Abstract
Introduction:
Currently available endothelin receptor antagonists for treating pulmonary arterial hypertension block either the endothelin (ET) receptor A or both A and B receptors. Transition from one endothelin receptor antagonist to another may theoretically alter side-effects or efficacy. We report our experience of a transition from sitaxsentan to ambrisentan, both predominant ETA receptor antagonists, in pulmonary arterial hypertension patients.
Methods:
At Baylor Pulmonary Hypertension Center, 18 patients enrolled in the open-label extension phase of the original sitaxsentan studies (Sitaxsentan To Relieve ImpaireD Exercise) were transitioned to ambrisentan (from July 2007 to September 2007) at the time of study closure. Pre-transition (PreT), 1 month (1Mth) and 1 year (1Yr) post-transition assessments of 6-minute walk distance (6MWD), brain naturetic peptide (BNP) levels, WHO functional class (WHO FC), Borg dyspnea score (BDS), oxygen saturation, liver function, and peripheral edema were compared.
Results:
6MWD was 356 ± 126 m at PreT, 361 ± 125 m at 1Mth, and 394 ± 114 m at 1Yr (mean ± SD). There was no difference in the walk distance at 1Mth and 1Yr post transition compared with PreT (P = 0.92, 0.41 respectively). Oxygen saturation was no different at 1Mth and 1Yr to PreT level (P = 0.49 and P = 0.06 respectively). BNP was 178 ± 44 pg/mL at PreT, 129 ± 144 pg/mL at 1Mth and 157 ± 201 at 1Yr. Peripheral edema was present in 7/18 patients at PreT, in 8/16 patients at 1Mth, and in 6/13 patients at 1Yr post transition. Proportions of patients with edema over these 3 time points did not change significantly (P = 0.803). At 1Yr, 2 patients had died, 1 had undergone lung transplantation, 1 had relocated, and 1 patient was started on intravenous prostacyclin therapy. Over 3 points (baseline, 1 month, and 1 year), there was no significant change in function class (P = 0.672).
Conclusion:
Our limited data suggest that ETA receptor antagonists can be switched from one to another with sustained exercise capacity and maintained WHO FC with no increase in incidence of peripheral edema.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease resulting from pulmonary vascular remodeling leading to right heart failure.Citation1 Endothelin 1 (ET-1), a modulator of pulmonary vascular remodeling, has an important role in the pathophysiology of this disease. ET-1 binds to 2 receptors, ETA and ETB. Endothelin receptor antagonists (ETRAs) block either A or A and B receptors are used to treat PAH. Two Food and Drug Administration (FDA) -approved ETRAs are available to treat PAH in the US. Sitaxsentan, a predominantly ETA receptor antagonist, was studied in the US, Europe, and Canada; sitaxsentan was approved in Europe and Canada but did not receive FDA approval in the US. Thus patients enrolled in open label sitaxsentan extension studies were transitioned to ambrisentan at the time of sitaxsentan study closure. We report our single-center experience of patients transitioning from sitaxsentan to ambrisentan after study closure.
To date indications for changing from one ETRA to another ETRA are anecdotal. Side effect profiles such as liver toxicity and drug interactions differ between these ETRAs.Citation2 Due to the predominant blockade of endothelin A receptor with sitaxsentan we decided to switch our patients from sitaxsentan to ambrisentan (both predominantly ETA receptor antagonists). We present the available long-term clinical data for 18 patients switched to ambrisentan at the time of sitaxsentan study closure.
Method
Eighteen PAH patients were enrolled in the open-label extension phase of the original sitaxsentan studies (Sitaxsentan To Relieve ImpaireD Exercise [STRIDE] −2, −3 and −6) at Baylor Pulmonary Hypertension Center. All patients were on 100 mg oral daily of sitaxsentan. Patients were enrolled in the open label extension phase of clinical studies, which permitted addition of other PAH medications as clinically indicated. These patients were changed to ambrisentan (5 mg oral daily) (between July 2007 and September 2007) at the time of study closure. This transition was necessary because sitaxsentan did not gain FDA approval in US and hence this drug was unavailable to patients after sitaxsentan study was closed.
Data was collected for each patient at pre-transition (PreT), 1 month (1Mth) (48 ± 28 days, range 19–123), and 1 year (1Yr) post transition (401 ± 73 days, range 252–489). Collected data included 6-minute walk distance (6MWD), brain naturetic peptide (BNP) plasma concentrations, WHO functional class (WHO FC), Borg dyspnea score (BDS), presence of peripheral edema, changes in diuretic dose, and liver function tests. Patient 2 was hospitalized at the time of transition for acute gastroenteritis and remained in the hospital till he died from worsening right heart failure. Hence limited data were available for patient 2. Patient 17 had only laboratory testing done at 1Mth and was unable to do the walk test at 1Yr due to severe hip pain. Patient 3 was taken off ambrisentan and started on intravenous treprostinil due to worsening pulmonary hypertension 9 month post transition and died 13 months post transition. This patient did not have a walk test done at 1Yr because of severe back pain, which was considered a treprostinil related side-effect by the treating physician. Patient 18 progressed to WHO FC IV at 1Mth from WHO FC III at baseline and subsequently underwent lung transplantation 5.5 months post transition. Patient 9 relocated 5 months post transition. Hence, 1Yr data available for 13 patients was included in this analysis.
Statistical analysis
All data are reported as mean ± standard deviation (SD) unless stated otherwise. Differences between groups were determined by using paired Student’s t-test. A chi-square test was used to evaluate the significance of change of WHO FC over 3 time points. We also used a chi-square test to examine the difference in proportions of patients who experienced edema at 3 time points. Analyses were conducted using SAS (v. 9.2;SAS Institute Inc., Cary, NC). Significance was accepted at P < 0.05 and all hypothesis testing was two-sided.
Results
Demographic and baseline characteristics are outlined in . There were 15 females and 3 males with a mean age of 52 ± 14 (mean ± SD). At 1Mth, the ambrisentan dose was increased in 7 patients to 10 mg and 10 patients were continued on 5 mg dose. Patient 2 was on ambrisentan 5 mg when he died in the hospital. At 1Yr, 8 patients were on 10 mg dose, 5 patients remained on 5 mg, and 1 patient was taken off ambrisentan for worsening disease and started on intravenous prostacyclin therapy (patient 3). Patient 11 had sildenafil added to ambrisentan 4 months prior to 1Yr. None of the patients discontinued ambrisentan due to liver function abnormalities (). Patient 15 had increased aspartate aminotransferase at 1Mth (3.5 × upper limit of normal) which returned to normal on repeat testing in 1 week without interruption in therapy.
Table 1 Demographics and baseline patient characteristics of transitioned patients
Table 2 Serum aminotransferase concentrations in transitioned patients
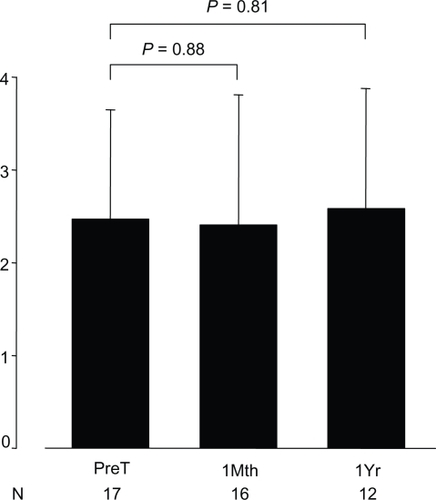
6MWD was 356 ± 126 m at PreT, 361 ± 125 m at 1Mth, and 394 ± 114 m at 1Yr. There was no difference in the 6MWD at 1Mth and at 1Yr compared with PreT (P = 0.92 and P = 0.41 respectively) (). BNP was not significantly different at 1Mth and at 1Yr compared with PreT (P = 0.50 and P = 0.83 respectively) (). BDS was 2.5 ± 1.2 (range 0 to 4) at PreT in 17 patients, 2.4 ± 1.4 (range 0 to 4) at 1Mth in 16 patients and 2.6 ± 1.3 (range 0.5 to 5) at 1Yr in 12 patients (). These values was not significantly different (P = 0.88 and 0.81 respectively).
Figure 1 Six-minute walk distance at transition and follow-up in PAH patients.
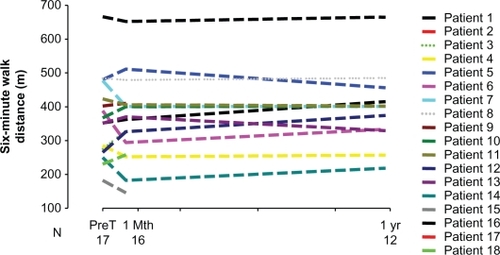
Figure 2 BNP levels at transition and follow-up in PAH patients. Data presented as mean ± standard error.
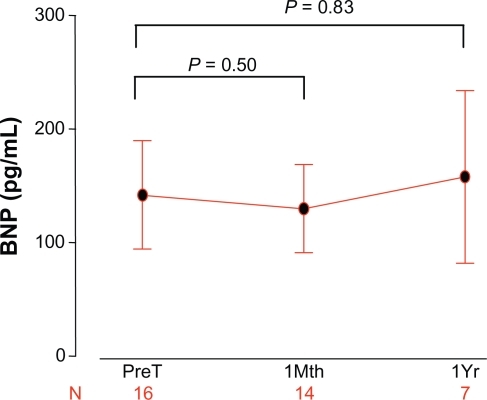
Figure 3 Borg dyspnea score at transition and follow-up in PAH patients. Data presented as mean ± standard deviation.
Eleven of the 18 patients were idiopathic PAH (IPAH) patients. Patients were divided into 2 groups – IPAH, and PAH associated with other conditions, and data was analyzed to determine whether our findings would differ in patients with different PAH etiologies. There was no significant difference between the 2 groups at PreT, 1Mth, and 1Yr in 6MWD (P = 0.42, 0.34, 0.27 respectively), BNP levels (P = 0.63, 0.90, 0.55 respectively), oxygen saturation (P = 0.35, 0.47, 0.06 respectively), and BDS (0.55, 0.45, 0.39 respectively).
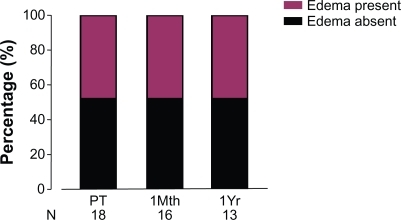
Peripheral edema was present in 7/18 patients at PreT, 8/16 patients at 1Mth, and in 6/13 patients at 1yr (). At 1Mth, the diuretic dose was increased in 2 of the 16 patients (patients 4 and 18). At 1Yr, a second diuretic was added for patient 4. Proportions of patients with edema over these 3 time points did not change significantly (P = 0.803).
Oxygen saturation was not different at 1Mth compared with PreT (P = 0.49). Oxygen requirements remained unchanged except for 1 patient who was on room air PreT and was started on 2 L oxygen at 1Mth and remained on 2 L at 1Yr. 1Yr data were available for 13 patients and oxygen saturation slightly improved compared with PreT (P = 0.06).
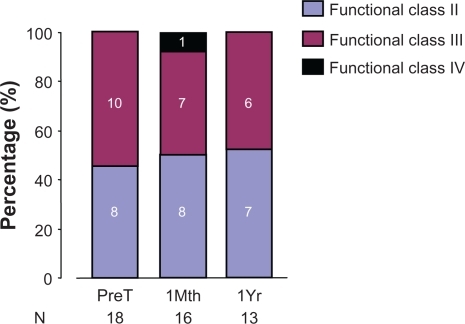
Over 3 points (PreT, 1Mth, and 1Yr), there was no significant change in WHO function class (P = 0.672) ().
Discussion
In this single-center retrospective study switching from sitaxsentan to ambrisentan was safe and efficacy was maintained. At the end of the open label extension phase of STRIDE studies, we switched all of the patients then enrolled to ambrisentan. In the open label extension phase, addition of PAH therapies was allowed. Prior to transitioning, 3 patients enrolled in this extension phase had died. Our data suggest that switching from a selective ETRA to another selective ETRA may be reasonable and safe. However, 3 patients in our cohort developed disease worsening and 2 patients died after this change, suggesting that individual responses to selective ET receptor antagonists may vary.
Ambrisentan and sitaxsentan are both ETA-selective antagonists. Based on in vitro competitive receptor binding assay, ambrisentan has a 77-fold affinity for ETA compared with ETB receptors.Citation3 On the other hand, sitaxsentan has a ∼6500-fold affinity for ETA compared with ETB receptors.Citation4,Citation5 Activation of the ETA receptor on smooth muscle cells causes sustained vasoconstriction and proliferation of these cells. However, activation of ETB on endothelial cells induces clearance of ET-1 from the circulation and mediates vasodilation through the activation of NO and prostacyclin. This selectivity of ETA receptor blockages has been proposed to prevent the deleterious effects of ETA, while allowing the favorable effects of ETB.Citation2 However, this difference in selectively has not translated into substantial differences in clinical efficacy, as shown by the published clinical studies.Citation6–Citation10 Our data show that the walk distance was maintained for up to 1 year after the switch in the ETRA. This suggests that selective ETRA can be used interchangeably as required by the clinical necessity. However, we caution against using this data for the dual receptor ETRA, because we switched our patients to a selective ETRA, ambrisentan, in our cohort.
Both ambrisentan and sitaxsentan have a long half-life and require once daily dosing.Citation7,Citation11,Citation12 Ambrisentan is a propanoic acid-based compound that is metabolized predominately by glucuronidation and transported via the bile. Sitaxsentan is derived from amidothiophenesulponamides by a series of chemical modifications. It is metabolized by the CYP2CP and CYP3A4 with 50% to 60% of the dose excreted in the urine and the remaining in feces. The incidence of liver function test abnormalities is similar between the two drugs (3% with both ambrisentan and sitaxsentan).Citation7,Citation11,Citation13
Transitioning from one ETRA to another ETRA may be required in different clinical situations. This may be because of side-effects, liver function abnormalities, or availability of the drug. McGoon et al have previously shown that patients who discontinued bosentan due to liver function test abnormalities tolerated ambrisentan and this change resulted in improvement in walk distance.Citation14 We have previously shown that carefully selected patients can be safely transitioned from intravenous prostacyclin to oral agents.Citation15 Our data was obtained from patients with normal liver function who were switched to ambrisentan when the sitaxsentan did not gain FDA approval in US. We included patients who died, were lost to follow-up, or underwent lung transplantation. We did not exclude any patient to prevent an introduction of bias in data collection. Patient 15 in our cohort developed transient elevation in transaminase, which resolved spontaneously without any medical intervention. This patient had a congenital heart defect (ventricular septal defect), Eisenmenger physiology, and Down syndrome. This patient was not on any additional PAH therapy due to significant underlying medical conditions and parents’ request. This patient did not develop increased edema at 1Mth and the parents felt that the breathing was unchanged. However, this patient developed an intolerable cough and 3 months post transition the walk distance had significantly deteriorated and therefore the patient was switched to bosentan and subsequently died 5 months post transition. It is conceivable that the clinical condition may have deteriorated when this patient was switched from sitaxsentan to ambrisentan. On the other hand, this patient was 45 years of age at the time of transition and had been diagnosed with PAH for the previous 37 years. Possibly this patient was at the end of the disease spectrum.
Peripheral edema is considered to be a class side-effect of ETRAs,Citation16 with a greater percentage of older patients (>65 years) experiencing higher rates of peripheral edema.Citation16 Some ETRAs were thought to be associated with more peripheral edema than others. However, our data suggest that at least in the selective ETRAs there was no significant worsening of peripheral edema. None of the patients required intravenous diuresis or hospitalization for worsening edema. One patient, however, who was already in the hospital at the time of study closure, developed worsening right heart failure and subsequently died. This could have been related to the progression of disease or, on the other hand, may have been related to worsening right heart failure as a consequence of increased fluid retention caused by a switch in the ETRA. Therefore, switching ETRA in patients with marginal clinical status should be undertaken cautiously. We do not recommend switching between ETRAs without a compelling reason such as when side-effects must be mitigated. Hence, such switches should be undertaken only when they confer a greater clinical benefit to the patient.
Acknowledgements
This study was supported by the National Institute of Health grant K23HL-093214 to ZS. Part of this study was presented at the European Respiratory Society Meeting, Barcelona 2010, and at the American Thoracic Society Meeting, New Orleans 2010 as an abstract.
The author thanks Degang Wang, PhD for his assistance in the statistical analysis, and Helena Purl, RN, and Ms Janice Brister for their help in preparing this manuscript.
Disclosure
ZS has been on Gilead Science, Actelion, United Therapeutics and Pfizer advisory boards and has provided consultancy for these companies.
References
- RubinLJPathology and pathophysiology of primary pulmonary hypertensionAm J Cardiol199575351A54A
- SafdarZTargeted oral therapies in the treatment of pulmonary arterial hypertensionClin Drug Investig20103012811826
- ValerioCJKabungaPCoghlanJGSafety and efficacy of ambrisentan in the treatment of pulmonary arterial hypertensionClin Med Ther20091541556
- WuCChanMFStavrosFDiscovery of TBC11251, a potent, long acting, orally active endothelin receptor-A selective antagonistJ Med Chem19974011169016979171878
- WuCDeckerERBlokNEndothelin antagonists: substituted mesitylcarboxamides with high potency and selectivity for ET(A) receptorsJ Med Chem199942224485449910579813
- OpitzCFEwertRKirchWPittrowDInhibition of endothelin receptors in the treatment of pulmonary arterial hypertension: does selectivity matter?Eur Heart J200829161936194818562303
- GalieNBadeschDOudizRAmbrisentan therapy for pulmonary arterial hypertensionJ Am Coll Cardiol200546352953516053970
- OudizRJTorresFFrostAEARIES-1: A placebo-controlled, efficacy and safety study of ambrisentan in patients with pulmonary arterial hypertensionChest20061304 121S-a-.
- GalieNOlschewskiHOudizRJAmbrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2Circulation2008117233010301918506008
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med20023461289690311907289
- BarstRJLanglebenDFrostASitaxsentan therapy for pulmonary arterial hypertensionAm J Respir Crit Care Med2004169444144714630619
- MotteSMcEnteeKNaeijeREndothelin receptor antagonistsPharmacol Ther2006110338641416219361
- LanglebenDCacoubPA review of STRIDE-2 and STRIDE-2X: the case for selective endothelin receptor blockadeEur J Clin Invest200939Suppl 2273119335744
- McGoonMDFrostAEOudizRJAmbrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalitiesChest2009135112212918812445
- SafdarZOutcome of pulmonary hypertension subjects transitioned from intravenous prostacyclin to oral bosentanRespir Med2009103111688169219539456
- KingmanMRuggieroRTorresFAmbrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertensionExpert Opin Pharmacother200910111847185819601701