Abstract
Paraoxonase-1 (PON1) is a high-density lipoprotein-associated esterase and is speculated to play a role in several human diseases including diabetes mellitus and atherosclerosis. Low PON1 activity has been associated with increased risk of major cardiovascular events, therefore a variety of studies have been conducted to establish the cardioprotective properties and clinical relevance of PON1. The major aim of this review was to highlight the important studies and to subsequently assess if PON1 has clinical relevance. A review of the literature showed that there is currently insufficient data to suggest that PON1 has clinical relevance. It is our opinion that robust studies are required to clarify the clinical relevance of PON1.
Introduction
Human serum paraoxonase-1 (PON1) is a calcium-dependent hydrolytic enzyme that is found in a variety of mammalian species. Abraham MazurCitation1 and Norman AldridgeCitation2 played a pivotal role in the identification and classification of PON1 in the mid-1940s to early 1950s. Initially, the enzymes were referred to as “A”-esterases, but later became universally known as paraoxonases due to their ability to detoxify the organophosphate compound paraoxon which is the toxic metabolite of parathion, a commonly used agricultural insecticide.Citation3
PON1 belongs to a family of three serum paraoxonases, including PON2 and PON3; however, PON1 remains the most popular member of this family.Citation4 This is largely due to the elegant studies by Mackness et al that described the role of high-density lipoprotein (HDL)-associated PON1 in decreasing lipid peroxide accumulation on low-density lipoprotein (LDL).Citation5–Citation7 This highlighted a link to PON1 and cardiovascular disease, which sparked the research interest in PON1, mainly to elucidate the precise physiological mechanisms of the enzyme. In addition, PON1 hydrolyzes homocysteine thiolactone. Homocysteine thiolactonase activity of PON1 protects against N-homocysteinylation, which is detrimental to protein structure and function.Citation8
Structure of PON1
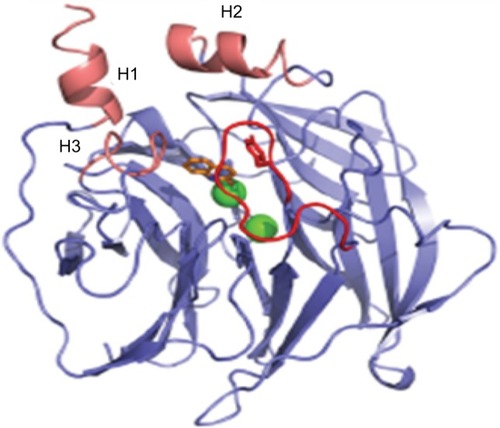
PON1 is a calcium-dependent enzyme consisting of 354 amino acids with a molecular mass of 43 kDa. Structural analysis using X-ray crystallography revealed the six-bladed β-propeller structure of PON1 (), with a central tunnel that houses two calcium ions.Citation9 Each calcium ion, depending on its location within the enzyme, plays an important part in the activity of PON1.Citation10 The calcium ion located deeper within the tunnel has a structural role that is critical for the conformational stability of PON1. The other calcium ion which lies at the bottom of the active site cavity has a catalytic role and is important for substrate positioning and ester bond activation. Three helices are located above the active site of PON1: H1, H2 and H3, where H1 and H2 have functions in PON1–HDL interactions.Citation9
Figure 1 Structure of recombinant PON1 illustrating the position of the Ca2+ ions (green) and the three helices (pink).
Abbreviation: PON1, Paraoxonase-1.
Genetics of PON1
The human PON1 gene is a member of a multigene family consisting of three members in total. PON1, PON2 and PON3 are located next to each other on chromosome 7 and share extensive structural homology. Interestingly, PON1 can be differentiated from PON2 and PON3 by the three extra nucleotide residues in exon 4.Citation11 The genes for this family are expressed in various mammalian tissues, with PON1 and PON3 primarily synthesized in the liver and mostly found associated with HDL in the plasma.Citation12,Citation13 PON1 forms part of a repertoire of HDL-associated enzymes, including lecithin-cholesterol acyltransferase and platelet-activating factor acetyl-hydrolase, responsible for the antioxidative activity of HDL.
PON1 polymorphisms
Human PON1 has many single-nucleotide polymorphisms (SNPs); eight have been identified on the promoter region and 176 within the gene sequence,Citation14 some of which exert changes in PON1 level and activity. These polymorphisms may also affect the risk for disease development and the severity of disease.Citation15 Studies have identified two common polymorphisms in the coding region (at positions 55 and 192) that have been reported to affect the activity and concentration of PON1.Citation16 The leucine/methionine polymorphism at position 55 of the amino acid sequence (L55M) has been associated with changes in PON1 serum concentrations, and an association with the occurrence of cardiovascular disease was also observed.Citation17 The glutamine/arginine polymorphism at position 192 (Q192R) has been shown to affect PON1 activity, where the Q192 isoform was demonstrated to hydrolyze paraoxon and metabolize oxidized LDL more effectively than the R192 isoform.Citation18 The Q192R polymorphism is regarded as the chief biomarker of oxidant status, where LDL oxidation is prevented the most in QQ homozygous patients and the least in RR patients.Citation19
In addition, three common SNPs (G-907C, A-162G and C-108T) were identified in the promoter sequence of the PON1 gene.Citation14 These polymorphisms are associated with considerable differences in PON1 concentration and activity, and they have also been implicated in the presence of coronary heart disease.Citation20 It has been well established that low PON1 activity is linked with an increased risk of cardiovascular disease, implicating PON1 as a physiologically important enzyme.
Clinical relevance
PON1 is an HDL-associated protein that has the ability to hydrolyze oxidized LDL-cholesterol, with potential atheroprotective effects.Citation21 Furthermore, PON1 can cleave phospholipid peroxidation adducts with potential cytoprotective functions.Citation22 Given these potential atheroprotective effects, and the large burden of atherosclerotic cardiovascular disease, considerable work has focused on elucidating the clinical relevance of PON1.
Animal studiesCitation23,Citation24 have suggested atheroprotective benefits of PON1. Transgenic mice overproducing human PON1 protected them from atherosclerosis, when compared to wild-type mice.Citation23 In addition, PON1-deficient mice are at greater risk of developing atherosclerosis than wild-type mice.Citation24 Animal studies have various limitations and direct extrapolation to humans cannot be made. However, they are useful for “transiting” from in vitro studies to clinical studies.
With respect to clinical studies, Mackness et alCitation20 investigated the effects of the C-108T and G-909C promoter polymorphisms on PON1 levels and the presence of coronary heart disease (CHD). It was a case–control study, with 417 people with CHD and 282 healthy controls. PON1 activity and concentration were significantly lower in the CHD population compared to controls, regardless of their C-108T and G-909C genotype (p<0.001). Both promoter polymorphisms were not associated with CHD presence and the authors concluded that PON1 status was significantly lower in people with CHD.
Azarsiz et alCitation25 investigated PON1 activity in patients with angiographically confirmed CHD. Twenty-four healthy volunteers and 101 patients were enrolled in the study; 68 patients had coronary artery disease, which was confirmed by coronary angiography. PON1 activities of patients with CHD were lower than those of controls; however, this difference was statistically insignificant. In contrast to Azarsiz et al’s study,Citation25 Sharma et alCitation26 demonstrated that serum PON1 activity was significantly low in CHD patients. Sharma et al investigated PON1 activity in 200 patients suffering from CHD and 150 normal individuals. CHD patients were classified into two groups on the basis of associated risk factors (diabetes mellitus, hypertension): group 1 (n=120; CHD patients with associated risk factors) and group 2 (n=80; CHD patients with no associated risk factors).Citation26
Smoking is an important independent risk factor for CHD, and a study by Han et alCitation27 showed that cigarette smoking status together with the presence of common PON1 SNPs play a role in the risk of developing CHD.Citation27 In this case–control study nested within the Singapore Chinese Health Study, which was a prospective cohort of 63,257 participants recruited over 5 years, Han et al evaluated 1,914 Singaporean Chinese: 688 cases and 1,226 controls. The participants were stratified according to cigarette smoking status as ever-smokers (n=813), who answered yes to smoking at least one cigarette a day for a year or longer, and never-smokers (n=1,101). The T allele of the PON1 rs662 polymorphism was shown to be associated with an increased risk of CHD among ever-smokers only (odds ratio [OR]=1.35, 95% CI 1.08–1.68; adjusted p=0.036), whereas another PON1 SNP, rs3735590, was shown to be associated with an increase in CHD among never-smokers only (OR=1.53, 95% CI 1.11–2.11; adjusted p=0.036),Citation27 highlighting that more research is required on PON1 polymorphisms and CHD.
Kunutsor et alCitation28 investigated the association of PON1 with cardiovascular risk. The study included prospectively measuring PON1 activity in 6,902 study subjects. The study subjects were followed up for a mean of 9.3 years, with 730 adverse cardiovascular events being recorded. There is an approximately log-linear inverse association between PON1 activity and CVD risk, which is partly dependent on HDL-cholesterol levels. Kunutsor et al did a further meta-analysis of six studies with 15,064 study subjects and 2,958 incident adverse cardiovascular events. Based on the findings of Kunutsor et al, although there is a negative relationship between PON1 and adverse cardiovascular events, PON1 does not add value to cardiovascular risk stratification, beyond conventional cardiovascular risk factors.Citation28
Given that atherosclerotic cardiovascular disease is a major cause of mortality, it was hypothesized that older individuals may have lower PON1 activity, when compared to younger individuals. Seres et alCitation29 investigated the relationship between age and serum PON1 activity. One hundred twenty-nine healthy subjects aged between 22 and 89 years were included in their study. Serum PON1 activity significantly decreased with age (r=−0.38, p<0.0001). HDL concentrations remained unchanged with age; however, Apo A1 concentration showed a slight negative, but significant correlation with age (r=−0.19, p<0.027). Moreover, the total cholesterol concentration was positively and significantly correlated with age (r=0.40, p<0.001). The authors also noted that HDL from elderly subjects was more susceptible to oxidation than HDL from young subjects, measured by higher lipid peroxidation rate. The study was limited by relatively small sample size, but did demonstrate reduction in PON1 activity with age.
Numerous further studies investigated whether PON1 is a longevity gene. Lescai et alCitation30 carried out a meta-analysis of these aforementioned studies that included 5,962 subjects: 2,795 young controls (<65 years of age) and 3,167 old subjects (>65 years of age). R carriers demonstrated a significant result with an overall OR of 1.16 (95% CI 1.04–1.30, p=0.006). The QR genotype also showed a significant result, with an overall OR of 1.14 (95% CI 1.02–1.27, p=0.016). The authors concluded that PON1 gene variants at codon 192 impact on the probability of attaining longevity, and those subjects carrying RR and QR genotypes (R+ carriers) are favored in reaching extreme ages. However, subsequent meta-analysis with larger number of patientsCitation31,Citation32 has suggested that there is no effect of PON1 on human longevity. However, population-specific effects could not be excluded. The aforementioned meta-analysis may be limited by publication bias and variations in analytical procedures used to measure PON1 activity.
Another meta-analysis based on 30 publications analyzed the risk of cancer in relation to the PON1 Q192R polymorphism.Citation33 Considering PON1 polymorphisms have been associated with several types of cancer, Zhang et al investigated the role of the genetic variant Q192R in cancer susceptibility. The authors conducted a thorough search of the literature and based on robust inclusion criteria, their meta-analysis examined 30 publications with a total of 8,112 cases and 10,037 controls. The results indicated that the PON1-192 R allele was associated with a reduced risk of overall cancers compared to the 192 Q allele (OR=0.842, 95% CI 0.725–0.979); however, when the results were analyzed according to the cancer types, an increased and decreased risk of cancer subtypes were observed under heterozygous, homozygous, dominant and recessive models.Citation33 It is well established that oxidative stress and increased free radicals may lead to an increased risk of cancer; therefore, the antioxidant properties of the genetic variants of PON1 should be studied in more detail to fully understand their role in cancer.
Diabetes mellitus is characterized by increased oxidative stress and damage, possibly due to the result of glycosylation of LDL by glucose.Citation34 Various studiesCitation35–Citation39 have demonstrated a reduction in PON1 in type 2 diabetic patients. Furthermore, reduced PON1 activity in type 2 diabetes mellitus has been associated with increased risk of cardiovascular disease.Citation40 Rozek et al postulated that reduced PON1 activity in diabetic patients results in reduced HDL protective activity against cell membrane peroxidation contributing to increased arteriosclerosis in diabetic patients.Citation41 Studies demonstrating reduction in PON1 levels in diabetic patients are contradicted by studies that show no changes in the levels of PON1 in diabetic subjects.Citation42–Citation44 However, although the aforementioned studies showed no absolute reductions in PON1 levels, they demonstrated qualitative reductions in PON1 activity.
Nie et al conducted a meta-analysis on the relationship of PON genes and Alzheimer’s disease.Citation45 Fifteen studies (involving five polymorphisms) were included in the meta-analysis. The authors concluded that the “SS genotype of PON2 S311C polymorphism had a significant association with Alzheimer’s disease in the studied population, and the A allele of PON1 rs705379 polymorphism was positively related to AD in the Caucasian population as well as the GG genotype decreased AD risk significantly in Caucasians.”Citation45 The meta-analysis is limited by the quality of studies included; further robust studies are required to elucidate the role of PON in Alzheimer’s disease.
Liu et al conducted a systematic review and meta-analysis of PON gene polymorphisms and ischemic stroke.Citation46 Twenty-eight studies were included in the meta-analysis. The R allele or RR genotype of PON1 Q192R polymorphism had an increased risk for ischemic stroke in the general population, but there was no significant association between other genetic variants of PON gene and ischemic stroke.Citation46 Again, the quality of the studies included in the meta-analysis and systematic review lack robustness, and thus, global inferences cannot be made from this study.
Organophosphates are chemicals commonly used in insecticides. They are also sometimes ingested by humans either accidently or intentionally to commit suicide. PON1 has shown activity against organophosphates, and individuals with higher levels of PON1 may be protected against the harmful effects of organophosphates.Citation47 However, PON1 levels are not employed routinely during the management of organophosphate poisoning, and probably will not be included in future management algorithms because they are unlikely to affect treatment.
illustrates the potential clinical role of PON, and lists the key teaching points.
Figure 2 The liver secretes HDL-associated PON into the systemic circulation. PON has antioxidant properties, stabilizes vulnerable atherosclerotic plaques and limits plaque rupture, with the potential limitation of atherosclerotic cardiovascular disease. PON has the potential to serve as a biomarker for cellular stress, vascular wall stress and insulin resistance.
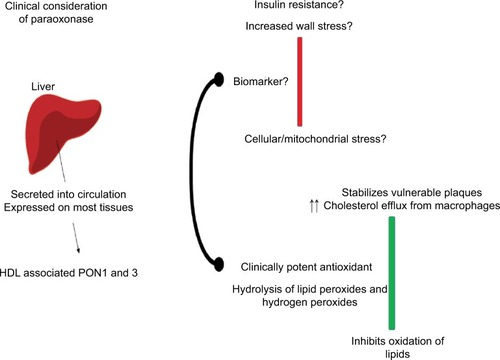
Table 1 Key teaching points
Pharmacological interactions of PON1
A detailed review of the relationship between PON1 and pharmacological agents is beyond the scope of this review. A comprehensive review by MahroozCitation48 describes the interactions of PON1 with cardiovascular drugs, antidiabetic drugs, antibiotics, anticancer drugs, antidepressants and contraceptives. There remains a large amount of incongruences in the study findings, and the clinical relevance of PON1 remains to be further investigated. Mahrooz attributes “dosage and type of drug, length of treatment, genetic variations, particularly loss-of-function polymorphisms, and the model used (cultured cells, animal studies, or human studies)” for the variability of study results.Citation48
As an example, we will describe the studies of PON1 and the antiplatelet drug, clopidogrel. Bouman et alCitation49 investigated the clinical relevance of the PON1 Q192R genotype in a population of individuals with coronary artery disease who underwent stent implantation and received clopidogrel therapy. PON1 QQ192 homozygous individuals showed a considerably higher risk than RR192 homozygous individuals of stent thrombosis, lower PON1 plasma activity, lower plasma concentrations of active metabolite and lower platelet inhibition.Citation49 The findings of Bouman et al were contradicted by a systematic review and meta-analysis.Citation50,Citation51 Given the aforementioned incongruent study findings, PON1 levels are currently not measured routinely during treatment with clopidogrel and are not included in mainstream clinical guidelines.
Conclusion
Although the preclinical and clinical studies around PON1 are intriguing, there is currently insufficient data to suggest that PON1 has clinical relevance. Furthermore, the study findings are incongruent and, in some instances, contradictory. Robust studies are required to clarify the clinical relevance of PON1.
Disclosure
PN is employed by Sanofi and NS is a previous employee of Sanofi. Sanofi has molecules in the lipid, antiplatelet, hypertension, diabetes, anticancer, anti-infective and antidepressant therapeutic areas. Neither PN nor NS own stocks in Sanofi. The other author reports no conflicts of interest in this work.
References
- MazurAAn enzyme in animal tissues capable of hydrolysing the phosphorus-fluorine bond of alkyl fluorophosphatesJ Biol Chem194616427128920989488
- AldridgeWNSerum esterases 2-An enzyme hydrolysing diethyl p-nitrophenylphosphate (E600) and its identity with the A-esterase of mammalian seraBiochem J19535311712413032042
- MacknessMMacknessBHuman Paraoxonase-1 (PON1): gene structure and expression, promiscuous activities and multiple physiological rolesGene20155671122125965560
- PrécourtLPAmreDDenisMCThe three-gene paraoxonase family: physiologic roles, actions and regulationAtherosclerosis20112141203620934178
- MacknessMIArrolSDurringtonPNParaoxonase prevents accumulation of lipoperoxides in low-density lipoproteinFEBS Lett19912861521541650712
- MacknessMIAbbottCAArrolSDurringtonPNThe role of high-density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidationBiochem J1993a2948298348379937
- MacknessMIArrolSAbbottCAProtection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonaseAtherosclerosis19931041291358141836
- Perła-KajánJJakubowskiHParaoxonase 1 and homocysteine metabolismAmino Acids20124341405141722643843
- Blaha-NelsonDKrügerDMSzelerKActive site hydrophobicity and the convergent evolution of paraoxonase activity in structurally divergent enzymes: the case of serum paraoxonase 1J Am Chem Soc201713931155116728026940
- VitariusJASultatosLGThe role of calcium in the hydrolysis of the organophosphate paraoxon by human serum A-esteraseLife Sci19955621251347823759
- Primo-ParmaSLSorensonRCTeiberJLa DuBNThe human serum araoxonase/arylesterase gene (PON1) is one member of a multigene familyGenomics1996334985098661009
- Rodríguez-SanabriaFRullABeltrán-DebónRTissue distribution and expression of paraoxonases and chemokines in mouse: the ubiquitous and joint localisation suggest a systemic and coordinated roleJ Mol Histol201041637938620931267
- AviramMRosenblatMParaoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis developmentFree Radic Biol Med20043791304131615454271
- NajafiMGohariLHFiroozraiMParaoxonase 1 gene promoter polymorphisms are associated with the extent of stenosis in coronary arteriesThromb Res2009123350351018433845
- Ponce-RuizNMurillo-GonzálezFERojas-GarcíaAETranscriptional regulation of human Paraoxonase 1 by nuclear receptorsChem Biol Interact2017268778428223025
- HumbertRAdlerDADistecheCMThe molecular basis of the human serum paraoxonase activity polymorphismNat Genet19933173768098250
- Blatter GarinMCJamesRWDussoixPParaoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetesJ Clin Invest199799162669011577
- CostaLGColeTBVitaloneAFurlongCEMeasurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicityClin Chim Acta20053521–2374715653099
- DounousiEBoubaISpotoBA genetic biomarker of oxidative stress, the Paraoxonase-1 Q192R gene variant, associates with cardiomyopathy in CKD: a longitudinal studyOxid Med Cell Longev20162016150727027313824
- MacknessBTurkieWMacknessMParaoxonase-1 (PON1) promoter region polymorphisms, serum PON1 status and coronary heartdiseaseArch Med Sci20139181323515649
- GetzGSReardonCAParaoxonase, a cardioprotective enzyme: continuing issuesCurr Opin Lipidol200415326126715166781
- ChistiakovDAMelnichenkoAAOrekhovANBobryshevYVParaoxonase and atherosclerosis-related cardiovascular diseasesBiochimie2017132192727771368
- TwardAXiaYRWangXPDecreased atherosclerotic lesion formation in human serum paraoxonase transgenic miceCirculation2002106448449012135950
- ShihDMGuLXiaYRMice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosisNature19983942842879685159
- AzarsizEKayikciogluMPayzinSYildirim SözmenEPON1 activities and oxidative markers of LDL in patients with angiographically proven coronary artery diseaseInt J Cardiol2003911435112957728
- SharmaRSinghBMahajanMPON1 activity is inversely related to LDL apoB carbonyl content in patients with coronary artery diseaseKaohsiung J Med Sci200723522523117525004
- HanYDorajooRKeTInteraction effects between Paraoxonase 1 variants and cigarette smoking on risk of coronary heart disease in a Singaporean Chinese populationAtherosclerosis20152401404525746376
- KunutsorSKBakkerSJJamesRWDullaartRPSerum paraoxonase-1 activity and risk of incident cardiovascular disease: the PREVEND study and meta-analysis of prospective population studiesAtherosclerosis201624514315426724525
- SeresIParaghGDescheneEFulopTJrKhalilAStudy of factors influencing the decreased HDL associated PON1 activity with agingExp Gerontol2004391596614724065
- LescaiFMarchegianiFFranceschiCPON1 is a longevity gene: results of a meta-analysisAgeing Res Rev20098427728419376276
- CaliebeAKleindorpRBlanchéHNo or only population-specific effect of PON1 on human longevity: a comprehensive meta-analysisAgeing Res Rev20109323824420362697
- WeiGZZhuMYWangFParaoxonase (PON1) polymorphisms Q192R and L55M are not associated with human longevity: a meta-analysisZ Gerontol Geriatr2016491243125962362
- ZhangMXiongHFangLParaoxonase 1 (PON1) Q192R gene polymorphism and cancer risk: a meta-analysis based on 30 publicationsAsian Pac J Cancer Prev201516104457446326028114
- BaynesJWRole of oxidative stress in development of complications in diabetesDiabetes1991404054122010041
- GuptaNBinukumarBKSinghSSerum paraoxonase-1 (PON1) activities (PONase/AREase) and polymorphisms in patients with type 2 diabetes mellitus in a North-West Indian populationGene2011487889521803130
- HedrickCCThorpeSRFuM-XGlycation impairs high density lipoprotein functionDiabetologia20004331232010768092
- SinghDVenketeshSVermaJSLellammaCOGoelRCParaoxonase (PON1) activity in north west Indian Punjabis with coronary artery disease & type 2 diabetes mellitusIndian J Med Res200712578378717704557
- El-SaidaNENasr-AllahaMMSadikaNASharafSAParaoxonase-1 activity in type 2 diabetes mellitus with and without nephropathyEgypt J Intern Med2015276368
- NamithaDNusrathARajeshwariARaniNASerum paraoxonase levels in type 2 diabetes mellitus patients: a case control studyJ Med Sci Health2015121418
- FlekacMSkrhaJZidkovaKLacinováZHilgertováJParaoxonase 1 gene polymorphism and enzyme activities in diabetes mellitusPhysiol Res20085771772617949258
- RozekLSHatsukamiTSRichterRJThe correlation of paraoxonase (PON1) activity with lipid and lipoprotein levels differs with vascular disease statusJ Lipid Res20054691888189515995178
- JarvikGPHatsukamiTSCarlsonCParaoxonase activity, but not haplotype utilizing the linkage disequilibrium structure, predicts vascular diseaseArterioscler Thromb Vasc Biol2003231465147112805074
- RectorRSWarnerSOLiuYExercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndromeAm J Physiol Endocrinol Metab2007293E500E50617473052
- AmunaRAMythiliSVShunmugamNStudy on Paraoxonase 1 in type 2 diabetes mellitusIndian J Physiol Pharmacol2014581131625464671
- NieYLuoDYangMA meta-analysis on the relationship of the PON genes and Alzheimer diseaseJ Geriatr Psychiatry Neurol201730630331028954597
- LiuHXiaPLiuMPON gene polymorphisms and ischaemic stroke: a systematic review and meta analysisInt J Stroke20138211112322631428
- RichardSAFrankEAD’SouzaCJCorrelation between cholinesterase and Paraoxonase 1 activities: case Series of pesticide poisoning subjectsBioimpacts20133311912224163803
- MahroozAPharmacological interactions of Paraoxonase 1 (PON1): a HDL-bound antiatherogenic enzymeCurr Clin Pharmacol201611425926427633038
- BoumanHJSchömigEvan WerkumJWParaoxonase-1 is a major determinant of clopidogrel efficacyNat Med201117111011621170047
- RenyJLCombescureCDaaliYFontanaPPON1 Meta-Analysis GroupPON1 Meta-Analysis Group. Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysisJ Thromb Haemost2012101242125122520065
- MegaJLCloseSLWiviottSDPON1 Q192R genetic variant and response to clopidogrel and prasugrel: pharmacokinetics, pharmacodynamics, and a meta-analysis of clinical outcomesJ Thromb Thrombolysis20164137438326573179
