Abstract
Purpose
Testicular cancer survivors who have received platinum-based chemotherapy are at risk for premature cardiovascular disease. The etiology of this risk is not well understood. This pilot study explores the impact of platinum-based chemotherapy on endothelial function.
Methods
Testicular cancer survivors <30 years old at the time of diagnosis who received platinum-based chemotherapy between 2002 and 2012, as well as 17 similarly aged male controls, were identified. Consented subjects underwent vascular assessment using the HDI/PulseWave CR-2000 Cardiovascular Profiling System and the Endo-PAT2000 system. Biomarkers and functional test markers were compared among cases, controls, and a group of historical controls using two sided two-sampled t-tests and Wilcoxon rank-sum tests.
Results
Thirteen survivors with a median age of 30.2 years and body mass index of 27.3 were enrolled, along with 17 healthy controls with a median age of 27.1 years and body mass index of 24.8. Median time from chemotherapy was 4.7 (range: 0.8–14) years. There was no statistical difference in reactive hyperemia peripheral arterial tonometry ratio between cases and controls (p = 0.574). There was no statistical difference in small or large artery elasticity between cases and controls (p = 0.086) or between cases and historical controls (p = 0.729). There was also no statistical difference in the blood levels of circulating endothelial cells, von Willebrand factor, and vascular cell adhesion molecules. There was a trend toward increased metabolic syndrome in cases (15%) as compared to recruited controls (6%), though this difference was not statistically significant (p = 0.565).
Conclusion
Testicular cancer survivors have no clinically significant difference in endothelial function compared to controls 4 years after the completion of chemotherapy. Further research is needed to explore the secondary modifiable causes that may contribute to the risk of premature cardiovascular disease.
Background
Testicular cancer is the most common malignancy among 15- to 40-year-old males.Citation1 In the past 3 decades, the 5-year survival rates of testicular cancer have increased from 63% to >90%.Citation1,Citation2 As cancer survivors are living longer, however, they are more at risk for subsequent therapy-related health complications. Recent studies have suggested that testicular cancer survivors are at risk for premature cardiovascular disease, myocardial infarction, and sudden death.Citation2–Citation5 Among Dutch testicular cancer survivors, the prevalence of cardiovascular disease is estimated to be 18% 20 years after completion of therapy.Citation6,Citation7 In evaluating 992 patients diagnosed in the UK between 1982 and 1992, at a median follow-up of 10.2 years, the relative risk of cardiac events was 2–3 folds higher for all survivors who received chemotherapy alone, radiation alone, or combined chemotherapy and radiation, as compared to those who received surgery alone.Citation8 Other studies have suggested a 5.7-fold increased risk of cardiovascular disease in those receiving platinum-based chemotherapy as compared to those receiving surgery alone.Citation5 The risk appears to be higher in nonseminomatous testicular cancer survivors.
The etiology of premature cardiovascular disease in testicular cancer survivors is not known.Citation3,Citation5,Citation10–Citation12 It has been hypothesized that platinum-based chemotherapy may cause direct, diffuse endothelial damage.Citation10 This damage is most notable in the first year after chemotherapy.Citation13,Citation14 Platinum-based chemotherapy has also been associated with components of metabolic syndrome: weight gain, hyperlipidemia, hypertension, and insulin resistance.Citation5,Citation6,Citation9 Secondary endothe-lial damage from metabolic syndrome may also contribute to premature cardiovascular disease.Citation11 In the present study, we sought to determine whether endothelial dysfunction underlies the etiology of premature cardiovascular disease among testicular cancer survivors. We hypothesized that testicular cancer survivors treated with chemotherapy could have a reduction in small artery elasticity (SAE), suggestive of early atherosclerotic disease, as compared to healthy, noncancerous controls. We, therefore, conducted a cross-sectional pilot study to assess endothelial function among testicular cancer survivors who received platinum-based chemotherapy as compared to healthy controls.
Methods
Subjects
Testicular cancer survivors <30 years of age at the time of diagnosis and who received platinum-based chemotherapy between 2002 and 2012 were identified at the Masonic Cancer Clinic, University of Minnesota in Minneapolis, MN, and Park Nicollet Health Services in Saint Louis Park, MN, USA. Eligible subjects had received platinum-based chemotherapy, were disease free, had been off therapy for at least 6 months, and were not currently on any ongoing myelosuppressive therapy or chemotherapy. Subjects with a cardiac history (myocardial infarction, congestive heart failure, or cardiac catheterization requiring intervention) were excluded. Subjects with a prior history of radiation therapy were excluded. Medical record abstraction for cases confirmed the diagnosis, stage at diagnosis, use of chemotherapy, use of radiation therapy, and personal medical history including history of cardiovascular diseases and medications.
Ethical approval
The University of Minnesota Institutional Review Board and Cancer Center Review Committee, as well as the Park Nicollet Institutional Review Board, approved this protocol. All patients provided written informed consent according to the Declaration of Helsinki.
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Controls
Seventeen healthy controls from the Minneapolis and Saint Paul area without a history of myocardial infarction, congestive heart failure, or cardiac catheterization were enrolled. After reviewing our data and determining that ~ 30% of our cases and 0% of our controls smoked and knowing that tobacco use can affect vascular disease, we chose to also compare SAE and large artery elasticity (LAE) data from the testicular cancer survivor cohort with a group of historical controls in which a similar number had smoked tobacco. The data from healthy, historical controls were obtained from the Hennepin County Medical Center and previously published.Citation15 Subjects were matched by age, sex, and smoking status. Subjects with a history of diabetes, hepatitis C, myocardial infarction, congestive heart failure, or cardiac catheterization requiring intervention (stent placement and angioplasty) were excluded.
Study recruitment
Potential subjects were mailed a letter of recruitment inviting them to participate in the study and have been previously described.Citation16 A follow-up phone call was placed to the subjects who did not respond. Consented subjects were invited to undergo vascular assessment and biomarker assessment. The consented subjects who completed the protocol received a $50 gift card.
Vascular assessment
The vascular assessment testing procedure has been previously described.Citation16 Testing was performed using the Endo-PAT 2000 and HDI/PulseWave CR-2000 Cardiovascular Profiling System (Hypertension Diagnostic Inc, Eagan, MN, USA). LAE and SAE are derived from the diastolic pulse contour analysis based on a modified Windkessel model of circulation (model HDI/PulseWave CR-2000 Cardiovascular Profiling System). Before and during the waveform assessment, an automated oscillatory blood pressure (BP) measurement was taken on the contralateral arm. This system has been standardized and correlated with cardiovascular risk factors. Grey et alCitation17 have demonstrated that SAE is predictive for coronary heart disease events and that LAE and SAE are predictive for heart failure events above and beyond arterial BP.Citation18 A reduction in reactive hyperemia, assessed by the Endo-PAT ratio (<1.67), has also been correlated with an increase in cardiac events independent of other traditional cardiac risk factors.Citation19
The flow-mediated dilation examination required the patient to be supine and at rest. Subjects were fasting for 8 hours. All vasoactive medications were withheld for at least 4 half-lives, when possible. The SonoSite Ultrasound system was equipped with vascular software for 2-dimensional imaging, color and spectral Doppler, an internal electrocardiogram (ECG) monitor, and a high-frequency vascular transducer.
All study assessments were conducted in a specifically designed research space for clinical vascular assessments. Blood collection and vascular functional measurements were conducted by the same trained vascular technician. The pulse waveform assessments were performed in triplicate, and the mean (average of 3 values for each participant) LAE and SAE indices were used in statistical analyses.
Biomarker assessment
Biomarkers were assessed using blood obtained from a fasting draw. Assessments of biomarkers for lipids and inflammatory changes included serum glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides (TGs), C-reactive protein, plasmino-gen activator 1, tissue-type plasminogen activator, circulating endothelial cells, vonWillebrand factor (vWF), soluble vascular cell adhesion molecule, soluble P-selectin, surface P-selectin, and surface vascular cell adhesion molecule. Blood samples were collected prior to vascular functional assessments.
Statistical analysis
Demographic and clinical characteristics of participants by group (cases, controls, and historical controls) were obtained. For triplicate measurements, the average value was used in analysis. Mean ± SD or median and range of the biomarkers and the functional test measurements are presented as appropriate. Differences between young adult survivors of testicular cancer and both control populations (recruited controls and historical controls) were analyzed in terms of the biomarkers and function tests using t-tests assuming unequal variances and Wilcoxon rank-sum tests as appropriate. Data were analyzed using SAS version 9.3 (SAS Institute Inc, Cary, NC, USA). A p-value of 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
Seventy testicular cancer survivors diagnosed between 2002 and 2013 were identified. Of these, 14 consented to participate in the study, resulting in an overall participation rate of 20%. One participant was subsequently removed from the analysis when it was determined that he had received radiation therapy alone and no chemotherapy, leaving a final cohort of 13 testicular cancer survivors. Median time between completion of platinum-based chemotherapy and study participation was 4.7 years. The majority of survivors had received combination chemotherapy with cisplatin, etoposide, and bleomycin, with only 1 survivor receiving single-agent carboplatin. Demographic and clinical characteristics of cases, recruited controls, and historical controls are presented in .
Table 1 Demographic and clinical characteristics of cases and controls at a median follow-up time of 4.7 years after completion of platinum-based chemotherapy
Cases and recruited controls
Mean age of cases was 30.2 ± 4.6 years as compared to 27.1 ± 8.3 years for controls (p = 0.2). Testicular cancer cases tended to be Caucasian (92% vs 56%; p = 0.044) and overweight in comparison to controls (mean body mass index [BMI] 27.3 ± 8.0 kg/m2 compared to 24.8 ± 3.6 kg/m2; p = 0.31). There was no statistical difference in lipid profiles between cases and controls. In addition, mean systolic blood pressure was elevated in cases (125 ± 11 mmHg vs 113 ± 11 mmHg, p = 0.01). There was no difference in free testosterone (11.4 vs 16.5 pg/mL, p = 0.10) or total testosterone (4.0 vs 5.4 ng/mL, p = 0.09) levels between cases and controls. Smoking status, however, between cases and controls differed markedly (31% vs 0%, p = 0.026). As a result of this difference in smoking status, we also compared cases with a historical control group that had a similar proportion of tobacco users. No other demographic or clinical differences between cases and historical controls achieved statistical significance.
Cases and historical controls with similar numbers of tobacco users
Cases and historical controls did not differ by age or smoking status (). Both cases and historical controls were overweight, with mean BMIs of 27.3 ± 8.0 kg/m2 and 27.8 ± 4.8 kg/m2, respectively. Systolic blood pressure, diastolic blood pressure, and total cholesterol were comparable between the 2 groups. There was a trend toward higher TG levels among cases (mean: 152.6 ± 124.5 mg/dL vs 120.7 ± 54.9 mg/dL); however, this trend was not statistically significant and was thought to be attributable to 1 outlier.
Vascular testing of cases, recruited controls, and historical controls
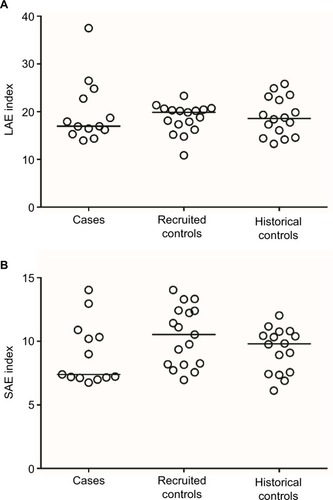
No statistically significant differences were found in LAE or SAE between cases and recruited controls or historical controls (). Median LAE for cases was 17.0 × 10 (range: 14.0–37.5) mL/mmHg and 19.9 × 10 (10.9–23.3) mL/mmHg for recruited controls (p = 0.679). Median SAE for cases was 7.4 × 100 (6.8–14.0) mL/mmHg and 10.5 × 100 (7.0–14.0) mL/mmHg for recruited controls (p = 0.086). Median LAE for historical controls was 18.6 × 10 mL/mmHg (13.3–25.8 mL/mmHg; p = 0.879, compared to cases). Median SAE for historical controls was 9.8 × 100 mL/mmHg (6.1–12.0 mL/mmHg; p = 0.729, compared to cases). When adjusted for age, BMI, and smoking status, the results continued to show no differences in SAE and LAE between cases and either the recruited controls or historical controls (all p > 0.6).
Figure 1 Distribution of LAE and SAE index values by disease status.
Abbreviations: LAE, large artery elasticity; SAE, small artery elasticity.
There was no difference in the median Endo-PAT reactive hyperemia peripheral arterial tonometry ratio between cases (ratio: 1.68) and controls (ratio: 1.54) (p = 0.574).
Given the small number of subjects, we were unable to evaluate the effect of time since treatment on vascular function testing.
Vascular testing
Risk factors for metabolic syndrome
A known risk factor for cardiovascular disease, metabolic syndrome is defined as ≥3 of the following characteristics: central or abdominal obesity, TGs ≥ 150 mg/dL, high-density lipoprotein ≤ 40 mg/dL, elevated BP > 130/85 mmHg, and fasting glucose ≥ 100 mg/dL. Although metabolic syndrome was more common in cases (15%) than in recruited controls (6%), this difference was not statistically significant (p = 0.565). Insufficient data on the characteristics of metabolic syndrome in our historical control group prevented direct comparisons with cases.
Biomarkers of premature cardiovascular disease
Biomarkers predictive of early vascular injury were assessed (). No statistically significant differences were seen between cases and recruited controls. Biomarker comparisons between cases and historical controls were not possible because data were not available for our historical controls.
Table 2 Primary measurements of vascular endothelium
Discussion
Although there may be a trend toward a reduction in SAE in testicular cancer survivors, there appears to be no clinically significant difference in SAE in survivors of testicular cancer treated with platinum-based chemotherapy as compared with controls 4 years after completing chemotherapy. Despite the risky behavior of tobacco use, we were unable to identify any difference in other cardiovascular risk factors (ie, metabolic syndrome and testosterone levels) between our cases and controls. In comparing our testicular cancer survivors to a group of tobacco-using controls, we also were unable to elucidate any difference in endothelial function.
Cisplatin can directly injure cardiac myocytes through oxidative stress and mitochondrial damage.Citation10 In prior investigations, studies have reported quantitative evidence of endothelial dysfunction and damage in testicular cancer survivors. Nuver et alCitation20 and van den Belt-Dusebout et alCitation7 measured serum markers of acute inflammation, including vWF, tissue-type plasminogen activator, plasminogen activator inhibitor-1, fibrinogen, and high-sensitivity C-reactive protein. These biomarkers were measured before and 10 weeks after chemotherapy. Acute elevations in these markers were identified. Feldman et alCitation21 also detected a reduction in SAE through endothelial reactivity at baseline and 34 weeks after chemotherapy (p = 0.07). Similarly, Dieckmann et alCitation10 detected acutely increased vWF after cisplatin-based chemotherapy. The vWF values normalized with time, suggesting that direct endothelial damage may occur but that it stabilizes over time after chemotherapy. The data of Dieckmann et alCitation10 support our hypothesis that the evidence of direct damage stabilizes over time. At 4 years after chemotherapy, as seen in our cohort, this damage seems to have normalized. Similarly, in a recent presentation using the Surveillance, Epidemiology, and End Results program, the risk of direct endothelial damage appears highest in the first year after chemotherapy.Citation13,Citation22 After chemotherapy, there is an increased cardiovascular mortality concentrated within Year 1 after testicular cancer diagnosis (standardized mortality ratio: 5.31, p < 0.05). This risk decreases with time since diagnosis.
Cisplatin-based chemotherapy may also indirectly lead to cardiovascular disease. Studies suggest that cisplatin-based chemotherapy promotes an increase in risk factors for cardiovascular disease, namely, metabolic syndrome.Citation2,Citation23,Citation24 Hyperlipidemia, central obesity, and insulin resistance have been previously described after cisplatin-based chemotherapy.Citation14 In testicular cancer survivors, metabolic syndrome occurs in 20%–30% of survivors and occurs at an earlier age than expected, generally 3–5 years after therapy.Citation24–Citation26 Metabolic syndrome is a known risk factor for cardiovascular death. In our study, survivors had a trend toward a higher incidence of metabolic syndrome than our control populations. However, in our study, there was no statistical difference in metabolic syndrome between the cases and controls, and no difference in testosterone or free testosterone levels between cases and controls was detected; it is possible that the control of both testosterone levels and risk factors of metabolic syndrome explains why no changes in endothelial function were observed in our study.
Features of metabolic syndrome have care targets that can be intervened upon, such as hyperlipidemia reduction, weight management, and glucose control. These risk factors are modifiable in cancer survivors as well. Other studies suggest that low testosterone levels were associated with higher rates of metabolic syndrome (OR: 4.4).Citation27 Additionally, tobacco use, well known to cause direct cardiovascular toxicity,Citation28–Citation30 was prevalent in our study in which 30% of our testicular cancer survivors smoked. Other studies have reported similar numbers in which tobacco use among young cancer survivors is highly prevalent,Citation31–Citation33 as well as being highly prevalent among testicular cancer survivors specifically.Citation33 Addressing these modifiable risk factors is vital in caring for these cancer survivors.Citation34
Our study is limited by its small sample size and low participation rate. If those survivors who did not respond to the study invitation were generally less healthy, this may have affected the study results, introducing some bias. This study is also limited by its cross-sectional nature in which longitudinal data are not available. Additionally, controlling for the discrepancy in race may be important, as other literature suggests that African Americans may have more arterial stiffness and changes in arterial elasticity at baseline;Citation35 the difference in race between our cohorts may account for the difficulty in interpreting these results. Given the suggestion of reduced SAE and the prevalence of risk factors for metabolic syndrome, longitudinal follow-up of this cohort is warranted. For future studies, however, we propose that interventions take into account race as well as address these modifiable risks factors at the time of completion of chemotherapy.
Conclusion
Previously published literature suggests that cisplatin-based chemotherapy causes direct endothelial toxicity. Our data do not support that endothelial damage exists at 4 years following completion of chemotherapy when there are no differences in testosterone levels or metabolic syndrome. Further follow-up, however, is needed. Long-term cardiovascular risk in testicular cancer survivors may be more related to indirect injury or secondary causes. Identifying these risk factors, such as metabolic syndrome, hypogonadism, and associated risky behaviors such as tobacco use, and intervening upon them needs to be done soon after the completion of chemotherapy. Collaboration with other health-care providers will likely be necessary in order to modify these long-term risk factors.
Acknowledgments
The results of this study were presented at the meeting of the American Society of Clinical Oncology in June 2014 as a poster presentation and published in abstract form in the Journal of Clinical Oncology. We greatly appreciate Dr Douglas Yee, for his time and effort in discussing the concept and providing insight on the overall project, as well as Dr Rachel Lerner and Dr Dylan Zylla, for their time and assistance in helping recruit subjects at the Park Nicollet Health Services. Finally, we thank Dr Jason Baker for sharing data on historical controls, as well as Natalia Florea for her contributions in performing vascular assessments. We also thank the following sources of funding for our project: AFLAC Young Investigator Award in Young Adult Oncology and Building Interdisciplinary Research Careers in Women’s Health (National Institutes of Health grant number K12-HD055887).
Disclosure
The authors report no conflicts of interest in this work.
References
- ShanmugalingamTSoultatiAChowdhurySRudmanSVan Hemel-rijckMGlobal incidence and outcome of testicular cancerClin Epidemiol2013541742724204171
- HaugnesHSWethalTAassNCardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year followup studyJ Clin Oncol201028304649465720855830
- VaughnDJPalmerSCCarverJRJacobsLAMohlerERCardiovascular risk in long-term survivors of testicular cancerCancer200811291949195318338810
- HashibeMAbdelazizSAl-TemimiMLong-term health effects among testicular cancer survivorsJ Cancer Surviv20161061051105727169992
- AlaneeSRFeldmanDRRussoPKonetyBLong-term mortality in patients with germ cell tumors: effect of primary cancer site on cause of deathUrol Oncol201432126.e9e15
- van den Belt-DuseboutAWde WitRGietemaJATreatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancerJ Clin Oncol200725284370437817906202
- van den Belt-DuseboutAWNuverJde WitRLong-term risk of cardiovascular disease in 5-year survivors of testicular cancerJ Clin Oncol200624346747516421423
- HuddartRANormanAShahidiMCardiovascular disease as a long-term complication of treatment for testicular cancerJ Clin Oncol20032181513152312697875
- TravisLBBeardCAllanJMTesticular cancer survivorship: research strategies and recommendationsJ Natl Cancer Inst2010102151114113020585105
- DieckmannKPGerlAWittJHartmannJTGerman Testicular Cancer Study GroupMyocardial infarction and other major vascular events during chemotherapy for testicular cancerAnn Oncol20102181607161120067918
- WillemsePMBurggraafJHamdyNAOsantoSReply: ‘Comment on Prevalence of the metabolic syndrome and cardiovascular disease risk in chemotherapy-treated testicular germ cell tumour survivors’Br J Cancer201310992503250424045664
- NuverJSmitAJvan der MeerJAcute chemotherapy-induced cardiovascular changes in patients with testicular cancerJ Clin Oncol200523369130913716301596
- FungCFossaSDMilanoMTSahasrabudheDMPetersonDRTravisLBCardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: a population-based studyJ Clin Oncol201533283105311526240226
- LubbertsSBoerHAltenaRVascular fingerprint and vascular damage markers associated with vascular events in testicular cancer patients during and after chemotherapyEur J Cancer20166318018827322917
- BakerJVDuprezDRapkinJUntreated HIV infection and large and small artery elasticityJ Acquir Immune Defic Syndr2009521253119731451
- BlaesABeckwithHFloreaNVascular function in breast cancer survivors on aromatase inhibitors: a pilot studyBreast Cancer Res Treat2017166254154728801846
- GreyEBratteliCGlasserSPReduced small artery but not large artery elasticity is an independent risk marker for cardiovascular eventsAm J Hypertens200316426526912670741
- DuprezDAHearstMOLutseyPLAssociations among lung function, arterial elasticity, and circulating endothelial and inflammation markers: the Multiethnic Study of AtherosclerosisHypertension2013611254254823283358
- RubinshteinRKuvinJTSofflerMAssessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse eventsEur Heart J20103191142114820181680
- NuverJSmitAJSleijferDTMicroalbuminuria, decreased fibrinoly-sis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancerEur J Cancer200440570170615010071
- FeldmanDRJacobsenEPWooKAcute changes in endothelial function with cisplatin among germ cell tumor patientsJ Clin Oncol2014325s Suppl; Abstr 9587
- FungCSessoHDWilliamsAMPlatinum Study GroupMulti-institutional assessment of adverse health outcomes among North American testicular cancer survivors after modern cisplatin-based chemotherapyJ Clin Oncol201735111211122228240972
- de HaasECZwartNMeijerCAssociation of PAI-1 gene polymorphism with survival and chemotherapy-related vascular toxicity in testicular cancerCancer2010116245628563620737565
- de HaasECAltenaRBoezenHMEarly development of the metabolic syndrome after chemotherapy for testicular cancerAnn Oncol201324374975523131388
- WillemsePPvan der MeerRWBurggraafJAbdominal visceral and subcutaneous fat increase, insulin resistance and hyperlipidemia in testicular cancer patients treated with cisplatin-based chemotherapyActa Oncol201453335136023957624
- RichieJPReply: ‘Early development of the metabolic syndrome after chemotherapy for testicular cancer’J Urol2014191498724703124
- BogeforsCIsakssonSBobjerJHypogonadism in testicular cancer patients is associated with risk factors of cardiovascular disease and the metabolic syndromeAndrology20175471171728544654
- AghaGLoucksEBTinkerLFHealthy lifestyle and decreasing risk of heart failure in women: the Women’s Health Initiative Observational StudyJ Am Coll Cardiol201464171777178525443698
- SchnohrPMarottJLKristensenTSRanking of psychosocial and traditional risk factors by importance for coronary heart disease: the Copenhagen City Heart StudyEur Heart J201536221385139325681607
- ChengSClaggettBCorreiaAWTemporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities StudyCirculation20141301082082825210095
- TarletonHPRyan-IbarraSInduniMChronic disease burden among cancer survivors in the California Behavioral Risk Factor Surveillance System, 2009–2010J Cancer Surviv20148344845924715532
- Karam-HageMCinciripiniPMGritzERTobacco use and cessation for cancer survivors: an overview for cliniciansCA Cancer J Clin201464427229024817674
- ReilleyMJJacobsLAVaughnDJPalmerSCHealth behaviors among testicular cancer survivorsJ Community Support Oncol201412412112824971421
- SmithWALiCNottageKALifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort StudyCancer2014120172742275025070001
- WasselCLJacobsDRJrDuprezDAAssociation of self-reported race/ethnicity and genetic ancestry with arterial elasticity: the Multi-Ethnic Study of Atherosclerosis (MESA)J Am Soc Hypertens20115646347221890448
