Abstract
Multiple conditions result in development of pulmonary hypertension. Pulmonary arterial hypertension (PAH) is the subclassification of pulmonary hypertension, in which known or unknown underlying conditions lead to similar intrinsic alterations in the pulmonary vasculature. PAH is a progressive condition characterized by restricted blood flow through the pulmonary circulation leading to poor survival in the absence of effective therapy. Over the last two decades, new therapeutic agents have substantially improved the course and prognosis for PAH patients. Three available classes of drugs, ie, prostacyclins, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors provide multiple options for treatment of PAH. Endothelin receptor antagonists and phosphodiesterase-5 inhibitors are administered orally, whereas prostacyclin therapies are delivered by continuous intravenous or subcutaneous infusion, or as aerosols by nebulization. Because of the risks and inconveniences associated with administration, prostacyclins are typically reserved for patients with more advanced disease or progression despite oral therapy. Inhaled administration may be a safer and easier route for prostacyclin administration. Treprostinil is a prostacyclin analog that has been demonstrated to be effective when administered by continuous subcutaneous or intravenous infusion, and more recently by nebulization.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by restricted blood flow through the pulmonary circulation. The current criteria for PAH include an elevated mean pulmonary artery pressure greater than 25 mmHg at rest, with a pulmonary artery occlusion pressure less than 15 mmHg and pulmonary vascular resistance greater than 3 Wood units.Citation1 Elevated pulmonary arterial pressure increases the work required of the right ventricle, ultimately leading to right heart failure and death. Prior to the development of modern treatments, the one-, three-, and five-year survival rates were 68%, 48%, and 34%, respectively.Citation2 With the development of modern therapeutic agents, these survival rates have improved to 86%, 69%, and 61%, respectively.Citation3
Epidemiology
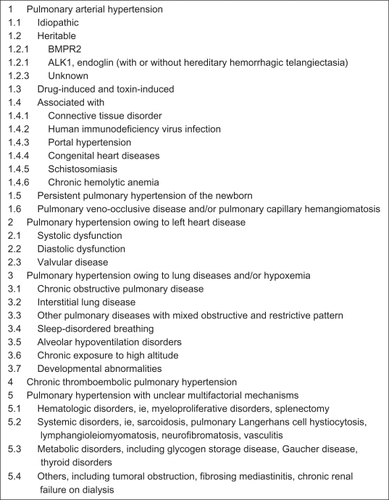
Pulmonary hypertension is an uncommon disease. Recent registries in the US, Scotland, and France put the overall prevalence of PAH at 15–56 cases per million.Citation4–Citation6 The recent 2008 World Health Organization (WHO) classification of pulmonary hypertension includes five general categories ().Citation7 The previous diagnostic category of primary pulmonary hypertension is now referred to as idiopathic PAH, and is included under the classification of WHO Class I or PAH. The recently published REVEAL study demonstrated that idiopathic PAH is the most prevalent form of PAH, accounting for 46.2% of patients.Citation8 Women have a 2–3 times greater prevalence of idiopathic PAH in most registries. Although previously thought to be a disease of young women, the peak prevalence seems to occur in the fourth, fifth, and sixth decades of life. Familial forms of PAH caused by mutations in bone morphogenic protein receptor-2 or activin-like kinase 1 represented only 2.7% of cases. Other etiologies of PAH include those associated with connective tissue disorders (25.3%), congenital heart disease (9.8%), liver disease (5.3%), human immunodeficiency virus infection (1.9%), and medication usage, including anorexigens (5.3%). Also included in the PAH classification are pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis, which account for less than 0.5% of cases. Pulmonary hypertension due to left-sided heart disease, hypoxemic lung disease, and thromboembolic disorders are each classified separately. Additionally, a miscellaneous class includes sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangiomatosis, fibrosing mediastinitis, and other tumors that compress the pulmonary arteries.
Figure 1 Updated clinical classification of pulmonary hypertension.Citation7
While there are many conditions associated with PAH, they share common pathophysiologic findings. PAH is a vasculopathy involving small arteries in the lung that regulate pulmonary blood flow.Citation9,Citation10 Affected arteries demonstrate intimal hyperplasia, medial hypertrophy, adventitial proliferation, thrombosis in situ, and plexiform arteriopathy. The adventitial proliferation is thought to be due to a decreased ratio of apoptosis to proliferation of pulmonary artery smooth muscle cells.Citation11 All contribute to narrowing of the vessel lumen, leading to increased pulmonary vascular resistance.
Endothelial dysfunction further contributes to elevated pulmonary artery resistance. Ordinarily, endothelial cells produce a balance of vasoconstrictive and vasodilatory mediators. In PAH, there is excessive production of endothelin and thromboxane, both of which are vasoconstrictors.Citation12–Citation14 Similarly, there is a deficit of the vasodilators, prostacyclin and nitric oxide.Citation13,Citation15 These molecules have become the targets of modern therapeutic interventions for PAH.
Evaluation and diagnosis
The symptoms of PAH are often nonspecific, thus a high index of suspicion is required for timely diagnosis. The most common symptoms include dyspnea (60%) and fatigue (19%).Citation16 Other common symptoms include chest pain (7%), syncope (8%), lower extremity edema (3%), and palpitations (5%). Typical signs on physical examination suggest right ventricular strain, including an accentuated P2, jugular venous distention, a holosystolic tricuspid murmur, and an S3 or S4 heart sound. Even with these signs and symptoms, there can still be a delay of several years before a definitive diagnosis is made.
When PAH is suspected in a patient with appropriate signs and symptoms, transthoracic Doppler echocardiography is the most appropriate initial study.Citation9,Citation17 By measuring the tricuspid regurgitant jet, the pulmonary artery systolic pressure can be estimated. The sensitivity and specificity for detecting PAH is 0.79 to 1.00 and 0.68 to 0.98, respectively. When a tricuspid regurgitant jet is difficult to visualize, agitated saline can be used to enhance its measurement. In studies of normal populations, only 5% of individuals have a pulmonary artery systolic pressure of greater than 37.9 mmHg, although this number increases with advanced age and obesity.Citation18 Thus, if pulmonary artery systolic pressure is measured greater than 40 mmHg, referral for further evaluation is warranted. Echocardiography can also detect other signs of pulmonary hypertension, including right ventricular hypertrophy, right ventricular enlargement, and impaired right ventricular function.
Further testing is directed at evaluating specific conditions associated with PAH and excluding secondary causes of pulmonary hypertension.Citation9,Citation10 Chest radiography may reveal enlarged pulmonary arteries and right heart structures. Electrocardiogram may demonstrate right ventricular enlargement and strain pattern. Pulmonary function testing can evaluate for significant airflow obstruction or restriction. In the appropriate clinical settings, serologic testing for mixed connective tissue disorders, chronic liver disease, human immunodeficiency virus infection testing, and screening for obstructive sleep apnea and nocturnal hypoxia should be undertaken. Ventilation-perfusion scanning and computed tomography scanning of the chest assess for chronic thromboembolic disease and other pulmonary parenchymal diseases.
The gold standard for diagnosis of PAH is right heart catheterization. As first described by Swan and Ganz in 1970, a catheter is introduced via a central vein through the right atrium and right ventricle into the pulmonary artery, and direct measurements of pulmonary artery pressures can be obtained.Citation19 PAH is defined by a mean pulmonary artery pressure greater than 25 mmHg, with a pulmonary artery occlusion pressure less than 15 mmHg and a calculated pulmonary vascular resistance of greater than 3 Wood units (240 dyne-sec/cm5). If the mean pulmonary artery pressure is greater than 25 mmHg but the pulmonary artery occlusion pressure is greater than 15 mmHg or the pulmonary vascular resistance is less than 3 Wood units, then the elevated pressure in the pulmonary arteries is more likely due to elevated left atrial pressure resulting from left-sided heart disease. These patients are not classified strictly as having pulmonary arterial pressure, but rather having WHO Class II pulmonary hypertension, and therapeutics are directed at improving left ventricular heart function.
Treatment
The goals for pulmonary hypertension treatment focus on the reduction of symptoms, enhancement of exercise tolerance, normalization of hemodynamic parameters, and improvement of survival. Comprehensive evidence-based guidelines for the treatment of PAH have recently been updated.Citation9,Citation10 These treatments can be divided into general measures and agents directed specifically at the pathophysiology of PAH. Included in these medications are endothelin receptor antagonists, phosphodiesterase inhibitors, and prostacyclin analogs, such as treprostinil.
General measures are directed toward minimizing hypoxemia and volume overload states that can exacerbate PAH. Supplemental oxygen should be provided to keep measured oximetry above 90%, with special attention to nocturnal hypoxemia and exposure to increased altitude/low atmospheric pressure, such as during commercial air travel. Influenza and pneumococcal vaccinations are recommended. Diuretics and a low sodium diet maintain euvolemia to prevent further right ventricular failure. Pregnancy should be avoided due to the volume expansion and increased blood flow demands normally experienced during pregnancy. In one series, the mortality associated with pregnancy in patients with pulmonary hypertension was 30%–56%.Citation20 Three observational studies suggest that anticoagulation should be considered in patients with severe PAH.Citation21–Citation23 One short-term study indicated that digoxin may be beneficial in patients with low cardiac output.Citation24
Calcium channel blockers may be used in selected individuals. During diagnostic right heart catheterization, a vasodilator trial with inhaled nitric oxide, intravenous epoprostenol, or adenosine is performed. A positive response involves a decrease in mean pulmonary artery pressure more than 10 mmHg to a level less than 40 mmHg and no decrement in cardiac output. Only about 10% of patients meet this criteria, and even fewer have a positive long-term response.Citation21,Citation25
Oral agents
Two classes of oral agents are recommended for the treatment of mild to moderate PAH (WHO functional class II/III).Citation9,Citation10 These include the endothelin receptor antagonists, bosentan, ambrisentan, and sitaxsentan, and the phosphodiesterase-5 inhibitors, sildenafil and tadalafil.
Endothelin is a 21-amino acid polypeptide first identified and isolated from cultured endothelial cells.Citation26,Citation27 Although it is produced primarily by endothelial cells, it is also expressed in epithelial cells, macrophages, fibroblasts, cardiomyocytes, and other tissues.Citation28 Endothelin secretion occurs both in a constitutive fashion and in response to various stimuli, including vascular shear stress, hypoxia, and peptide mediators of inflammation. In contrast, nitric oxide, natriuretic peptides, and dilator prostanoids inhibit endothelin release. Endothelin mediates its biologic effect through two G protein-coupled plasma membrane receptors, ET-A and ET-B. ET-A is found primarily on vascular smooth muscle cells and leads to vasoconstriction, proliferation, and fibrosis. ET-B is found in endothelial cells, as well as liver, lung, and kidney tissues. Occupancy of endothelial ET-B receptors leads primarily to vasodilation and induction of nitric oxide and prostacyclin production. ET-B binding in lung, liver, and kidney parenchyma is believed to be one mechanism of clearance of endothelin. Patients with pulmonary hypertension have increased levels of endothelin, and these levels correlate with poorer outcomes.Citation13,Citation29 Thus, antagonism of this peptide presents a target for treatment of pulmonary hypertension.
Two endothelin receptor antagonists are currently approved by the US Food and Drug Administration for treating PAH. Bosentan is an orally available, nonpeptide endothelin receptor antagonist that blocks both ET-A and ET-B receptors. Ambrisentan is an ET-A specific receptor antagonist. Sitaxsentan is another ET-A specific antagonist, but is currently not approved for use in the US. The efficacy of these medications is the subject of a recent Cochrane review.Citation30
Bosentan represents the first endothelin receptor antagonist for the treatment of PAH. Channick et al demonstrated in 2001 that bosentan given twice daily for at least 12 weeks to 32 patients with severe disease led to an improvement in six-minute walk distance (6MWD) of 76 meters over placebo.Citation31 Additionally, there were improvements in cardiac output, pulmonary vascular resistance, and reduced BORG dyspnea index scores, without a significant number of adverse events. This was followed by the larger BREATHE-1 (Bosentan Randomized Trial of Endothelin Antagonist Therapy) study in 2002, in which 213 patients with severe disease received bosentan for at least 12 weeks.Citation32 Again, patients receiving bosentan had improved 6MWD (42 meters), BORG dyspnea index, and WHO functional class. Bosentan also increased the time to clinical worsening, defined as the combined endpoint of death, lung transplantation, hospitalization for pulmonary hypertension, lack of clinical improvement, or worsening, leading to discontinuation, need for epoprostenol therapy, or atrial septostomy. Subsequently, several longer-term studies, up to three years in duration, have demonstrated a significant survival benefit of bosentan compared with historic controls.Citation33,Citation34 Patients taking bosentan had survival rates of 92%, 89%, and 79% at years 1, 2, and 3, respectively, compared with expected survival of 71%, 61%, and 51%. In 2008, Galie et al demonstrated that bosentan benefited patients with milder, WHO functional class II disease.Citation35 These studies demonstrated the most common adverse effect was an elevation in hepatic enzymes that was thought to be due to ET-B receptor antagonism leading to decreased clearance. This led to the development of the ET-A specific agents, sitaxsentan and ambrisentan.
Sitaxsentan was the first ET-A specific receptor antagonist for the treatment of PAH. It has about 6500 times greater affinity for ET-A than for ET-B receptors.Citation36 The STRIDE-1 (Sitaxsentan To Relieve Impaired Exercise) study examined the safety and efficacy of sitaxsentan in 118 patients over a 12-week period.Citation37 Although a secondary endpoint, there was an improvement in 6MWD of 35 and 33 meters with daily administration of 100 mg and 300 mg of sitaxsentan, respectively. However, elevations in hepatic aminotransferases were observed in 10% of the 300 mg group compared with 3% for the placebo arm. The STRIDE-2 study examined the effects of lower doses of sitaxsentan, from 50 mg to 100 mg.Citation38 Again, improvements in 6MWD of 24.2 and 31.4 meters were seen for 50 mg and 100 mg doses, respectively. The rates of elevated hepatic aminotransferases were similar to placebo (3%–5% versus 6%). An extension of the STRIDE-1 in 11 patients for one year demonstrated that 100 mg of sitaxsentan daily led to a sustained improvement in 6MWD of 50 meters, improved functional class, and hemodynamics compared with baseline.Citation39 There were no reports of hepatotoxicity in this study.
Ambrisentan is an oral, nonsulfonamide ET-A selective receptor antagonist with a half-life of 9–15 hours, allowing once-daily dosing. The safety and efficacy of ambrisentan was first shown in 2005.Citation40 In 64 patients with PAH, daily administration of ambrisentan led to an improvement of 6MWD of 36.1 meters, improved dyspnea, WHO functional class, and cardiopulmonary hemodynamics. Subsequently, the larger ARIES-1 and 2 studies demonstrated improved 6MWD, dyspnea scores, and time to clinical worsening in 202 and 192 patients, respectively.Citation41 Neither of these studies showed an elevation in liver enzymes greater than three times the upper limit of normal. However, peripheral edema was observed to a greater extent in patients taking ambrisentan. An extension of the ARIES study showed improved 6MWD in 280 patients after 48 weeks of treatment.
The phosphodiesterase inhibitors, sildenafil and tadalafil, represent the second class of oral agents for the treatment of PAH. In the lung, nitric oxide acts as an important vasodilator via stimulation of cyclic guanidine monophosphate (cGMP) synthesis. Cyclic GMP activates protein kinase G, which mediates lower intracellular calcium in vascular smooth muscle and facilitates vascular relaxation, antiproliferation, and apoptosis.Citation42 Phosphodiesterase-5 hydrolyzes cGMP, which prevents protein kinase G activation and its downstream effects. In PAH, there is increased expression of phosphodiesterase-5 in the lung and decreased expression of nitric oxide synthase.Citation43,Citation44 Both the reduction in cGMP production and its increased hydrolysis lead to pulmonary smooth muscle vasoconstriction and proliferation. Antagonism of phosphodiesterase-5 with either sildenafil or tadalafil increases cGMP levels, leading to relaxation of pulmonary vascular smooth muscle and decreased pulmonary vascular resistance.
The safety and efficacy of sildenafil for the treatment of PAH was demonstrated in the SUPER (Sildenafil Use in Pulmonary Arterial Hypertension) trial.Citation45 Published in 2005, this was a double-blind, placebo-controlled study of 278 patients with symptomatic PAH sponsored by Pfizer. During 12 weeks of treatment with 20 mg, 40 mg, or 80 mg three times daily, sildenafil treatment led to improvements in 6MWD of 45, 46, and 50 meters, respectively. At all doses, mean pulmonary artery pressure was decreased and WHO functional class was improved. A follow-up study of 222 patients who continued to take 80 mg of sildenafil showed an improved 6MWD of 51 meters after one year of treatment. Additionally, health-related quality of life measurements also improved with sildenafil treatment at 24 weeks.Citation46
Tadalafil is a once-daily dosed selective inhibitor of phosphodiesterase-5. Previously approved for erectile dysfunction, the utility of tadalafil in PAH was demonstrated by the PHIRST (Pulmonary Arterial Hypertension and Response to Tadalafil) study.Citation47 In this 16-week, double-blind, randomized, placebo-controlled study sponsored by Eli Lilly, 405 patients received varied doses of tadalafil or placebo. Tadalafil lead to improvements in 6MWD in a dose-dependent fashion. Although there was no improvement in WHO functional class, the time to clinical worsening was longer with tadalafil 40 mg daily. At the highest doses, tadalafil treatment also improved cardiac output and reduced pulmonary vascular resistance.
Prostacyclin analogs
Prostacyclin is an endogenous vasodilator produced by prostacyclin synthase in endothelial cells of the pulmonary vasculature. Prostacyclin acts locally to promote vasodilation and reduce pulmonary vascular resistance. In PAH, there is imbalance between this vasodilator and other vasoconstrictor molecules, with decreased expression of prostacyclin synthase and increased levels of thromboxane A2.Citation12,Citation15 In addition to vasodilation, prostacyclin has an antiproliferative effect on smooth muscle cells in vitro.Citation48 Abnormal levels of prostacyclin facilitate the medial hypertrophy seen in PAH. Furthermore, prostacyclin prevents platelet activation and aggregation by thromboxane A2.Citation49 Reduced levels of prostacyclin allow for thrombosis in situ also seen in PAH. Thus, pharmacologic restoration of prostacyclin levels would prevent the vasoconstriction, smooth muscle cell proliferation, thrombosis in situ, and plexiform arteriopathy that are hallmarks of PAH.
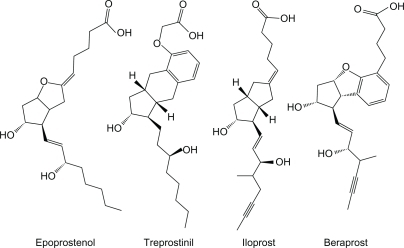
Three prostacyclin analogs are approved for the treatment of PAH in the US, ie, epoprostenol, treprostinil, and iloprost (). Beraprost is not currently approved by the Food and Drug Administration. Epoprostenol was the first prostacyclin approved for the treatment of PAH. Due to its short half life (<six minutes) and poor stability at pH < 10.5, epoprostenol must be refrigerated for storage and administered as a continuous infusion through a central venous catheter. These limitations led to the development of the tricyclic benzidine prostacyclin, treprostinil. Treprostinil may be given either as a continuous intravenous or subcutaneous infusion with similar bioavailability.Citation50 The half-life of treprostinil is 4.4 and 4.6 hours by intravenous and subcutaneous infusion, respectively. More recently, an inhaled form has become available with four times daily dosing. Iloprost also may be inhaled, as often as 6–9 times per day due to its short half-life (20–30 minutes).
Three seminal studies have demonstrated the clinical effectiveness of epoprostenol for PAH.Citation51–Citation53 In 81 patients with functional class III/IV primary pulmonary hypertension, Barst et al demonstrated that epoprostenol infusion improved 6MWD, mean pulmonary artery pressure, pulmonary vascular resistance, and survival. However, four episodes of catheter-related sepsis and one thrombotic event were noted.Citation53 Subsequently, McLaughlin et al demonstrated that epoprostenol therapy not only improved hemodynamic parameters, but also improved survival over a mean 36.1-month follow-up period in 162 patients compared with historic controls.Citation51 Sitbon et al similarly demonstrated a survival benefit of intravenous epoprostenol therapy in a separate study of 178 patients with moderate to severe PAH.Citation52 Badesch et al extended the utility of epoprostenol beyond patients with primary pulmonary hypertension in 2000. In 111 patients with pulmonary hypertension due to scleroderma, epoprostenol improved pulmonary vascular hemodynamics and exercise capacity over a 12-week treatment course.Citation54 In addition, nonrandomized studies have suggested that epoprostenol treatment may be effective in patients with PAH due to congenital left-to-right shunts, portopulmonary hypertension, and human immunodeficiency virus infection.Citation55–Citation58
The pharmacodynamics of treprostinil were recently reviewed in this journal.Citation59 Unlike epoprostenol, treprostinil is chemically stable at room temperature and at neutral pH. This facilitates its use in both intravenous and subcutaneous infusion. Simonneau et al evaluated the efficacy of continuous subcutaneous treprostinil in 470 patients over a 12-week period.Citation60 In this study, 6MWD improved in a dose-dependent fashion, as did dyspnea indices and hemodynamics. Infusion site pain and reactions were common, complicating therapy in 85% and 83% of patients, respectively. The effects of long-term subcutaneous treprostinil were separately examined in 99 patients with PAH and 23 patients with chronic thromboembolic pulmonary hypertension.Citation61 After a mean follow-up of 26.2 months, patients treated with subcutaneous treprostinil demonstrated increased 6MWD as well as improved dyspnea score, functional class, and overall survival. Barst et al followed 806 patients for up to four years on subcutaneous treprostinil therapy and demonstrated improved survival versus historic controls.Citation62 Of the 196 patients who discontinued the medication, 34% stopped within the first year of treatment due to infusion site pain. Intravenous treprostinil therapy was studied in a 12-week, open-label study in 16 patients with PAH.Citation63 Continuous intravenous treprostinil improved 6MWD (82 meters compared with baseline), hemodynamics, and BORG dyspnea score.
Iloprost is an inhaled carbacyclin prostacyclin analog used to treat pulmonary hypertension.Citation64 Early studies comparing inhaled epoprostenol and iloprost demonstrated short-term efficacy of both agents, but iloprost had a significantly longer duration of action (10–30 minutes versus 60–120 minutes).Citation65 In an uncontrolled trial, Olschewksi et al demonstrated the benefit of inhaled iloprost nebulized 6–12 times daily over the course of three months.Citation66 A large, randomized controlled study of iloprost confirmed this benefit.Citation67 Over a period of 12 weeks, patients treated with inhaled iloprost six or nine times daily demonstrated increased 6MWD (36.4 meters), improved hemodynamics, and functional class. In this study, syncope, flushing, and cough significantly increased with inhaled iloprost. The long-term efficacy of inhaled iloprost was studied in 24 patients over the course of one year.Citation68 Patients had a sustained improvement in 6MWD, cardiac output, and pulmonary vascular resistance. However, in a separate study, after 12 months, only 32 of 76 patients were stabilized on iloprost monotherapy, most requiring additional therapy (23 patients) or transplant (two patients).Citation69
Beraprost is a stable, cyclopentabenzofuranyl prostacyclin analog that enables oral administration. ALPHABET (Arterial Pulmonary Hypertension and Beraprost European Trial) was a randomized, placebo-controlled study of 130 patients with PAH over 12 weeks.Citation70 Beraprost administered at a dose of up to 120 μg four times daily improved exercise capacity in patients with idiopathic PAH although it did not significantly alter the hemodynamic outcomes measured. Headache, flushing, jaw pain, diarrhea, and nausea were frequent during the titration period but were less common in the maintenance phase. In contrast, Barst et al failed to demonstrate sustained improvement in exercise tolerance or disease progression in 116 patients with WHO functional class II or III PAH due to collagen vascular disease or congenital heart disease.Citation71 A retrospective study of 58 patients with idiopathic PAH demonstrated a three-year survival rate of 76% compared with 44% with conventional therapy.Citation72 Beraprost is currently approved for treatment of PAH in Japan.
Inhaled treprostinil
The difficulty with injection site pain and potential pharmacologic benefits of inhaled therapy led to the development of aerosolized treprostinil therapy for PAH. Initial investigations compared the effects of inhaled and intravenous treprostinil in sheep with PAH induced by 9,11-dideoxy-9, 11-epoxymethanoprostaglandin F2 (U44069), a PGH2 analog that led to a four-fold increase in pulmonary vascular resistance.Citation73 Both inhaled and intravenous treprostinil reversed this effect in a dose-dependent fashion. However, only inhaled treprostinil at 1000 ng/kg/min fully reversed the pulmonary arterial vasoconstriction and normalized pulmonary artery pressures. At this dose, there was no significant change in systemic blood pressure, cardiac output, or heart rate. The duration of effect of inhaled treprostinil was about three times greater than that of inhaled epoprostenol, thereby demonstrating the efficacy of inhaled treprostinil in an animal model of PAH.
The first published trial of inhaled treprostinil in humans characterized its effects in three patients with severe PAH.Citation74 After right heart catheterization, each patient received a single administration of 15 μg of treprostinil in three breaths through an ultrasonic inhalation device. This single dose reduced pulmonary vascular resistance by 45.2% ± 17.5%, with sustained effects observed for longer than 180 minutes after inhalation. Subsequently, two patients continued on inhaled treprostinil therapy, 15 μg four times daily, on a compassionate treatment basis. After three months, both had improvement in functional class and 6MWD.
Further studies in humans compared inhaled treprostinil with iloprost. Voswinckel et al compared the effects of these medications in 123 patients with different forms of PAH.Citation75 Administration of either medication led to a rapid decrease in pulmonary vascular resistance and pulmonary artery pressure. A comparison of three inhaled treprostinil regimens, ie, 7.5 μg over six minutes, 15 μg over six minutes, or 15 μg over three minutes, showed no significant difference in pulmonary vascular resistance reduction. When compared with iloprost, the maximum effect of inhaled treprostinil was comparable. However, the peak effect of iloprost (8 ± 1 minutes) was observed more quickly than for inhaled treprostinil (18 ± 2 minutes), while the inhaled treprostinil led to a more prolonged effect extending beyond the three-hour experimental window. Peak plasma treprostinil levels were observed 10–15 minutes after inhalation, consistent with the drug’s peak effect. In this small study, the most common side effects of treprostinil were cough (six of 48 patients), headache (two of 48 patients), and jaw pain (1 of 48 patients).
Additional clinical trials have been performed to evaluate the combination of inhaled treprostinil with other PAH therapies. In a 12-week, open-label safety and efficacy trial, 12 patients with PAH and functional class III or IV symptoms despite oral bosentan administration received additional inhaled treprostinil.Citation76 Two dosing regimens were used, ie, 30 μg (six inhalations) four times daily and 45 μg (nine inhalations) four times daily, via a hand-held ultrasonic, single-breath nebulizer. Acutely, the effect of inhaled treprostinil peaked at 45 minutes and returned to baseline by 180 minutes. After 12 weeks of therapy, significant reductions in mean pulmonary artery pressure and pulmonary vascular resistance were observed at peak post-inhalation, with a trend toward improvement in cardiac output. Notably, both pre- and post-inhalation 6MWD was improved with therapy.
The acute effects of the combination of sildenafil and inhaled treprostinil have also been studied.Citation77 Fifty patients with moderate to severe pulmonary hypertension received sildenafil plus 15 μg or 30 μg inhaled treprostinil. Inhaled treprostinil induced a significant reduction in pulmonary vascular resistance and pulmonary arterial pressure beyond the treatment effect from sildenafil alone that was present up to two hours after inhalation. While the 15 μg and 30 μg doses were equally effective, the 30 μg dose had a longer duration of action. Interestingly, a subgroup analysis of patients with either PAH or chronic thromboembolic pulmonary hypertension demonstrated similar results. Approximately 10% of patients reported a cough, but the known side effects of sildenafil including headache, musculoskeletal pain, dizziness, and hypotension, were not potentiated by treprostinil inhalation. Also, ventilation/perfusion matching was not significantly altered by treprostinil administration.
Recently, a metered dose inhaler (MDI) formulation of treprostinil was evaluated.Citation78 This device has the advantages of a smaller size and improved ease of use compared with the ultrasonic nebulizer. The aerodynamic aerosol diameter of MDI treprostinil is 4–5 μm, which is at the upper size limit for alveolar deposition. Doses of 30 μg, 45 μg, or 60 μg of MDI treprostinil lowered mean pulmonary artery pressure and pulmonary vascular resistance. Pulmonary vasodilation extended beyond the observation period of two hours. Pulmonary vascular resistance reduction by MDI was comparable with that previously observed with ultrasonic nebulizer. There was no observed decrease in ventilation/perfusion matching by treprostinil delivered by MDI.
Currently, treprostinil is delivered using an ultrasonic pulse-delivery nebulizer system.Citation79 Initial dosing begins at three breaths or 18 μg four times daily, and may be increased at 1–2-week intervals up to a maximum dose of nine breaths (54 μg) four times daily. Cough, headache, throat irritation, nausea, and flushing are common side effects of inhaled treprostinil.
Conclusion
The number of available treatments for PAH continues to increase. Three classes of drugs, ie, prostacyclins, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors, affecting different mechanisms involved in the pathogenesis of PAH, are currently available. Endothelin receptor antagonists and phosphodiesterase-5 inhibitors are administered orally at a frequency that varies from once to three times daily. Prostacyclin therapies are delivered by continuous intravenous infusion (epoprostenol and treprostinil), by continuous subcutaneous infusion (treprostinil), or as aerosols by nebulization (iloprost and treprostinil). Typically, oral therapies are utilized for patients with milder disease, whereas the prostacyclins are initiated in patients with more advanced disease, or progression despite oral therapy. The use of inhaled agents reduces the risks and inconveniences associated with infused treatments, and further expand the potential role for prostacyclin therapies. Continued experience and evaluation of inhaled treprostinil, particularly in combination with oral therapies, will help to provide further assessment of its long-term safety and efficacy.
Disclosure
The authors report no conflicts of interest in this work.
References
- HoeperMDefinition, classification and epidemiology of pulmonary arterial hypertensionSemin Respir Crit Care Med20093036937519634076
- D’AlonzoGFBarstRJAyresSMSurvival in patients with primary pulmonary hypertensionAnn Intern Med19911153433491863023
- ThenappanTShahSRichSTianLArcherSGomberg-MaitlandMContemporary survival in patients with pulmonary arterial hypertension: A reappraisal of the NIH risk stratification equationEur Respir J2010351079108720032020
- HumbertMSitbonOChaouatAPulmonary arterial hypertension in France: Results from a national registryAm J Respir Crit Care Med20061731023103016456139
- PeacockAMurphyNMcMurrayJCaballeroLStewartSAn epidemiological study of pulmonary arterial hypertensionEur Respir J20073010410917360728
- ThenappanTShahSRichSGomberg-MaitlandMA USA-based registry for pulmonary arterial hypertension: 1982–2006Eur Respir J2007301103111017804449
- SimmoneauGRobbinsIBeghettiMUpdated clinical classification of pulmonary hypertensionJ Am Coll Cardiol200954S43S5419555858
- BadeschDRaskobGElliottCPulmonary arterial hypertension – baseline characteristics from the REVEAL registryChest201013737638719837821
- McLaughlinVArcherSBadeschDACCF/AHA 2009 expert consensus document on pulmonary hypertensionCirculation20091192250229419332472
- GalieNHoeperMHumbertMGuidelines for the diagnosis and treatment of pulmonary hypertensionEur Respir J2009341219126319749199
- FarberHLoscalzoJPulmonary arterial hypertensionN Engl J Med20043511655166515483284
- ChristmanBMcPhersonCNewmanJAn imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertensionN Engl J Med199232770751603138
- GiaidAYanagisawaMLanglebenDExpression of endothelin-1 in the lungs of patients with pulmonary hypertensionN Engl J Med1993328173217398497283
- StewartDLevyRCernacekPLanglebenDIncreased plasma endothelin-1 in pulmonary hypertension: Marker or mediator of disease?Ann Intern Med19911144644691994793
- TuderRCoolCGeraciMProstacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertensionAm J Respir Crit Care Med19991591925193210351941
- RichSDantzkerDAyresSPrimary pulmonary hypertension – a national prospective studyAnn Intern Med19871072162233605900
- McGoonMGuttermanDSteenVScreening, early detection and diagnosis of pulmonary arterial hypertensionChest2004126Suppl 114S34S15249493
- McQuillanBPicardMLeavittMWeymanAClinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjectsCirculation20011042797280211733397
- SwanHGanzWForresterJMarcuHDiamondGChonetteDCatheterization of the heart in man with use of a flow-directed balloon-tipped catheterN Engl J Med19702834474515434111
- WeissBZempLSeifertBHessOOutcome of pulmonary vascular disease in pregnancy: A systematic overview from 1978 through 1996J Am Coll Cardiol199831165016579626847
- RichSKaufmanELevyPThe effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertensionN Engl J Med199232776811603139
- FrankHMlczochJHuberKSchusterEGurtnerHKneusslMThe effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertensionChest19971127147219315805
- FusterVSteelePEdwardsWGershBMcGoonMFryeRPrimary pulmonary hypertension: Natural history and the importance of thrombosisCirculation1984705805876148159
- RichSSeidlitzMDodinEThe short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertensionChest19981147877929743167
- SitbonOHumbertMJaisXLong-term response to calcium channel blockers in idiopathic pulmonary arterial hypertensionCirculation20051113105311115939821
- HickeyKRubanyiGPaulRHighsmithRCharacterization of a coronary vasoconstrictor produced by cultured endothelial cellsAm J Physiol1985248C5505563993773
- YanagisawaMKuriharaHKimuraSA novel potent vasoconstrictor peptide produced by vascular endothelial cellsNature19883324114152451132
- KedzierskiRYanagisawaMEndothelin system: The double-edged sword in health and diseaseAnnu Rev Pharmacol Toxicol20014185187611264479
- RubensCEwertRHalankMBig endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertensionChest20011201562156911713135
- LiuCChenJGaoYDengBLiuKEndothelin receptor antagonists for pulmonary arterial hypertensionCochrane Database Syst Rev20093CD00443419588356
- ChannickRSimonneauGSitbonOEffects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebo controlled studyLancet20013581119112311597664
- RubinLBadeschDBarstRBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- McLaughlinVSitbonOBadeschBSurvival with first-line bosentan in patients with primary pulmonary hypertensionEur Respir J20052524424915684287
- ProvencherSSitbonOHumbertMCabrolSJaisXSimonneauGLong-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertensionEur Heart J20062758959516431875
- GalieNRubinLHal-HitiPTreatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): A double-blind, randomised controlled trialLancet20083712093210018572079
- WuCChanMStavrosFDiscovery of TBC11251, a potent, long acting, orally active endothelin receptor-a selective antagonistJ Med Chem199740169016979171878
- BarstRLanglebenDFrostASitaxsentan therapy for pulmonary arterial hypertensionAm J Respir Crit Care Med200416944144714630619
- BarstRLanglebenDBadeschDTreatment of pulmonary arterial hypertension with the selective endothelin-a receptor antagonist sitaxsentanJ Am Coll Cardiol2006472049205616697324
- LanglebenDHirschAShalitELezenkoLBarstRSustained symptomatic, functional, and hemodynamic benefit with the selective endothelin-a receptor antagonist, sitaxsentan, in patients with pulmonary arterial hypertensionChest20041261377138115486408
- GalieNBadeschDOudizRAmbrisentan therapy for pulmonary arterial hypertensionJ Am Coll Cardiol20054652953516053970
- GalieNOlschewskiHOudizRAmbrisentan for the treatment of pulmonary arterial hypertensionCirculation20081173010301918506008
- ArcherSMichelakisEPhosphodiesterase type 5 inhibitors for pulmonary arterial hypertensionN Engl J Med20093611864187119890129
- GiaidASalehDReduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertensionN Engl J Med19953332142217540722
- MurrayFMacLeanMPyneNIncreased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertensionBr J Pharmacol20021371187119412466227
- GalieNGhofraniHTorbickiASildenafil citrate therapy for pulmonary arterial hypertensionN Engl J Med20053532148215716291984
- Pepke-ZabaJGilbertCCollingsLBrownMSildenafil improves health-related quality of life in patients with pulmonary arterial hypertensionChest200813318318918187744
- GalieNBrundageBGhofraniHTadalafil therapy for pulmonary arterial hypertensionCirculation20091192894290319470885
- ZuckerTBunischDHasseAGrosserTWeberASchrorKTolerance development to antimitogenic actions of prostacyclin but not of prostaglandin E1 in coronary artery smooth muscle cellsEur J Pharmacol19983452132209600640
- ChengYAustinSRoccaBRole of prostacyclin in the cardiovascular response to thromboxane A2Science200229653954111964481
- LaliberteKArnesonCJeffsRHuntTWadeMPharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteersJ Cardiovasc Pharmacol20044420921415243302
- McLaughlinVShilingtonARichSSurvival in primary pulmonary hypertension: The impact of epoprostenol therapyCirculation20021061477148212234951
- SitbonOHumbertMNunesHLong-term intravenous epoprostenol infusion in primary pulmonary hypertensionJ Am Coll Cardiol20024078078812204511
- BarstRRubinLLongWA comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertensionN Engl J Med19963342963018532025
- BadeschDTapsonVMcGoonMContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of diseaseAnn Intern Med200013242543410733441
- AguilarRFarberHEpoprostenol (prostacyclin) therapy in HIV-associated pulmonary hypertensionAm J Respir Crit Care Med20001621846185011069824
- PlotkinJKuoPRubinLSuccessful use of chronic epoprostenol as a bridge to liver transplantation in severe portopulmonary hypertensionTransplantation199844574599500616
- TanHMarkowitzJMontgomeryRLiver transplantation in patients with severe portopulmonary hypertension treated with preoperative chronic intravenous epoprostenolLiver Transpl2001774574911510023
- RosenzweigEKersteinDBarstRLong-term prostacyclin for pulmonary hypertension with associated congenital heart defectsCirculation1999991858186510199883
- Skoro-SajerNLangINaeijeRTreprostinil for pulmonary hypertensionVasc Health Risk Manag2008450751318827901
- SimonneauGBarstRGalieNContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertensionAm J Respir Crit Care Med200216580080411897647
- LangIGomez-SanchezMKneusslMEfficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertensionChest20061291636164316778286
- BarstRGalieNSimonneauGJeffsRArnesonCRubinLLong-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinilEur Heart J20062811951203
- TapsonVGomberg-MaitlandMMcLaughlinVSafety and efficacy of IV treprostinil for pulmonary arterial hypertensionChest200612968368816537868
- KrugSSablotzkiAHammerschmidtSWirtzHSeyfarthHInhaled iloprost for the control of pulmonary hypertensionVasc Health Risk Manag2009546547419475782
- OlschewskiHWalmrathDSchermulyRGhofraniAGrimmingerFSeegerWAerosolized prostacyclin and iloprost in severe pulmonary hypertensionAnn Intern Med19961248208248610951
- OlschewskiHGhofraniASchmehlTInhaled iloprost to treat severe pulmonary hypertensionAnn Intern Med200013243544310733442
- OlschewskiHSimonneauGGalieNInhaled iloprost for severe pulmonary hypertensionN Engl J Med200234732232912151469
- HoeperMSchwarzeMEhlerdingSLong-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogueN Engl J Med20003421866187010861321
- OpitzCWenselRWinklerJClinical efficacy and survival with first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertensionEur Heart J2005261895190215888496
- GalieNHumbertMVachieryJEffects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: A randomized, double-blind, placebo controlled trialJ Am Coll Cardiol2002391496150211985913
- BarstRMcGoonMMcLaughlinVBeraprost therapy for pulmonary arterial hypertensionJ Am Coll Cardiol2003412119212512821234
- NagayaNUematsuMOkanoYEffect of orally active prostacyclin analogue on survival of outpatients with primary pulmonary hypertensionJ Am Coll Cardiol1999341188119210520811
- SandiferBBrighamKLawrenceEMottolaDCuppelsCParkerRPotent effects of aerosol compared with intravenous treprostinil on the pulmonary circulationJ Appl Physiol2005992363236816141385
- VoswinckelRGhofraniHGrimmingerFSeegerWInhaled treprostinil for treatment of chronic pulmonary arterial hypertensionAnn Intern Med200614414915016418424
- VoswinckelREnkeBReichenbergerRFavorable effects of inhaled treprostinil in severe pulmonary hypertension: Results from randomized controlled pilot studiesJ Am Coll Cardiol2006481672168117045906
- ChannickROlschewskiHSeegerWStaubTVoswinckelRRubinLSafety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertensionJ Am Coll Cardiol2006481433143717010807
- VoswinckelHReichenbergerFEnkeBAcute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertensionPulm Pharmacol Ther20082182483218657627
- VoswinckelRReichenbergerFGallHMetered dose inhaler delivery of treprostinil for the treatment of pulmonary hypertensionPulm Pharmacol Ther200922505619071225
- Tyvaso Prescribing InformationResearch Triangle Park, NCUnited Therapeutics Corp2010