62,Citation70–Citation76 This overview of the evidence emphasizes the poor quality of data that characterizes all aspects of PVI and its associated clinical presentations.
Summary
Chronic pelvic pain is a major health problem that affects the lives of millions of women. The available evidence suggests that the management of CPP can be complex, and that symptom profiles are inconsistent and multifaceted. Investigations can be extensive, but can still fail to lead to effective treatment, with many women continuing to experience chronic pain despite seeking medical care. PVI may be an under-investigated, under-researched cause of CPP in women.
The optimal investigation and treatment of PVI to alleviate CPP is beginning to emerge. There is a multitude of low-quality evidence in the literature to support the hypotheses that TVDU is a useful screening tool for patients who may benefit from transvenous occlusion, and to also support the use of that treatment itself. However, the significant methodological flaws contained within the majority of these studies cannot be ignored. Similarly weak studies are unlikely to add to our knowledge and understanding of PVI; therefore, researchers should concentrate on producing higher quality data using comparative methodologies. Our group is currently undertaking a robust case–control study to define the frequency of PVI in women with CPP and in healthy volunteers. Our parallel randomized controlled trial of venography or venography plus coil/foam occlusion for women with CPP and PVI is also in progress. We will recruit 100 patients to the study, and compare pain scores, quality-of-life scores, and health economic data in both arms of the study.
We are proud to have formed the Manchester Pelvic Vein Study Group from an experienced multidisciplinary team of academic surgeons, radiologists, gynecologists, nurses, health economists, women’s health experts, and patient representatives, and are excited to explore this important condition, which may affect millions of women.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
DMR and CM are partially funded by a National Institute of Health Research, Research for Patient Benefit grant (grant number PB-PG-0214-33102). We are grateful to Miss Rae Larmour who reviewed the images used in this review.
Disclosure
The authors report no conflicts of interest in this work.
References
- Royal College of Obstetricians and GynaecologistsThe initial management of chronic pelvic pain: Green Top Guideline No. 412012 Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_41.pdfAccessed November 01, 2017
- ZondervanKTYudkinPLVesseyMPDawesMGBarlowDHKennedySHPrevalence and incidence of chronic pelvic pain in primary care: evidence from a national general practice databaseBr J Obstet Gynaecol1999106111149115510549959
- LatthePLattheMSayLGülmezogluMKhanKSWHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidityBMC Public Health2006617716824213
- HowardFMThe role of laparoscopy in chronic pelvic pain: promise and pitfallsObstet Gynecol Surv19934863573878327235
- MathiasSDKuppermannMLibermanRFLipschutzRCSteegeJFChronic pelvic pain: prevalence, health-related quality of life, and economic correlatesObstet Gynecol19968733213278598948
- AhangariAPrevalence of chronic pelvic pain among women: an updated reviewPain Physician2014172E141E14724658485
- DaviesLGangarKFDrummondMSaundersDBeardRWThe economic burden of intractable gynaecological painJ Obstet Gynaecol199212Suppl 2S54S56
- HansraniVMorrisJCaressALPayneKSeifMMcCollumCNIs pelvic vein incompetence associated with symptoms of chronic pelvic pain in women? A pilot studyEur J Obstet Gynecol Reprod Biol2016196212526656197
- PalmerSKZamudioSCoffinCParkerSStammEMooreLGQuantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancyObstet Gynecol1992806100010061448242
- NascimentoABMitchellDGHollandGOvarian veins: magnetic resonance imaging findings in an asymptomatic populationJ Magn Reson Imaging200215555155611997896
- RozenblitAMRicciZJTuviaJAmisESJrIncompetent and dilated ovarian veins: a common CT finding in asymptomatic parous womenAJR Am J Roentgenol2001176111912211133549
- AsciuttoGMummeAAsciuttoKCGeierBOestradiol levels in varicose vein blood of patients with and without pelvic vein incompetence (PVI): diagnostic implicationsEur J Vasc Endovasc Surg201040111712120202867
- LiHWangZWuRChanges in endothelin-1 and atrial natriuretic peptide in peritoneal fluid of pelvic venous congestion syndrome after tubal sterilizationZhonghua Fu Chan Ke Za Zhi1996319533536 Chinese9275424
- BeardRWHighmanJHPearceSReginaldPWDiagnosis of pelvic varicosities in women with chronic pelvic painLancet1984284099469496149342
- BeardRWReginaldPWWadsworthJClinical features of women with chronic lower abdominal pain and pelvic congestionBr J Obstet Gynaecol19889521531613349005
- HalliganSCampbellDBartramCITransvaginal ultrasound examination of women with and without pelvic venous congestionClin Radiol2000551295495811124075
- ChampaneriaRShahLMossJThe relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectivenessHealth Technol Assess20162051108
- AlmeidaECNogueiraAACandido dos ReisFJRosa e SilvaJCCesarean section as a cause of chronic pelvic painInt J Gynaecol Obstet200279210110412427392
- HebbarSChawlaCRole of laparoscopy in evaluation of chronic pelvic painJ Minim Access Surg20051311612021188008
- LynnNEMTheinHTSMyaWWStudy of laparoscopic findings in patients with chronic pelvic pain in Central Women’s Hospital, Yangon, MyanmarJ Obstet Gynaecol Res201541S198109 Available from: http://onlinelibrary.wiley.com/doi/10.1111/jog.12874/epdfAccessed November 10, 2017
- MarshPHoldstockJHarrisonCSmithCPriceBAWhiteleyMSPelvic vein reflux in female patients with varicose veins: comparison of incidence between a specialist private vein clinic and the vascular department of a National Health Service District General HospitalPhlebology200924310811319470861
- GeierBBarberaLMummeAReflux patterns in the ovarian and hypogastric veins in patients with varicose veins and signs of pelvic venous incompetenceChir Ital200759448148817966768
- BarrosFSPerezJMGZandonadeEEvaluation of pelvic varicose veins using color Doppler ultrasound: comparison of results obtained with ultrasound of the lower limbs, transvaginal ultrasound, and phlebographyJ Vasc Bras201091523
- ParkSJLimJWKoYTDiagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonographyAJR Am J Roentgenol2004182368368814975970
- GuptaAMcCarthySPelvic varices as a cause for pelvic pain: MRI appearanceMagn Reson Imaging19941246796818057774
- AsciuttoGMummeAMarpeBKösterOAsciuttoKCGeierBMR venography in the detection of pelvic venous congestionEur J Vasc Endovasc Surg200836449149618718774
- YangDMKimHCNamDHJahngGHHuhCYLimJWTime-resolved MR angiography for detecting and grading ovarian venous reflux: comparison with conventional venographyBr J Radiol2012851014e117e12221385913
- CoakleyFVVargheseSLHricakHCT and MRI of pelvic varices in womenJ Comput Assist Tomogr199923342943410348450
- KimCYMillerMJJrMerkleEMTime-resolved MR angiography as a useful sequence for assessment of ovarian vein refluxAJR Am J Roentgenol20091935W458W46319843728
- LeiberLMThouvenyFBouvierAMRI and venographic *aspects of pelvic venous insufficiencyDiagn Interv Imaging201495111091110224630150
- SterlingKMBaglaSVan BredaAMagnetic resonance imaging (MRI) findings in patients with pelvic congestion syndrome (PCS)Cardiovasc Intervent Radiol201336Suppl 359
- ReginaldPWBeardRWKoonerJSIntravenous dihydroergotamine to relieve pelvic congestion with pain in young womenLancet1987285553513532886820
- ReginaldPWAdamsJFranksSWadworthJBeardRWMedroxyprogesterone acetate in the treatment of pelvic pain due to venous congestionBr J Obstet Gynaecol19899610114811522531610
- ShokeirTAmrMAbdelshaheedMThe efficacy of Implanon for the treatment of chronic pelvic pain associated with pelvic congestion: 1-year randomized controlled pilot studyArch Gynecol Obstet2009280343744319190927
- BurakFGunduzTSimsekMTaskinOChronic pelvic pain associated with pelvic congestion syndrome and the benefit of Daflon 500 mg: a reviewPhlebolymphology2009163290294
- SimsekMBurakFTaskinOEffects of micronized purified flavonoid fraction (Daflon) on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trialClin Exp Obstet Gynecol2007342969817629162
- SoysalMESoysalSVicdanKOzerSA randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestionHum Reprod200116593193911331640
- FarquharCMRogersVFranksSPearceSWadsworthJBeardRWA randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestionBr J Obstet Gynaecol19899610115311622531611
- GrabhamJABarrieWWLaparoscopic approach to pelvic congestion syndromeBr J Surg199784912649313709
- GargiuloTMaisVBrokajLCossuEMelisGBBilateral laparoscopic transperitoneal ligation of ovarian veins for treatment of pelvic congestion syndromeJ Am Assoc Gynecol Laparosc200310450150414738638
- ChungMHHuhCYComparison of treatments for pelvic congestion syndromeTohoku J Exp Med2003201313113814649734
- AksungurEBalliTHAikimbaevKAkgulEYalcinCClinical outcome and efficiency of mechanical and combined mechanical/chemical embolization in patients with pelvic venous congestion syndromeCardiovasc Intervent Radiol20151S232S233 Available from: https://link.springer.com/content/pdf/10.1007%2Fs00270-015-1173-5.pdfAccessed November 10, 2017
- AsciuttoGAsciuttoKCMummeAGeierBPelvic venous incompetence: reflux patterns and treatment resultsEur J Vasc Endovasc Surg200938338138619574069
- GandiniRChiocchiMKondaDTreatment of symptomatic high-flow female varicocele with balloon-occluded retrograde transvenous foam sclerotherapy (B-ORTFS) using sodium-tetradecyl-sulphate foamCardiovasc Intervent Radiol201134584 Available from: https://link.springer.com/content/pdf/10.1007%2Fs00270-011-0216-9.pdfAccessed November 10, 2017
- GandiniRChiocchiMKondaDPampanaEFabianoSSimonettiGTranscatheter foam sclerotherapy of symptomatic female varicocele with sodium-tetradecyl-sulfate foamCardiovasc Intervent Radiol200831477878418172712
- GandiniRKondaDAbrignaniSTreatment of symptomatic high-flow female varicoceles with stop-flow foam sclerotherapyCardiovasc Intervent Radiol20143751259126724190634
- KwonSHOhJHKoKRParkHCHuhJYTranscatheter ovarian vein embolization using coils for the treatment of pelvic congestion syndromeCardiovasc Intervent Radiol200730465566117468903
- LabordaAMedranoJde BlasIUrtiagaICarnevaleFCde GregorioMAEndovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patientsCardiovasc Intervent Radiol20133641006101423456353
- MaleuxGStockxLWilmsGMarchalGOvarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical resultsJ Vasc Interv Radiol200011785986410928522
- MonederoJLEzpeletaSZPerrinMPelvic congestion syndrome can be treated operatively with good long-term resultsPhlebology201227Suppl 1657322312070
- NasserFCavalcanteRNDe FinaBResults in endovascular treatment of pelvic congestion syndromeJ Vasc Interv Radiol2014253S69
- PieriSAgrestiPMorucciMDe’MediciLIl trattamento percutaneo nella sindrome della congestione pelvicaRadiol Med20031051–27682 Italian12700549
- PyraKWoźniakSRomanTEvaluation of effectiveness of endovascular embolisation for the treatment of pelvic congestion syndrome--preliminary studyGinekol Pol201586534635126117971
- SanchezMLabordaAUrtiagaIEndovascular treatment of pelvic congestion syndrome: retrospective study in 132 patientsCardiovasc Intervent Radiol200932348 Available from: https://link.springer.com/content/pdf/10.1007%2Fs00270-009-9650-3.pdfAccessed November 10, 2017
- ScultetusAHVillavicencioJLGillespieDLKaoTCRichNMThe pelvic venous syndromes: analysis of our experience with 57 patientsJ Vasc Surg200236588188812422096
- VenbruxACChangAHKimHSPelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic painJ Vasc Interv Radiol2002132 Pt 117117811830623
- HansraniVAbbasABhandariSCaressALSeifMMcCollumCNTrans-venous occlusion of incompetent pelvic veins for chronic pelvic pain in women: a systematic reviewEur J Obstet Gynecol Reprod Biol201518515616325590499
- CapassoPSimonsCTrotteurGDondelingerRFHenroteauxDGaspardUTreatment of symptomatic pelvic varices by ovarian vein embolizationCardiovasc Intervent Radiol19972021071119030500
- CordtsPREclaveaABuckleyPJDeMaioribusCACockerillMLYeagerTDPelvic congestion syndrome: early clinical results after transcatheter ovarian vein embolizationJ Vasc Surg19982858628689808854
- CretonDHennequinLKohlerFAllaertFAEmbolisation of symptomatic pelvic veins in women presenting with non-saphenous varicose veins of pelvic origin – three-year follow-upEur J Vasc Endovasc Surg200734111211717336555
- d’ArchambeauOMaesMDe SchepperAMThe pelvic congestion syndrome: role of the “nutcracker phenomenon” and results of endovascular treatmentJBR-BTR20048711815055326
- GreinerMGilling-SmithGLLeg varices originating from the pelvis: diagnosis and treatmentVascular2007152707817481367
- KimHSMalhotraADRowePCLeeJMVenbruxACEmbolotherapy for pelvic congestion syndrome: long-term resultsJ Vasc Interv Radiol2006172 Pt 128929716517774
- Leal MonederoJZubicoa EzpeletaSCastro CastroJCalderon OrtizMSellers FernandezGEmbolization treatment of recurrent varices of pelvic originPhlebology2006211311
- MenesesLFavaMDiazPEmbolization of incompetent pelvic veins for the treatment of recurrent varicose veins in lower limbs and pelvic congestion syndromeCardiovasc Intervent Radiol201336112813222547030
- RichardsonGDDriverBOvarian vein ablation: coils or surgery?Phlebology20062111623
- TarazovPGProzorovskijKVRyzhkovVKPelvic pain syndrome caused by ovarian varices. Treatment by transcatheter embolizationActa Radiol1997386102310259394662
- TropeanoGDi StasiCAmorosoSCinaAScambiaGOvarian vein incompetence: a potential cause of chronic pelvic pain in womenEur J Obstet Gynecol Reprod Biol2008139221522118313828
- van der VleutenCJvan KempenJASchultze-KoolLJEmbolization to treat pelvic congestion syndrome and vulval varicose veinsInt J Gynecol Obstet20121183227230
- AshourMASolimanHEKhougeerGARole of descending venography and endovenous embolization in treatment of females with lower extremity varicose veins, vulvar and posterior thigh varicesSaudi Med J200728220621217268690
- ColucciFGiulianiMSPillonSMinucciSRole of pelvic varices sclerotherapy in treatment of venous insufficiency in lower limbsCardiovasc Intervent Radiol201336S361 Available from: https://link.springer.com/content/pdf/10.1007%2Fs00270-013-0701-4.pdfAccessed November 10, 2017
- GiannoukasADDacieJELumleyJSRecurrent varicose veins of both lower limbs due to bilateral ovarian vein incompetenceAnn Vasc Surg200014439740010943794
- HartungOEmbolization is essential in the treatment of leg varicosities due to pelvic venous insufficiencyPhlebology2015301 Suppl818525729072
- HobbsJTVaricose veins arising from the pelvis due to ovarian vein incompetenceInt J Clin Pract200559101195120316178988
- JinKNLeeWJaeHJYinYHChungJWParkJHVenous reflux from the pelvis and vulvoperineal region as a possible cause of lower extremity varicose veins: diagnosis with computed tomographic and ultrasonographic findingsJ Comput Assist Tomogr200933576376919820508
- WhiteleyAMTaylorDCDos SantosSJWhiteleyMSPelvic venous reflux is a major contributory cause of recurrent varicose veins in more than a quarter of womenJ Vasc Surg Venous Lymphat Disord20142441141526993547
