Abstract
Objectives:
An impaired heart rate response during exercise (chronotropic incompetence) and an impaired heart rate recovery (HRR) after exercise are predictors of cardiovascular risk and mortality. Cystatin C is a novel marker for cardiovascular disease. We aimed to investigate exercise electrocardiographic responses in patients with metabolic syndrome who were without overt diabetes mellitus, in addition to the association of serum cystatin C levels with the exercise electrocardiographic test results.
Method:
Forty-three consecutive patients admitted to a cardiology outpatient clinic without angina pectoris were recruited if they met criteria for metabolic syndrome but did not have overt diabetes mellitus. Serum cystatin C levels were measured, and all participants underwent exercise electrocardiographic testing. Patients who were found to have ischemia had a coronary angiography procedure.
Results:
The mean cystatin C level of patients was higher in metabolic syndrome group than healthy controls (610.1 ± 334.02 vs 337.3 ± 111.01 μg/L; P < 0.001). The percentage of patients with ischemia confirmed by coronary angiography was 13.9% in the metabolic syndrome group. Cystatin C levels in the ischemic patients of the metabolic syndrome group were higher than that in nonischemic patients (957.00 ± 375.6 vs 553.8 ± 295.3 μg/L; P = 0.005). Chronotropic incompetence was observed in 30.2% of the patients with metabolic syndrome compared with 16.7% in the control group (P = 0.186). Chronotropic response indices were 0.8 ± 0.18 versus 0.9 ± 0.10 for the two groups, respectively (P = 0.259). HRR was significantly lower in the metabolic syndrome patients compared with the controls (20.1 ± 8.01 vs 25.2 ± 4.5 per min; P < 0.001), and the ST-segment adjustment relative to heart rate(ST/HR index ratio) was 1.4 ± 1.34 versus 0.4 ± 0.31 μV/beat (P < 0.001), respectively. Cystatin C was negatively correlated with the chronotropic response index (CRI) and HRR and was positively correlated with ST/HR index in the entire study population (R = −0.658, −0.346, 0.388, respectively; P < 0.05).
Conclusions:
A substantial proportion of metabolic syndrome patients without overt diabetes mellitus had silent coronary ischemia in addition to impairment of objective exercise electrocardiographic parameters. In the metabolic syndrome patients without overt diabetes mellitus, cystatin C levels were found to be elevated and the elevation was more pronounced in the subgroup with silent ischemia. Cystatin C was also correlated with HRR and CRI.
Introduction
Metabolic syndrome, which consists of abdominal obesity, low levels of high-density lipoprotein, high blood pressure, high blood glucose, and high triglyceride levels, has become an increasingly important risk factor for cardiovascular morbidity and mortality.Citation1 Recently, it was reported that exercise electrocardiographic responses are strong predictors of cardiovascular risk and mortality in the presence of metabolic syndrome components and this relationship increased with the number of present metabolic syndrome components.Citation2
Chronotropic incompetence, (CI) defined as ‘the inability to achieve 85% of the age-predicted maximum heart rate’, has been shown to be associated with cardiovascular risk and cardiac mortality.Citation3 It has also been reported that autonomic nervous system–related chronotropic incompetence during exercise is influenced by endothelial dysfunction as represented by flow-mediated dilation.Citation4 Heart rate recovery (HRR), another parameter evaluated during exercise testing, is a marker of impaired parasympathetic reactivity and has been studied in patients with metabolic syndrome or metabolic risk factors.Citation5,Citation6
Cystatin C, which is a novel indicator of renal glomerular filtration rate, has recently been shown to be associated with metabolic syndrome, diabetes, and prediabetes.Citation7–Citation9 Cystatin C levels have been found to be superior to creatinine or creatinine-based formulas for the prediction of cardiovascular events and all-cause mortality.Citation10 The association of cystatin C with insulin resistance, metabolic syndrome, and endothelial dysfunction,Citation11 in addition to a number of inflammatory biomarkers,Citation12,Citation13 is the likely link between cardiovascular disease and cystatin C.
We chose to study metabolic syndrome patients without known coronary artery disease or overt diabetes mellitus because diabetes is considered to be a coronary artery disease risk equivalent. We sought to investigate the impact of metabolic syndrome on exercise electrocardiographic stress test parameters, especially regarding chronotropic incompetence, chronotropic response index (CRI), and HRR. In addition, we examined the association of cystatin C in metabolic syndrome without overt diabetes and the prementioned exercise test parameters regarding the early detection of cardiovascular disease and risk prediction.
Patients and methods
Forty-three consecutive patients admitted to the outpatient clinic of the Cardiology Department of Gazi University Faculty of Medicine Hospital between September 2009 and February 2010 were recruited only if they did not have angina pectoris or any angina equivalent and if they met the criteria for metabolic syndrome but did not exhibit overt diabetes mellitus. Patients were enrolled if they had impaired glucose tolerance. Metabolic syndrome was defined according to the American Heart Association and the National Heart, Lung, and Blood Institute criteria.Citation14 Patients with three or more of the following risk factors were considered to have metabolic syndrome: 1) abdominal obesity (>102 cm in men and >88cm in women), 2) low high-density lipoprotein levels (<40 mg/dL for men and <50 mg/dL for women), 3) high triglyceride levels (>150 mg/dL), 4) high fasting plasma glucose levels (>100 mg/dL), and 5) high blood pressure (≥130/85 mm Hg). Baseline biochemical examinations were performed after 12 h of fasting. Blood pressures were recorded in the outpatient clinic after 10 min of rest. Waist circumference was measured at the level of the umbilicus using a standard anthropometric tape measure. Patients with known diabetes mellitus, previous coronary artery disease, cerebrovascular disease, moderate to severe valvular pathology, heart failure, hyperthyroidism, hypothyroidism, left bundle branch block, Wolff–Parkinson–White syndrome, any rhythm other than sinus rhythm on baseline electrocardiography (atrial fibrillation, atrial flutter, etc.), impaired renal function (serum creatinine >1.4 mg/dL), or impaired liver function (aspartate aminotransferase/alanine aminotransferase elevation >3 times the upper limit) were excluded.
The control group comprised healthy individuals admitted to the institutional blood transfusion center of the hospital as voluntary blood donors. The study was conducted according to the guidelines of the Declaration of Helsinki, and it was approved by the Institutional Ethics Committee. All of the participants provided written informed consent.
Blood samples for cystatin C measurements were centrifuged and kept at −80°C until they were measured using the Human Cystatin C ELISA assay (BioVendor, Modrice, Czech Republic) according to the manufacturer’s protocols. Both intra-assay and inter-assay coefficient of variations were <5%.
Exercise testing was performed according to the Bruce protocol with an AT-10 Exercise Testing System (Schiller, Baar, Switzerland). A 12-lead electrocardiography study was obtained throughout the procedure, and heart rate was monitored from the beginning of testing to the end of the recovery period. Blood pressure measurements were made at the beginning of testing, at every stage, and during the recovery period. The target heart rate for each participant was calculated before the procedure according to the following formula for age-predicted maximum heart rate: 220 − age. Exercise capacity was represented as the maximal metabolic equivalent (MET) achieved during exercise. The Duke score, which has been validated as an important predictor of mortality,Citation15 was calculated for each participant according to the following formula: Duke treadmill score (DTS) = the duration of exercise (in minutes) − (5 × maximal ST segment deviation) −(4 × angina score). (Angina score: 0 = no angina, 1 = nontest-limiting angina, 2 = exercise-limiting angina.) Chronotropic incompetence was defined as a ‘failure to achieve 85% of age predicted maximum heart rate’. The CRI, which is a measure of maximal heart rate in relation to chronotropic reserve, was calculated using the formula: (peak heart rate − resting heart rate)/(age − age predicted maximum heart rate − resting heart rate).Citation16 HRR was defined as a decrease in the heart rate from peak exercise rate to 1 min after cessation of exercise.Citation17 Exercise test results regarding ischemia were interpreted as either ischemic, equivocally ischemic, or normal. The criteria for classifying an exercise stress test as ‘ischemic’ have been provided elsewhere.Citation18 We categorized an exercise stress test result as ‘equivocal’ if ST segment depressions of 0.5–1 mm were observed to persist for more than 0.08 sec. Participants with ischemic or equivocal test results underwent coronary angiography by Judkins technique, which was performed using a Toshiba Infinix CSI device (Toshiba, Tokyo, Japan) using 6F catheters via femoral route. Coronary angiographic evaluation was performed by two blinded cardiologists offline; in the case of any disagreement, a third cardiologist was consulted. The presence of >70% coronary stenosis was defined as definite ischemia.
Statistical analysis
SPSS for Windows (version 15; SPSS, Chicago, IL) was used for statistical analyses. Continuous variables, such as cystatin C levels, CRI, HRR, and the ST/HR index, were expressed as means±standard deviation, whereas percentages and frequencies were used to express categorical variables, such as the presence of ischemia or metabolic syndrome. A χ2 test and Fischer’s exact test were used for the analysis of categorical variables. For continuous variables, parametric test conditions were first tested. The Shapiro–Wilk test was used to examine whether continuous variables were normally distributed. For the analysis of the difference between two groups, Student’s t-test and Mann–Whitney U test were employed according to the parametric test conditions. The degree of association between continuous variables was calculated by a Spearman’s ‘rho’ correlation coefficient. The area under curve (AUC) and 95% confidence intervals for cystatin C were evaluated by receiver operating characteristic (ROC) analysis. The best cut-off point for cystatin C was also calculated. A P value < 0.05 was considered to be statistically significant.
Results
Baseline characteristics of the study population are provided in . The mean age of metabolic syndrome patients (52.4 ± 10.5) was higher than the control group (38.0 ± 6.5), whereas the ratio of males to females were comparable (23.3% vs 36.7%, P=0.213). The mean cystatin C levels were higher in metabolic syndrome patients than in healthy controls (610.1 ± 334.02 vs 337.3 ± 111.01 μg/L; P < 0.001). When a ROC curve analysis was performed, the cut-off value for cystatin C was 374.5μg/L (). The AUC was 0.824 (95% CI 0.728–0.921, P < 0.001). According to this cut-off point, 81.4% of the patients with metabolic syndrome had ‘high’ cystatin C levels, whereas only 23.3% of the patients without metabolic syndrome had levels >374.5 μg/L. When cystatin C levels were grouped according to the number of metabolic syndrome components present, it was found that cystatin C levels increased with the number of components (). However, the difference in cystatin C levels of patients who met at least three criteria for metabolic syndrome was nonsignificant.
Figure 1 Receiver operating characteristic (ROC) curve analysis for cystatin C levels. The cut-off point for cystatin C was 374.5 μg/L.
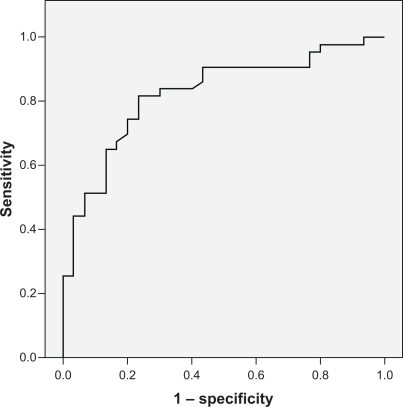
Table 1 Baseline characteristics of the study population
Table 2 Mean cystatin C levels with respect to the number of metabolic syndrome components present
Of the patients with metabolic syndrome, 55.8% completed the exercise test at stage III, and 63.4% of healthy controls achieved the same level. In contrast, 14% of metabolic syndrome patients and 33% of healthy controls reached stage IV. shows the exercise electrocardiographic results of the study and control groups. Chronotropic incompetence was observed in 30.2% of patients with metabolic syndrome compared with 16.7% in the control group (P = 0.186). Chronotropic response indices were 0.8 ± 0.18 versus 0.9 ± 0.10, respectively (P = 0.259). HRR was significantly lower in the metabolic syndrome patients compared with the controls (20.1 ± 8.01 vs 25.2 ± 4.5 per min, P < 0.001). The ST/HR index ratio was 1.4 ± 1.34 versus 0.4 ± 0.31 μV/beat, respectively (P < 0.001). shows the results from the exercise test regarding ischemia (ischemic, normal, or equivocal). The percentage of patients with an equivocal or ischemic exercise test was 30.2% in the metabolic syndrome population, but only 13.9% of metabolic syndrome patients had definite ischemia detected by coronary angiography. None of the participants in the control group had definite ischemia. shows the exercise test parameters of metabolic syndrome patients and the entire population with or without ischemia. We were unable to show significant differences in the mean CRI and the HRR time between ischemic and nonischemic patients within the metabolic syndrome group; however, these parameters were significantly different in the entire study population.
Table 3 Exercise electrocardiographic test results of participants with and without metabolic syndrome
Table 4 Exercise test results of the study population with and without metabolic syndrome
Table 5 Exercise electrocardiographic test results according to the presence or absence of ischemia in metabolic syndrome patients and the entire study population
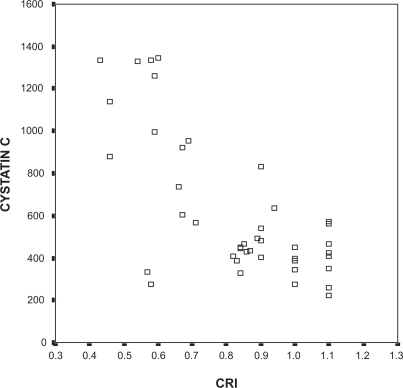
Cystatin C levels in the ischemic patients of the metabolic syndrome group were higher than levels in the nonischemic patients (957.00 ± 375.6 vs 553.8 ± 295.3 μg/L; P = 0.005). Cystatin C was negatively correlated with the CRI and HRR and was positively correlated with the ST/HR index in entire study population (R = −0.658, R = −0.346, and R = 0.388, respectively; P < 0.05). When patients with metabolic syndrome were separately evaluated, cystatin C levels were negatively correlated with the CRI (R = −0.737, P < 0.05) (), but the correlation with HRR and the ST/HR index did not reach statistical significance.
Discussion
The major finding of this study was the presence of silent ischemia in 13.9% of patients with metabolic syndrome but without overt diabetes mellitus. The mean cystatin C level was higher in metabolic syndrome patients than in healthy controls. Moreover, cystatin C levels in ischemic patients within the metabolic syndrome group were higher than in nonischemic patients. The 13.9% prevalence of silent ischemia in our study is lower than percentages reported for diabetic patients.Citation19,Citation20 To our knowledge, this study is the first to report the frequency of silent ischemia in metabolic syndrome patients without overt diabetes mellitus. Although our report highlights the presence of coronary ischemia in a substantial proportion of the target population, this issue should be a topic for future research in larger study populations to confirm these results.
When objective parameters for the interpretation of exercise stress testing were analyzed, we observed that HRR was significantly impaired when compared with healthy controls. However, the difference between the percentage of participants with and without chronotropic incompetence and the chronotropic response indices of participants with or without metabolic syndrome did not reach statistical significance. Cystatin C was negatively correlated with the CRI and HRR and was positively correlated with ST/HR index.
Cystatin C, a low molecular weight plasma protein that functions as an inhibitor of cysteine proteases, is produced by all nucleated cells, freely filtered by renal glomeruli and metabolized in the proximal tubules.Citation21 Cystatin C has been shown to be a strong independent risk factor for cardiovascular mortality and a powerful prognostic factor among patients with known coronary artery disease.Citation22,Citation23 High cystatin C concentrations also predict cardiovascular risks in patients without microalbuminuria and with normal glomerular filtration rates, in addition to patients with renal damage.Citation10 We have demonstrated an association between cystatin C and the presence of metabolic syndrome without overt diabetes mellitus. In addition, we report a positive correlation between this molecule and silent ischemia as well as some of the above-mentioned exercise electrocardiographic parameters. Recently cystatin C has been reported to be associated with endothelial dysfunction as represented by flow-mediated dilatation,Citation24 in addition to its correlation with insulin resistance and inflammatory biomarkers.Citation12,Citation13,Citation25 Although we cannot definitely suggest a causal relationship between cystatin C and silent ischemia or any of the exercise electrocardiographic findings due to the cross-sectional design of the study, endothelial dysfunction probably constitutes an important role in this process due to the fact that endothelium is the chief regulator of vascular homeostasisCitation26 and plays a role in all stages of atherosclerosis.Citation27 In a recent study, 899 patients with known coronary artery disease and stable angina pectoris had underwent an exercise treadmill test with stress echocardiography, and elevated levels of cystatin C were associated with inducible ischemia.Citation28
HRR after maximal exercise testing is a predictor of mortality and cardiovascular disease independent of the angiographic severity of coronary artery disease.Citation29,Citation30 Studies investigating HRR have expanded to include diabetic patients, patients with insulin resistance, and patients with metabolic risk factors.Citation31–Citation34 It has been suggested that short-term HRR (1-min HRR) reflects cardiac parasympathetic outflow, whereas a subsequent slow heart rate decay is associated with the withdrawal of sympathetic activity.Citation35 There have been studies suggesting that autonomic dysfunction is associated with hyperinsulinemia, insulin resistance, and obesityCitation36,Citation37 and that the central nervous system contributes to the development of metabolic syndrome. However, the CARDIA study has revealed that a slower HRR does not precede the development of the metabolic syndrome, but, rather appears after components of the syndrome are present.Citation5 In a recent study by Giallauria et al, in a population consisting of young women with polycystic ovary syndrome, abnormal HRR was associated with elevated white blood cell counts and C-reactive protein, a well-known inflammatory marker.Citation38 Another report demonstrated that in patients with established coronary artery disease, Cystatin C concentrations were associated with decreased exercise capacity and lower HRR;Citation39 however, the participants in that study were largely elderly men. Our results support previous studies that suggest an impairment of HRR in the presence of metabolic disturbances or inflammation, but this relationship has now been demonstrated for the first time in an asymptomatic patient group with metabolic syndrome, without overt diabetes.
Chronotropic incompetence is defined by an attenuated heart rate response to exercise that has been shown to predict cardiovascular risk and mortality in healthy populations; even after adjusting for age, standard risk factors, and ST segment changes during exercise.Citation40 Chronotropic incompetence has been believed to reflect a modulation of autonomic tone. It has been suggested that frequent activation of sympathetic nerves alters β-adrenergic sensitivity and causes postsynaptic β desensitization, which subsequently leads to chronotropic incompetence during exercise.Citation41,Citation42 Another proposed mechanism is compensatory vagal hyperactivity during exercise.Citation43 Huang et al reported that among patients who had undergone a treadmill exercise test for typical and atypical angina pectoris, the subgroup with chronotropic incompetence had endothelial dysfunction and enhanced systemic inflammation.Citation4 We observed that cystatin C was correlated with chronotropic incompetence and the CRI, although the cross-sectional design of this study prevents a causal interpretation of this relationship.
In conclusion, in metabolic syndrome patients without overt diabetes mellitus, we have demonstrated the presence of silent ischemia in a substantial percentage of the study population as well as impairment in most of the exercise electrocardiographic parameters and an association between them and elevated serum cystatin C levels. Cystatin C, an emerging biomarker for cardiovascular morbidity and mortality, may be used to identify higher risk subgroups within asymptomatic metabolic syndrome patients. These patients require aggressive risk factor modification and antiatherosclerotic treatment. Our findings are, therefore, significant, especially for clinicians and practitioners who often treat these patients in their clinical practice.
Acknowledgements
The ELISA assay used for the detection of serum Cystatin C levels was supplied by the IMSED (Internal Medicine Post Graduation Education Society)/Ankara/TURKEY.
Disclosure
The authors report no conflicts of interest in this work.
References
- ServaisAGiralPBernardMBruckertEDerayGIsnard BagnisCIs serum cystatin-C a reliable marker for metabolic syndrome?Am J Med2008121542643218456039
- LyerlyGWSuiXChurchTSLavieCJHandGABlairSNMaximal exercise electrocardiographic responses and coronary heart disease mortality among men with metabolic syndromeMayo Clin Proc201085323924620160139
- FukumaNOikawaKAisuNImpaired baroreflex as a cause of chronotropic incompetence during exercise via autonomic mechanism in patients with heart diseaseInt J Cardiol200497350350815561340
- HuangPHLeuHBChenJWComparison of endothelial vasodilator function, inflammatory markers, and N-terminal pro-brain natriuretic peptide in patients with or without chronotropic incompetence to exercise testHeart200692560961416159987
- KizilbashMACarnethonMRChanCJacobsDRSidneySLiuKThe temporal relationship between heart rate recovery immediately after exercise and the metabolic syndrome: the CARDIA studyEur Heart J200627131592159616728422
- LinLYKuoHKLaiLPLinJLTsengCDHwangJJInverse correlation between heart rate recovery and metabolic risks in healthy children and adolescents: insight from the National Health and Nutrition Examination Survey 1999–2002Diabetes Care20083151015102018268066
- RetnakaranRConnellyPWHarrisSBZinmanBHanleyAJCystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youthPediatr Nephrol20072271007101317394021
- DonahueRPStrangesSRejmanKRafalsonLBDmochowskiJTrevisanMElevated cystatin C concentration and progression to pre-diabetes: the Western New York StudyDiabetes Care20073071724172917456840
- LeeSHParkSAKoSHInsulin resistance and inflammation may have an additional role in the link between cystatin C and cardiovascular disease in type 2 diabetes mellitus patientsMetabolism201059224124619765773
- IxJHShlipakMGChertowGMWhooleyMAAssociation of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul studyCirculation2007115217317917190862
- SurendarJIndulekhaKAravindhanVGanesanAMohanVAssociation of cystatin-C with metabolic syndrome in normal glucose-tolerant subjects (CURES-97)Diabetes Technol Ther2010121190791220879967
- ShlipakMGKatzRCushmanMCystatin-C and inflammatory markers in the ambulatory elderlyAm J Med200511812141616378798
- KellerCKatzRCushmanMFriedLFShlipakMAssociation of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA)BMC Nephrol20089918681974
- GrundySMCleemanJIDanielsSRAmerican Heart AssociationNational Heart, Lung and Blood InstituteDiagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summaryCardiol Rev200513632232716708441
- MarkDBShawLHarrellFEJrPrognostic value of a treadmill exercise score in outpatients with suspected coronary artery diseaseN Engl J Med1991325128498531875969
- OkinPMLauerMSKligfieldPChronotropic response to exercise. Improved performance of ST-segment depression criteria after adjustment for heart rate reserveCirculation19969412322632318989133
- ColeCRBlackstoneEHPashkowFJSnaderCELauerMSHeart-rate recovery immediately after exercise as a predictor of mortalityN Engl J Med1999341181351135710536127
- GibbonsRJBaladyGJBeasleyJWACC/AHA guidelines for exercise testing: executive summary A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing)Circulation19979613453549236456
- PuelJValensiPVanzettoGALFEDIAM; SFC. Identification of myocardial ischemia in the diabetic patient. Joint ALFEDIAM and SFC recommendationsDiabetes Metab2004303 Pt 33S33S1815289742
- WackersFJYoungLHInzucchiSEDetection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD studyDiabetes Care20042781954196115277423
- BorgesRLHirotaAHQuintoBMRRibeiroABZanellaMTBatistaMCIs cystatin C a useful marker in the detection of diabetic kidney disease?Nephron Clin Pract20101142c127c13419887833
- ShlipakMGSarnakMJKatzRCystatin C and the risk of death and cardiovascular events among elderly personsN Engl J Med2005352202049206015901858
- KoenigWTwardellaDBrennerHRothenbacherDPlasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rateClin Chem200551232132715563478
- ReffelmannTKrebsAIttermannTMild renal dysfunction as a non-traditional cardiovascular risk factor?-Association of cystatin C-based glomerular filtration rate with flow-mediated vasodilationAtherosclerosis2010211266066620399428
- CurhanGCystatin C: a marker for renal function or something more?Clin Chem200551229329415681558
- VitaJAKeaneyJFJrEndothelial function: a barometer for cardiovascular risk?Circulation2002106664064212163419
- Alsheikh-AliAAKitsiosGDBalkEMLauJIpSThe vulnerable atherosclerotic plaque: scope of the literatureAnn Intern Med2010153638739520713770
- DeoDShlipakMGIxJHAliSSchillerNBWhooleyMAAssociation of cystatin C with ischemia in patients with coronary heart diseaseClin Cardiol20093211E18E2219816865
- VivekananthanDPBlackstoneEHPothierCELauerMSHeart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary diseaseJ Am Coll Cardiol200342583183812957428
- Morshedi-MeibodiALarsonMGLevyDO’DonnellCJVasanRSHeart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study)Am J Cardiol200290884885212372572
- ChengYJLauerMSEarnestCPHeart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetesDiabetes Care20032672052205712832312
- NilssonGHedbergPJonasonTLönnbergIOhrvikJHeart rate recovery is more strongly associated with the metabolic syndrome, waist circumference, and insulin sensitivity in women than in men among the elderly in the general populationAm Heart J20071543460. e1460.e717719290
- LauerMSExercise testing for assessment of autonomic functionAm Heart J2002144458058212360151
- PanzerCLauerMSBriekeABlackstoneEHoogwerfBAssociation of fasting plasma glucose with heart rate recovery in healthy adults: a population-based studyDiabetes200251380380711872683
- BuchheitMPapelierYLaursenPBAhmaidiSNoninvasive assessment of cardiac parasympathetic function: postexercise heart rate recovery or heart rate variability?Am J Physiol Heart Circ Physiol20072931H8H1017384128
- PetersonHRRothschildMWeinbergCRFellRDMcLeishKRPfeiferMABody fat and the activity of the autonomic nervous systemN Engl J Med198831817107710833352710
- GottsäterAAhmedMFernlundPSundkvistGAutonomic neuropathy in Type 2 diabetic patients is associated with hyperinsulinaemia and hypertriglyceridaemiaDiabet Med1999161495410229293
- GiallauriaFOrioFLombardiGRelationship between heart rate recovery and inflammatory markers in patients with polycystic ovary syndrome: a cross-sectional studyJ Ovarian Res20092319187547
- McManusDShlipakMIxJHAliSWhooleyMAAssociation of cystatin C with poor exercise study and heart rate recovery: data from the Heart and Soul studyAm J Kidney Dis200749336537217336697
- LauerMSOkinPMLarsonMGEvansJCLevyDImpaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart StudyCirculation1996938152015268608620
- LauerMSPashkowFJLarsonMGLevyDAssociation of cigarette smoking with chronotropic incompetence and prognosis in the Framingham Heart StudyCirculation19979638979039264498
- SrivastavaRBlackstoneEHLauerMSAssociation of smoking with abnormal exercise heart rate responses and long-term prognosis in a healthy, population-based cohortAm J Med20001091202610936474
- EllestadMHChronotropic incompetence. The implications of heart rate response to exercise (compensatory parasympathetic hyperactivity?)Circulation1996938148514878608613