Abstract
Combination therapy is an effective strategy to increase antihypertensive efficacy in those patients with poor blood pressure (BP) control. In order to achieve BP targets, at least 75% of patients may require combination therapy, and European guidelines advocate this approach, particularly in those patients with a high cardiovascular risk. Evidence from large, randomized controlled trials, and the European hypertension treatment guidelines is supportive of the use of an angiotensin receptor blocker (ARB) with a calcium channel blocker (CCB). Fixed-dose combination formulations of olmesartan medoxomil, an ARB, and the CCB amlodipine are approved in several European countries for patients with essential hypertension. The olmesartan/amlodipine combination has demonstrated greater efficacy than its component monotherapies in reducing BP in patients with mild-to-severe hypertension. Significantly greater reductions in seated diastolic BP were observed between baseline and after eight weeks of treatment with olmesartan/amlodipine, compared with equivalent doses of olmesartan or amolodipine monotherapy (P < 0.001), in the factorial Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure (COACH) trial. About 85% of the maximal BP reductions after the 8-week treatment period were already observed after two weeks. Uptitration as necessary, with or without hydrochlorothiazide, allowed the majority of patients to achieve BP control in a 44-week open-label extension treatment period to the COACH trial. The use of olmesartan/amlodipine allowed up to 54% of patients, with previously inadequate responses to amlodipine or olmesartan monotherapy, to achieve their BP goals. Data from post-registration studies using tight BP control and forced titration regimens have further demonstrated the high efficacy of olmesartan/amlodipine in achieving BP goal rates. Moreover, consistent reductions in BP were observed over the 24-hour dosing interval using ambulatory measurements. Olmesartan/amlodipine was generally well tolerated over the short- and long-term, with a lower frequency of peripheral edema with olmesartan/amlodipine 40/10 mg than with amlodipine 10 mg monotherapy.
Introduction
Arterial hypertension is the single largest contributor to global mortality,Citation1 and is responsible for approximately 7.1 million deaths each year.Citation2 In 2000, it was estimated that nearly 1 billion people worldwide had hypertension, and it was predicted that the prevalence would increase to over 1.5 billion by 2025.Citation3 The prevalence of hypertension among people aged 35–64 years is about 30% in the US population,Citation4 and about 44% in European countries.Citation5 Hypertension continues to be underdiagnosed and undertreated.Citation6
Raised blood pressure (BP) is a major risk factor for stroke, heart disease and renal failure.Citation7–Citation9 Many clinical trials have shown that BP reduction by a variety of strategies reduces the risk of stroke by approximately 35%, congestive heart failure by 42%, and coronary heart disease by 28%.Citation10–Citation14 Current European guidelines recommend a target systolic BP (SBP) and diastolic BP (DBP) of <140/90 mmHg in the general population.Citation9 However, despite these recommendations and the well-documented relationship between hypertension and the increased cardiovascular (CV) and renal risk, BP control rates remain poor, particularly in Europe.Citation15,Citation16 Therefore, the primary aim of an effective antihypertensive treatment strategy is to lower elevated BP to target levels and to achieve a maximum reduction in risk. The recent reappraisal of the European guidelines on hypertension management recommends that it may be prudent to lower BP to values within the range of 130–139/80–85 mmHg in the majority of hypertensive patients, including those with diabetes.Citation9 In these guidelines, both angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs) are recommended for first-line therapy either as monotherapy or in combination. This article reviews the rationale for fixed-dose combination therapy with the ARB olmesartan medoxomil and the CCB amlodipine.
Fixed-dose combination therapy versus monotherapy
Among the many factors that may contribute to suboptimal BP control rates are nonadherence of patients to therapy and clinical inertia, where physicians fail to increase the dosage of existing antihypertensive medication or prescribe combinations of antihypertensive drugs when patients do not achieve their BP goal.Citation17–Citation19 Increasing the dose of a single antihypertensive agent in an attempt to achieve an adequate response may lead, however, to an increase in side-effects, which can lead to noncompliance and exacerbation of the BP control problem. Hypertension is a complex multifactorial condition comprising multiple pathways involved in BP control.Citation20 The rationale behind combination therapy, using two or more drugs with different and complementary mechanisms of action, is the potential to improve BP control by the combined effects and, by allowing lower doses of the drugs, to reduce unwanted side-effects.Citation21 A recent meta-analysis of 10,968 patients from 42 trials has shown that the average antihypertensive effect of combining two drugs from different classes (thiazides, beta-blockers, angiotensin-converting enzyme inhibitors [ACEIs] and CCBs) is approximately additive.Citation22 The authors estimated that the additional reduction in BP produced by combining two drugs from different classes was approximately five times greater than that achieved by doubling the dosage of either drug. Therefore, combination therapy is a simple and effective strategy to increase antihypertensive efficacy and, therefore, control BP in hypertensive patients.
In the past, monotherapy has been the standard initial treatment approach in most patients with hypertension, with combination therapy being initiated when stepwise increases in the dose of the single agent fail to achieve the required BP reduction. More recently, a number of clinical trials have clearly demonstrated that most patients receiving antihypertensive combination therapy are indeed able to achieve adequate BP control.Citation23–Citation26 The available data suggest that, overall, at least 75% of patients with hypertension will require combination therapy to achieve BP targets.Citation27 Accordingly, recent European treatment guidelines recommend the use of combination therapy as an alternative to monotherapy as initial treatment, particularly in patients at high CV risk.Citation9
Single-pill combinations of two antihypertensive drugs, known as fixed-dose combinations, are now widely available, often combining an ACEI or an ARB as agents that target the renin–angiotensin system (RAS) with either a thiazide diuretic or a CCB.Citation28,Citation29 At low doses, fixed-dose combinations may have greater efficacy and better tolerability than the respective high-dose monotherapies.Citation29 Fixed-dose combinations can simplify the treatment schedule and improve compliance and persistence with therapy compared with two antihypertensive drugs given separately.Citation30 It is reasonable to expect that this may result in improvements in BP control and reduction in the incidence of CV events. Importantly, combination antihypertensive therapy comprising either an ACEI or ARB is favorable since, unlike drugs from other classes, these agents can be used at higher doses to increase efficacy without compromising tolerability.Citation31 Consequently, this poses the question of “what should be combined with a RAS blocker?”
Studies have shown that combination therapy with an ACEI (benazepril) and a CCB (amlodipine) provides superior BP-lowering efficacy compared with either agent as monotherapy.Citation32,Citation33 Subsequently, the ACCOMPLISH (Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension) trial was one of the first major studies to investigate the effects of fixed dose combination therapy and demonstrated the benefits of combination treatment comprising a RAS blocker/CCB and RAS blocker/thiazide diuretic by the achievement of very high levels of BP control. In this large, randomized, double-blind clinical trial, the effects of benazepril plus amlodipine were compared with those of benazepril plus the thiazide diuretic hydrochlorothiazide (HCTZ) in reducing CV morbidity and mortality in approximately 11,500 patients at high risk of CV events.Citation24 The study drugs were taken as a single-capsule formulation. Drug doses were force-titrated to attain recommended BP goals. BP control (<140/90 mmHg) was achieved by 75.4% of patients receiving benazepril/amlodipine and 72.4% of patients receiving benazepril/HCTZ. Notably, the primary composite endpoint, including death from CV causes and CV events, were significantly (P < 0.001) reduced by approximately 20% in the benazepril/amlodipine arm compared with the benazepril/HCTZ arm. However, these results should not be extrapolated to the general hypertensive population in regard to assuming that a RAS blocker/CCB combination is per se superior to a RAS blocker/thiazide diuretic combination since the patient population in ACCOMPLISH was not typical of the general hypertensive population: there was a high level of obesity and approximately 60% of patients were diabetic. Nonetheless, the combination of a RAS blocker plus a CCB was undoubtedly an effective combination in these patients, and supports the use of combination therapy comprising a RAS blocker and CCB to control BP and reduce CV risk in patients with hypertension, especially those with features of the metabolic syndrome such as obesity and diabetes.
Another randomized trial, ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial), demonstrated that the ARB telmisartan was equally as effective as the ACEI ramipril in reducing the incidence of CV events in high-risk patients.Citation34 Importantly, there was a lower incidence of cough and angio-edema in patients who received telmisartan compared with those who received ramipril. This result is consistent with a large-scale observational study of more than 195,000 patients in the US Veterans Affairs Health Care System who initiated ACE therapy. The study found an increase in the incidence of angioedema associated with the use of ACEIs (1.97 cases/1000 person years) compared with other antihypertensive medications (0.51 cases/1000 person years), and that the risk of angioedema remained elevated with longer-term use, even beyond one year.Citation35 Taken together these findings support the rationale for combining an ARB and a CCB as an antihypertensive strategy. This notion is reflected by the recent European hypertension treatment guidelines in which combination therapy with an ARB or ACEI plus a CCB is indeed a recommended strategy.Citation9,Citation36
Olmesartan/amlodipine combination therapy
Since ARBs inhibit the activity of the RAS by blocking the angiotensin II type 1 (AT1) receptor, the efficacy of the ARBs depend upon their ability to inhibit AT1 receptor activation by angiotensin II. Pharmacodynamic studies have shown that ARBs, when given in their recommended doses, differ in their ability to block the AT1 receptor. These differences in AT1 receptor blockade may translate into differences between ARBs in their ability to control BP over 24 hours. This is in line with an independent meta-analysis of studies which used ambulatory BP monitoring (ABPM) to measure 24-hour BP control with ARBs. This meta-analysis found that the size of reduction in ambulatory SBP depended upon the drug used, and that the dose used affected the duration of the antihypertensive activity for both systolic and diastolic BP.Citation37
In this regard, the ARB olmesartan medoxomil (hereafter referred to as olmesartan) is of interest since it has been shown in pharmacodynamic studies to produce a strong level of AT1 receptor blockade in relation to dose.Citation38–Citation40 Furthermore, direct comparison with several other ARBs has shown that olmesartan produces robust antihypertensive efficacy over 24 hours, the daytime, night-time, and end-of-dosing interval periods relative to losartan, candesartan or valsartan monotherapy, and was at least as efficacious as irbesartan.Citation41–Citation43
Clinical data suggest that olmesartan may protect against end-organ damage and, in this regard, renoprotective and anti-atherosclerotic effects have been reported in clinical and experimental studies. As with other members of this drug class, olmesartan has shown excellent, placebo-like tolerability in clinical studies.Citation44 Taken together, the efficacy and excellent tolerability of olmesartan make it highly suitable for use in combination therapy.
Fixed-dose combination formulations of olmesartan and amlodipine (olmesartan/amlodipine 20/5 mg, 40/5 mg or 40/10 mg) are approved in several European countries for once-daily administration in patients with essential hypertension who have responded inadequately to either drug as monotherapy, or who are receiving separate tablets as combination therapy. Like olmesartan, amlodipine provides effective BP control and exhibits organ-protective properties.Citation45
Therapeutic efficacy of olmesartan/amlodipine
The efficacy of olmesartan/amlodipine has been evaluated in three key randomized, double-blind trials. The factorial Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure (COACH) trial evaluated the efficacy of dual combination therapy with olmesartan/amlodipine compared with its component mono-therapies in patients with mild-to-severe hypertension.Citation46–Citation48 Two add-on trials evaluated the efficacy of olmesartan plus amlodipine in patients with moderate-to-severe hypertension who responded inadequately to amlodipineCitation49 or olmesartanCitation50 monotherapy.
Two other studies have evaluated the efficacy of olmesartan/amlodipine-based titration regimens in patients with hypertension. The BP-CRUSH (Blood Pressure Control in All Subgroups with Hypertension) trial was a study that evaluated rates of BP goal achievement in patients who responded inadequately to antihypertensive monotherapy and were switched to olmesartan/amlodipine-based therapy.Citation51 The AZTEC (AZOR Trial Evaluating Blood Pressure Reduction and Control) study used ABPM to determine the efficacy of a fixed-dose combination of olmesartan/amlodipine over the 24-hour dosing interval in patients with hypertension who did not respond adequately to amlodipine monotherapy.Citation52
Only treatment regimens involving olmesartan/amlodipine dosages approved for use in Europe are reviewed here with regard to the results obtained in the overall population in each study, respectively.
COACH trial
The COACH trial was a multicenter, randomized, double-blind, placebo-controlled study with a factorial design.Citation46 Eligible patients were aged ≥18 years, were naïve to antihypertensive therapy or underwent a 2-week washout period, and had a seated DBP (SeDBP) of 95–120 mmHg. Patients (n = 1940) were randomized to eight weeks of olmesartan monotherapy (10, 20 or 40 mg/day), amlodipine mono-therapy (5 or 10 mg/day), each possible combination of the corresponding olmesartan and amlodipine doses, or placebo. The primary endpoint was the change from baseline in mean trough SeDBP (measured before taking the daily dose of study medication) after eight weeks of treatment in the intent-to-treat (ITT) population (patients with a BP measurement at baseline and at least one BP measurement after taking at least one dose of study medication) with last-observation-carried-forward (LOCF) imputation. Secondary endpoints included change from baseline in seated SBP (SeSBP), and the proportion of patients achieving the BP target (<140/90 mmHg for patients with uncomplicated hypertension; <130/80 mmHg for patients with diabetes). BP was recorded at weeks 2, 4, 6 and 8 respectively.
A total of 1923 patients were included in the primary efficacy analysis, of which 1689 completed the 8-week treatment period. All combination and monotherapy dosages and placebo were associated with statistically significant reductions in SeDBP from baseline to week 8 (P < 0.001) (). The reductions in SeDBP at week 8 seen with each monotherapy increased as the dosage of monotherapy rose. The combinations of olmesartan/amlodipine also produced dose-dependent reductions in SeDBP at week 8, and these were significantly greater than those achieved with the equivalent doses of olmesartan or amlodipine monotherapy (P < 0.001). Changes in SeSBP from baseline to week 8 followed a similar pattern to the changes in SeDBP (). The largest reductions in SeDBP and SeSBP were achieved after two weeks of active treatment (). Thus, about 85% of the maximum BP reductions observed at the end of the 8-week treatment period had been observed after two weeks of treatment ().Citation47 The benefits of combination therapy were observed irrespective of baseline hypertension stage.Citation48 Furthermore, prior use of antihypertensive agents did not appear to affect efficacy.
Figure 1 Mean change in seated systolic blood pressure (SeSBP) from baseline to weeks 2, 4, 6 and 8 with olmesartan (OLM) and amlodipine (AML) monotherapy and olmesartan/amlodipine combination therapy.
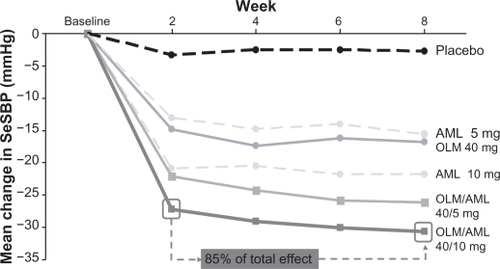
Table 1 The COACH trial – change in seated diastolic blood pressure (SeDBP) and seated systolic blood pressure (SeSBP) from baseline to week 8 in the intent-to-treat population (last observation carried forward)Citation46
Significantly greater proportions of patients receiving olmesartan/amlodipine achieved the BP target at week 8 than patients receiving monotherapy (). The proportions of patients reaching the BP goal were 42.5%, 51.0% and 49.1% for olmesartan/amlodipine 20/5 mg, 40/5 mg and 40/10 mg, respectively.
Table 2 The COACH trial – patients achieving the blood pressure target (<140/90 mmHg for patients with uncomplicated hypertension; <130/80 mmHg for patients with diabetes) after eight weeks of treatment (last observation carried forward)Citation46
At the end of the 8-week randomized phase of the COACH trial, 1684 patients entered a 44-week open-label extension period in which they received olmesartan/amlodipine 40/5 mg once-daily initially.Citation53 Uptitration to olmesartan/amlodipine 40/10 mg, followed by addition of HCTZ 12.5 mg and then 25 mg, was permitted if patients did not achieve the BP goal. Back-titration was also possible. Mean BP decreased from 164/102 mmHg at baseline to 131/82 mmHg at the end of this open-label extension period, while overall 66.7% of patients achieved the BP goal.
A total of 525 patients remained on olmesartan/amlodipine 40/5 mg throughout the extension period, and 80.0% of these achieved the BP goal. Uptitration to olmesartan/amlodipine 40/10 mg alone was necessary in 378 patients, of whom 70.6% achieved the BP goal. Addition of HCTZ at a dose of 12.5 mg/day (n = 287) or 25 mg/day (n = 419) resulted in 66.6% and 46.3% of the respective patients achieving their BP goal. Thus, treatment with olmesartan/amlodipine and up-titration as necessary, with or without HCTZ, allowed the majority of patients to achieve BP control.
Trial in patients with inadequate response to amlodipine monotherapy
This randomized, double-blind, multicenter study evaluated the efficacy of olmesartan/amlodipine in patients aged ≥18 years with moderate-to-severe hypertension who failed to respond adequately to amlodipine monotherapy.Citation49 Patients received open-label amlodipine 5 mg/day monotherapy for eight weeks. At the end of the monotherapy phase, patients with BP ≥ 140/90 mmHg were randomized to eight weeks of double-blind daily treatment with amlodipine 5 mg plus placebo or olmesartan/amlodipine 10/5 mg, 20/5 mg or 40/5 mg. At the end of the double-blind period, patients who had achieved the target BP of <140/90 mmHg continued on randomized therapy for a further eight weeks. Patients with BP ≥ 140/90 mmHg had their medication uptitrated to olmesartan/amlodipine 20/5 mg, 40/5 mg or 40/10 mg during this period.
The primary endpoint was the change in mean trough SeDBP from the end of the open-label run-in period (baseline) to the end of double-blind treatment (week 8) in the ITT population (defined as in the COACH trial) with LOCF imputation. Key secondary endpoints included the mean changes in trough SeDBP (baseline to week 4) and trough SeSBP (baseline to weeks 4 and 8), and the additional mean changes in SeDBP and SeSBP that occurred with further double-blind treatment (week 8 to week 16). The proportions of patients achieving the BP goal (defined as in the COACH trial) at weeks 8 and 16 of the double-blind phase were assessed.
A total of 755 patients were randomized to double-blind treatment, and 746 were included in the primary efficacy analysis. Compared with patients who were randomized to continue with amlodipine 5 mg, patients who were randomized to each olmesartan/amlodipine regimen showed significantly greater reductions in mean SeDBP from baseline to week 8 of the double-blind phase. The additional reductions in SeDBP achieved with olmesartan/amlodipine 20/5 and 40/5 mg compared with amlodipine 5 mg were 3.7 and 3.8 mmHg respectively (P < 0.0001) (). Patients receiving olmesartan/amlodipine also experienced greater reductions in mean SeSBP from baseline to week 8 of the double-blind phase. The additional reductions in SeSBP achieved with olmesartan/amlodipine 20/5 and 40/5 mg compared with amlodipine 5 mg were 5.8 and 7.1 mmHg respectively (P < 0.0001) (). All treatment regimens demonstrated a reduction in mean SeDBP and SeSBP after four weeks of double-blind treatment. In the second half of the double-blind phase, patients who had not achieved BP control had their treatment uptitrated and showed further significant increases in BP reduction by the end of this second 8-week treatment phase. Uptitration of amlodipine 5 mg to olmesartan/amlodipine 20/5 mg, olmesartan/amlodipine 20/5 to 40/5 mg, and olmesartan 40/5 to 40/10 mg resulted in further mean reductions of SBP () and DBP: −8.2, −6.2 and −8.2 mmHg respectively.
Figure 2 Olmesartan/amlodipine combination therapy versus amlodipine monotherapy – mean change from baseline in seated blood pressure after eight weeks of randomized, double-blind treatment.Citation49
Abbreviations: AML, amlodipine; OLM, olmesartan; SeBP, seated blood pressure; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure.
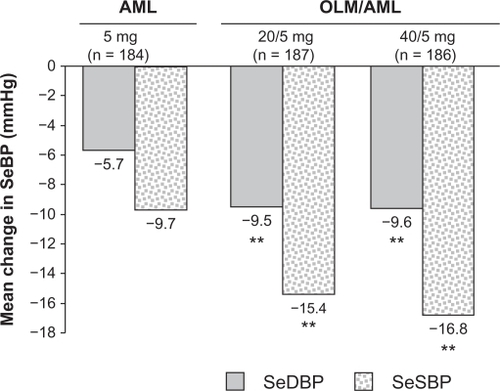
Figure 3 Olmesartan/amlodipine combination therapy versus amlodipine monotherapy – mean change from baseline in seated systolic blood pressure after eight weeks of randomized, double-blind, uptitrated treatment.Citation49
Abbreviations: AML, amlodipine; OLM, olmesartan; SeSBP, seated systolic blood pressure.
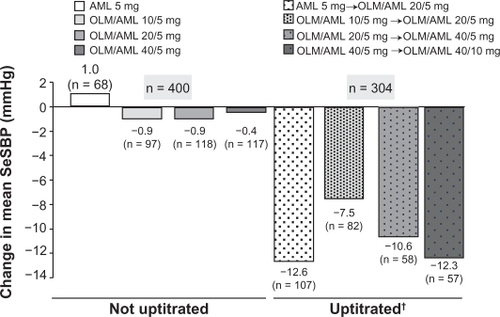
The proportion of patients reaching their BP goal at week 8 of double-blind treatment was significantly higher for olmesartan/amlodipine 20/5 mg (54%) and 40/5 mg (51%) compared with amlodipine monotherapy (30%) (P < 0.0001). Continuation of combination therapy for an additional eight weeks, without uptitration, resulted in BP goal achievement by over 70% of patients. Uptitration of medication resulted in an additional 36%–47% of patients achieving their BP goal. Overall, 469 of 746 patients (63%) achieved their BP goal at the end of 16 weeks of double-blind therapy, with or without uptitration.
This study also included ABPM measurements at the start and end of the first 8-week, double-blind phase, and after the additional eight weeks of randomized treatment with uptitration as necessary. During the first 8-week, double-blind period, each dose of olmesartan/amlodipine significantly reduced 24-hour, daytime and night-time DBP and SBP, compared with amlodipine 5 mg plus placebo.Citation54,Citation55 In patients who did not achieve their BP goal with their initial dosage of combination therapy, uptitration led to further reductions in 24-hour, daytime and night-time BP.Citation56 Taken together, the ABPM measurements were in agreement with the scheduled office BP measurements. Moreover, the detected BP reductions were consistent over the 24-hour dosing interval.Citation55
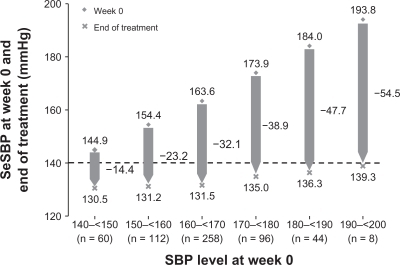
Patients who completed the 16 weeks of double-blind combination therapy entered a 28-week, open-label phase in which they received olmesartan/amlodipine 40/5 mg once daily (n = 691).Citation57 After 4, 10 and 19 weeks in the open-label phase, patients with inadequately controlled BP had their doses increased in a stepwise manner, with addition of HCTZ as necessary, to: olmesartan/amlodipine 40/10 mg; olmesartan/amlodipine/HCTZ 40/10/12.5 mg; and olmesartan/amlodipine/HCTZ 40/10/25 mg. The majority of patients remained on olmesartan/amlodipine 40/5 mg without uptitration, and 74.3% of these patients achieved their BP goal. Additional patients achieved with each successive uptitration. Overall, 66.9% of patients achieved their BP goal during this 28-week, open-label phase. Analysis of the final reductions in SBP, observed at the end of the overall active treatment period of 52 weeks, revealed that SBP reductions were related to the initial SBP level at the start of the study.Citation58 Thus, patients with higher baseline SBP levels achieved larger reductions in SBP (). Furthermore, despite the substantial reductions in BP achieved with olmesartan/amlodipine in this study, it is notable that the incidence of treatment-related hypotension was very low. Among the 578 patients who completed the 28-week, open-label phase, and received olmesartan/amlodipine 40/5 mg or 40/10 mg without the addition of HCTZ, there were four reports of hypotension (0.7%), all involving patients receiving olmesartan/amlodipine 40/5 mg.
Figure 4 Mean levels of seated systolic blood pressure (SeSBP) at the start (week 0) and end of treatment (week 52) according to baseline SeSBP in all patients treated with olmesartan/amlodipine combination therapy in a randomized, double-blind study.Citation58
Trial in patients with inadequate response to olmesartan monotherapy
Findings of a multicenter, randomized, double-blind trial conducted in patients aged ≥18 years with moderate-to-severe hypertension demonstrated that the addition of amlodipine to olmesartan lowered BP to a greater extent and enabled more patients to achieve their BP goal compared with olmesartan monotherapy.Citation50
After eight weeks of randomized, double-blind treatment, the additional reduction in SeDBP and SeSBP achieved with olmesartan/amlodipine 20/5 mg compared with olmesartan 20 mg monotherapy was 2.7 mmHg (P = 0.0006) and 5.3 mmHg (P < 0.0001), respectively. Furthermore, the proportion of patients achieving their BP goal was significantly higher for olmesartan/amlodipine 20/5 mg (44.5%) compared with olmesartan 20 mg monotherapy (28.5%) (P = 0.0011).
AZTEC and BP-CRUSH – efficacy of amlodipine/olmesartan-based titration regimens
AZTEC and BP-CRUSH are postregistration studies designed to obtain further information on the efficacy of olmesartan/amlodipine, both of which used tight BP control and forced titration regimens.
The AZTEC study was an open-label, multicenter, single-arm, dose-titration study in 185 patients with hypertension, consisting of a 3–4-week placebo run-in period and a 12-week active treatment period.Citation52 Initially, patients received amlodipine 5 mg/day. If SeBP remained ≥120/80 mmHg, as assessed using conventional office-based BP measurements, medication was uptitrated at 3-weekly intervals to olmesartan/amlodipine 20/5, 40/5 and 40/10 mg. The change from baseline in mean 24-hour ambulatory SBP/DBP at week 12 (the primary endpoint), as assessed by ABPM, was −21.4/−12.7 mmHg (P < 0.0001 vs baseline). The reduction in BP was consistent across the 24-hour dosing interval. The proportions of patients achieving the prespecified mean 24-hour ambulatory BP target of <130/80 mmHg was 70.9%. Dose-dependent reductions in office-based SeBP from baseline were observed with the stepwise olmesartan/amlodipine treatment algorithm, with the largest reductions in SeBP seen with the olmesartan/amlodipine 40/10 mg combination for which, cumulatively, 76.8% of patients achieved a SeBP goal of <140/90 mmHg.
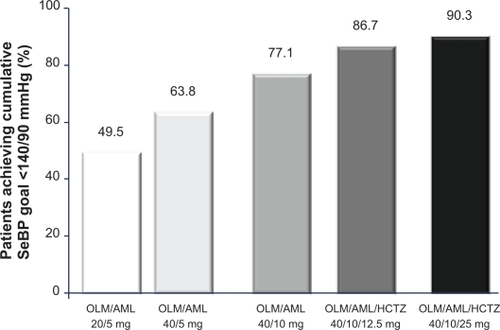
BP-CRUSH was an open-label, multicenter, single-arm, dose-titration study with a 20-week active-treatment period, the aim of which was to demonstrate that patients with hypertension who had previously failed to achieve BP control on monotherapy were able to achieve their BP goal with an olmesartan/amlodipine-based treatment regimen, which also included the addition of HCTZ.Citation59 On day 1, patients (n = 999) were switched from antihypertensive monotherapy to olmesartan/amlodipine 20/5 mg. If BP remained > 120/70 mmHg, medication was uptitrated at 4-weekly intervals to olmesartan/amlodipine 40/5 mg, olmesartan/amlodipine 40/10 mg, olmesartan/amlodipine/HCTZ 40/10/12.5 mg and olmesartan/amlodipine/HCTZ 40/10/25 mg. The primary efficacy endpoint, the proportion of patients achieving the SeBP goal (<140 mmHg; <130 mmHg for patients with diabetes) at the end of 12 weeks of olmesartan/amlodipine therapy, was 75.8%. Mean changes from baseline in BP at the end of each titration period ranged from −14.2/−7.7 mmHg for olmesartan/amlodipine 20/5 mg to −25.1/−13.7 mmHg for olmesartan/amlodipine/HCTZ 40/10/25 mg. The BP goal (<140/90 mmHg) was achieved by 90.3% of patients who received olmesartan/amlodipine/HCTZ 40/10/25 mg ().Citation51 ABPM measurements taken in a subgroup of patients (n = 243) at baseline and 12 and 20 weeks after treatment showed that BP reductions were sustained throughout the 24-hour dosing interval.Citation51
Figure 5 Proportion of patients who achieved the cumulative seated blood pressure (SeBP) goal of 140/90 mmHg in the BP-CRUSH study.Citation59
Tolerability of olmesartan/amlodipine
Olmesartan/amlodipine was generally well tolerated in clinical trials in patients with mild to severe hypertension. In the COACH trial, 521 of the 1940 randomized patients (26.9%) experienced a drug-related treatment-emergent adverse event (TEAE), with an overall incidence of 19.9% to 33.1% across the active-treatment groups receiving approved dosages and 29.6% for placebo-treated patients.Citation46 The majority of these adverse events were mild in severity. Peripheral edema was the most common TEAE, affecting 385 of the 1940 patients (19.8%). Other commonly reported TEAEs were headache (130/1940 [6.7%]), dizziness (76/1940 [3.9%]) and fatigue (62/1940 [3.2%]), with no consistent differences between the active-treatment groups. Headache occurred most frequently in the placebo group (23/162 [14.2%]). Overall, 3.8% (74/1940) of patients were withdrawn from the trial because of drug-related TEAEs. The only serious drug-related TEAE was a nonfatal cerebrovascular accident occurring in a patient receiving olmesartan 20 mg/day, in whom BP was not fully controlled. Hypotension was reported in 0.5% (9/1940) of patients across the treatment groups. Seven patients had drug-related hypotension, of which two were withdrawn from the trial because of moderate or severe hypotension. In the 44-week open-label extension of the COACH trial, the adverse event profile was similar to that observed during the double-blind phase.
In the trials comparing olmesartan/amlodipine with the respective monotherapies, drug-related TEAEs were reported in 5.3%–7.7% of patients receiving approved dosages of olmesartan/amlodipine compared with 7.4% and 8.9% of patients receiving amlodipine or olmesartan monotherapy, respectively.Citation49,Citation50 Few patients receiving combination therapy in either trial discontinued due to a drug-related TEAE, and no serious drug-related TEAEs were observed in either trial.
Peripheral edema represents a common side effect of CCBs such as amlodipine, because these drugs may increase capillary pressure in peripheral tissues by inducing precapillary vasodilation of resistance arteries.Citation60 Peripheral edema may be ameliorated by coadministration of an ARB or ACEI, as these agents may lower capillary pressures by decreasing postcapillary resistance in veins.Citation46 The COACH trial assessed patients specifically for peripheral edema, rating its presence on a 5-point severity scale at all scheduled clinic visits.Citation46,Citation53 At baseline, 264 of the 1940 randomized patients (13.6%) had peripheral edema, which was predominantly graded as mild.Citation46 During the 8-week, randomized, double-blind treatment phase, the frequency of edema was greatest among patients receiving amlodipine 10 mg monotherapy (60/163 [36.8%]), and affected 12.3% (20/162) of patients receiving a placebo. As expected, the frequency of peripheral edema was lower in the olmesartan/amlodipine 40/10 mg group (38/162 [23.5%]) than in the amlodipine 10 mg group (P = 0.011). Most cases of edema were mild or moderate in severity. Severe edema occurred in one patient (0.6%) in the amlodipine 5 mg group, two patients (1.2%) in the amlodipine 10 mg group and one patient (0.6%) in the olmesartan/amlodipine 40/10 mg group. Edema was also reported in the trials comparing olmesartan/amlodipine with the respective monotherapies, but the frequencies were lower than in the COACH trial.Citation49,Citation50
Conclusion
In randomized, double-blind trials, olmesartan/amlodipine has demonstrated greater efficacy than the respective monotherapies in reducing BP, including a reduction within two weeks of initiation in the COACH trial, and achieving their BP goals, including over 24 hours, in patients with moderate-to-severe hypertension who had responded inadequately to olmesartan or amlodipine monotherapy. Up to 54% of patients who had failed to respond adequately to olmesartan or amlodipine monotherapy achieved their BP goal during eight weeks of treatment with olmesartan/amlodipine. Uptitration of olmesartan/amlodipine provided additional BP reductions, allowing even more patients to achieve their BP goal, while the incidence of hypotension remained very low. Furthermore, treat-to-target studies have demonstrated the power of olmesartan/amlodipine-based treatment in achieving high BP goal rates. Olmesartan/amlodipine was generally well tolerated over short- and long-term therapy and this observation was not affected by uptitration. Peripheral edema was significantly less common with olmesartan/amlodipine 40/10 mg than with amlodipine 10 mg monotherapy. In Europe, a fixed-dose combined olmesartan/amlodipine formulation is available in three dosages (20/5, 40/5 and 40/10 mg), allowing flexible dosing and uptitration.
Acknowledgements
Editorial assistance was provided by Phil Jones and Amy McCallum (inScience Communications, a Wolters Kluwer business, Chester, UK), and funded by Daiichi Sankyo.
Disclosure
The author has received honoraria for lectures and consultancy fees from Daiichi Sankyo, Berlin-Chemie AG, and Menarini International.
References
- EzzatiMLopezADRodgersAVander HoornSMurrayCJSelected major risk factors and global and regional burden of diseaseLancet200236093431347136012423980
- World Health Organization. Reducing Risks, Promoting Healthy LifeThe World Health Report Available at: http://www.who.int/whr/2002/en/whr02_en.pdf. Accessed September 17, 2008.
- KearneyPMWheltonMReynoldsKMuntnerPWheltonPKHeJGlobal burden of hypertension: analysis of worldwide dataLancet2005365945521722315652604
- OngKLCheungBMManYBLauCPLamKSPrevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004Hypertension2007491697517159087
- Wolf-MaierKCooperRSBanegasJRHypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United StatesJAMA2003289182363236912746359
- SmithDHComparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertensionDrugs20086891207122518547132
- KannelWBBlood pressure as a cardiovascular risk factor: prevention and treatmentJAMA199627520157115768622248
- KlagMJWheltonPKRandallBLBlood pressure and end-stage renal disease in menN Engl J Med1996334113187494564
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force documentJ Hypertens2009272121215819838131
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research GroupMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA2002288232981299712479763
- DolorRJYancyWSJrOwenWFHypertension Improvement Project (HIP): study protocol and implementation challengesTrials2009101319245692
- GueyffierFFromentAGoutonMNew meta-analysis of treatment trials of hypertension: improving the estimate of therapeutic benefitJ Hum Hypertens1996101188642184
- PsatyBMSmithNLSiscovickDSHealth outcomes associated with antihypertensive therapies used as first–line agents. A systematic review and meta-analysisJAMA199727797397459042847
- SHEP Cooperative Research GroupPrevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research GroupJAMA199126524325532642046107
- WangYRAlexanderGCStaffordRSOutpatient hypertension treatment, treatment intensification, and control in Western Europe and the United StatesArch Intern Med2007167214114717242314
- Wolf-MaierKCooperRSKramerHHypertension treatment and control in five European countries, Canada, and the United StatesHypertension2004431101714638619
- CushmanWCBasileJAchieving blood pressure goals: why aren’t we?J Clin Hypertens (Greenwich)200681286587217170612
- PhillipsLSBranchWTCookCBClinical inertiaAnn Intern Med2001135982583411694107
- SprangerCBRiesAJBergeCARadfordNBVictorRGIdentifying gaps between guidelines and clinical practice in the evaluation and treatment of patients with hypertensionAm J Med20041171141815210383
- KreutzRPharmacogenetics of antihypertensive drug responseCurr Hypertens Rep200461152014972084
- NeutelJMLow-dose antihypertensive combination therapy: its rationale and role in cardiovascular risk managementAm J Hypertens1999128 Pt 273S79S10478700
- WaldDSLawMMorrisJKBestwickJPWaldNJCombination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trialsAm J Med2009122329030019272490
- DahlofBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet2005366948989590616154016
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med2008359232417242819052124
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436394262022203115207952
- CushmanWCEvansGWByingtonRPEffects of intensive blood-pressure control in type 2 diabetes mellitusN Engl J Med2010362171575158520228401
- GradmanAHBasileJNCarterBLCombination therapy in hypertensionJ Am Soc Hypertens201042909820400053
- BramlagePFixed-dose combinations of renin-angiotensin blocking agents with calcium channel blockers or hydrochlorothiazide in the treatment of hypertensionExpert Opin Pharmacother200910111755176719538001
- SchmiederREThe role of fixed-dose combination therapy with drugs that target the renin-angiotensin system in the hypertension paradigmClin Exp Hypertens2010321354220144071
- GuptaAKArshadSPoulterNRCompliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysisHypertension201055239940720026768
- LawMRWaldNJMorrisJKJordanREValue of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trialsBr Med J20033267404142712829555
- FrishmanWHRamCVMcMahonFGComparison of amlodipine and benazepril monotherapy to amlodipine plus benazepril in patients with systemic hypertension: a randomized, double-blind, placebo-controlled, parallel-group study. The Benazepril/Amlodipine Study GroupJ Clin Pharmacol19953511106010668626878
- KuschnirEAcunaESevillaDTreatment of patients with essential hypertension: amlodipine 5 mg/benazepril 20 mg compared with amlodipine 5 mg, benazepril 20 mg, and placeboClin Ther1996186121312249001838
- YusufSTeoKKPogueJTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med2008358151547155918378520
- MillerDROliveriaSABerlowitzDRFinckeBGStangPLillienfeldDEAngioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitorsHypertension20085161624163018413488
- National Institute for Health and Clinical ExcellenceHypertension: Management of hypertension in adults in primary care Available at: http://www.nice.org.uk/nicemedia/live/10986/30114/30114.pdf. Accessed June 9, 2010.
- FabiaMJAbdillaNOltraRFernandezCRedonJAntihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoringJ Hypertens20072571327133617563549
- ColtamaiLMaillardMSimonAVogtBBurnierMComparative vascular and renal tubular effects of angiotensin II receptor blockers combined with a thiazide diuretic in humansJ Hypertens201028352052620104189
- HaslerCNussbergerJMaillardMForclazABrunnerHRBurnierMSustained 24-hour blockade of the renin-angiotensin system: a high dose of a long-acting blocker is as effective as a lower dose combined with an angiotensin-converting enzyme inhibitorClin Pharmacol Ther200578550150716321616
- JonesMRSealeyJELaraghJHEffects of angiotensin receptor blockers on ambulatory plasma renin activity in healthy, normal subjects during unrestricted sodium intakeAm J Hypertens200720890791617679042
- BrunnerHRStumpeKOJanuszewiczAAntihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertensionClin Drug Invest2003237419430
- OparilSWilliamsDChrysantSGMarburyTCNeutelJComparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertensionJ Clin Hypertens (Greenwich)20013528329131811588406
- SmithDHDubielRJonesMUse of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartanAm J Cardiovasc Drugs200551415015631537
- ScottLJMcCormackPLOlmesartan medoxomil: a review of its use in the management of hypertensionDrugs20086891239127218547134
- HariaMWagstaffAJAmlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular diseaseDrugs19955035605868521773
- ChrysantSMelinoMKarkiSLeeJHeyrmanRThe combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety studyClin Ther200830458760418498909
- ChrysantSMelinoMKarkiSLeeJHeyrmanROnset of antihypertensive effect in patients with mild-to-severe hypertension treated with a combination of amlodipine besylate and olmesartan medoxomil compared with component monotherapy and placeboJ Clin Hypertens2008a26378 Suppl 1378
- OparilSLeeJKarkiSMelinoMSubgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: evaluation by baseline hypertension stage and prior antihypertensive medication useJ Cardiovasc Pharmacol200954542743619730391
- VolpeMBrommerPHaagUMieleCEfficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig20092911125
- BarriosVBrommerPHaagUCalderonAEscobarCOlmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig2009297427439
- WeirMRHsuehWANesbittSDEffects on BP control of amlodipine (AML)/olmesartan medoxomil (OM), with or without hydrochlorothiazide (HCTZ), in patients not controlled by prior antihypertensive monotherapy (late breaker at the American Society of Hypertension 25th Annual Scentific Meeting, New York)J Clin Hypertens (Greenwich)2010127536
- PunziHNeutelJMKereiakesDJEfficacy of amlodipine and olmesartan medoxomil in patients with hypertension: the AZOR Trial Evaluating Blood Pressure Reductions and Control (AZTEC) studyTher Adv Cardiovasc Dis20104420922120519261
- ChrysantSGOparilSMelinoMKarkiSLeeJHeyrmanREfficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertensionJ Clin Hypertens (Greenwich)200911947548219751459
- HeagertyAMLaeisPHaagUOlmesartan medoxomil/amlodipine (OLM/AML) provides 24-hour antihypertensive efficacy – additional effect by uptitration in patients with moderate-to-severe hypertensionJ Hypertens200927Suppl 4S283
- ParatiGBiloGOlmesartan medoxomil/amlodipine (OLM/AML) improves blood pressure (BP) control throughout 24 hours compared with AML monotherapy in patients with moderate-to-severe hypertensionJ Hypertens201028e111
- HeagertyAMLaeisPHaagUOlmesartan medoxomil/amlodipine (OLM/AML) provides 24-hour antihypertensive efficacy – additional effect by uptitration in patients with moderate-to-severe hypertensionJ Hypertens200927Suppl 4S283
- VolpeMMieleCHaagUEfficacy and safety of a stepped-care regimen using olmesartan medoxomil, amlodipine and hydrochlorothiazide in patients with moderate-to-severe hypertension: an open-label, long-term studyClin Drug Investig2009296381391
- MouradJJLe JeuneSEffective systolic blood pressure reduction with olmesartan medoxomil/amlodipine combination therapy: post hoc analysis of data from a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig2009296419425
- WeirMHsuehWANesbittSDEffects on BP control of amlodipine (AML)/olmesartan medoxomil (OM), with or without hydrochlorothiazide (HCTZ), in patients not controlled by prior anti-hypertensive monotherapy2010: Presented as a late breaker at the American Society of Hypertension 25th Annual Scentific Meeting and Exposition2010New York
- OpieLHPharmacological differences between calcium antagonistsEur Heart J199718Suppl AA71A799049541