Abstract
Aim:
Analyzing safety aspects of a drug from individual studies can lead to difficult-to-interpret results. The aim of this paper is therefore to assess the general safety and tolerability, including incidences of the most common adverse events (AEs), of vildagliptin based on a large pooled database of Phase II and III clinical trials.
Methods:
Safety data were pooled from 38 studies of ≥12 to ≥104 weeks’ duration. AE profiles of vildagliptin (50 mg bid; N = 6116) were evaluated relative to a pool of comparators (placebo and active comparators; N = 6210). Absolute incidence rates were calculated for all AEs, serious AEs (SAEs), discontinuations due to AEs, and deaths.
Results:
Overall AEs, SAEs, discontinuations due to AEs, and deaths were all reported with a similar frequency in patients receiving vildagliptin (69.1%, 8.9%, 5.7%, and 0.4%, respectively) and patients receiving comparators (69.0%, 9.0%, 6.4%, and 0.4%, respectively), whereas drug-related AEs were seen with a lower frequency in vildagliptin-treated patients (15.7% vs 21.7% with comparators). The incidences of the most commonly reported specific AEs were also similar between vildagliptin and comparators, except for increased incidences of hypoglycemia, tremor, and hyperhidrosis in the comparator group related to the use of sulfonylureas.
Conclusions:
The present pooled analysis shows that vildagliptin was overall well tolerated in clinical trials of up to >2 years in duration. The data further emphasize the value of a pooled analysis from a large safety database versus assessing safety and tolerability from individual studies.
Introduction
Vildagliptin is an orally effective dipeptidyl peptidase-4 (DPP-4) inhibitor that has been studied in a large clinical program as monotherapy and combination therapy.Citation1 It binds covalently to the catalytic site of DPP-4, eliciting prolonged enzyme inhibition. This raises intact glucagon-like peptide-1(GLP-1) levels both after meal ingestion and in the fasting state. By increasing concentrations of active GLP-1, vildagliptin improves β- and α-cell sensitivity to glucose.Citation2 This results in glucose-sensitive modulation of insulin and glucagon secretion, improving both fasting and postprandial glycemia, with a low risk for hypoglycemia and no weight gain.
Areas of potential safety concern related to type 2 diabetes (T2DM) itself (ie, cardiovascular and hepatic safety), as well as potential safety concerns specific to DPP-4 inhibitors (ie, immune system, skin, and pancreatitis), have been analyzed previously for vildagliptin based on a large pooled database, with no increased risks identified versus comparators.Citation3,Citation4 However, other safety aspects, such as general safety and tolerability, including incidences of most common specific adverse events (AEs), have so far been reviewed in the literature from individual studies only.Citation1,Citation5,Citation6 Although this is often the only possible approach early in the development of a new drug, such data from single and often relatively small studies are less reliable than analyses from larger datasets. Furthermore, specific design features, study duration, sample size, and, in particular, the comparator chosen for individual studies can influence the AE reporting rates in a specific study, which needs to be weighed against the overall experience in a clinical trial program. For example, one issue that arose from an individual study with vildagliptin was related to edema. In contrast to other studies, Bolli et al,Citation7 in a trial comparing vildagliptin and pioglitazone as add-on therapy with metformin, reported peripheral edema as the most common AE for vildagliptin with an incidence even somewhat higher than for the thiazolidinedione (TZD) itself.
Based on these considerations, it was of interest to assess the general safety and tolerability of vildagliptin, as well as the specific risk of edema-related AEs with vildagliptin treatment, using the previously described large pooled database of vildagliptin Phase II and III clinical studies.Citation4 We report here the results of these new pooled safety analyses.
Methods
Populations
The safety analyses are based on the previously reported pool of 38 Phase II and Phase III studies that used vildagliptin as monotherapy or in combination with metformin, TZDs, sulfonylureas (SUs), or insulin for ≥12 weeks up to ≥104 weeks.Citation4
For the analysis of overall AEs, AEs by system organ class (SOC) or preferred term (PT), serious AEs (SAEs), discontinuations due to AEs, and deaths, as well as edema-related AEs, the “all studies (excluding open-label) safety population”, which excludes open-label studies in order to minimize reporting bias, was used. Supplementary Table 1 briefly describes each of the studies included in this pooled dataset.
Peto odds ratios (ORs) were additionally calculated for edema-related AEs, as a single study had previously reported a higher incidence with vildagliptin.Citation7 Calculation of ORs requires a comparator; thus, calculation of ORs and the resulting Forest plot for edema-related AEs used data pooled from all controlled studies excluding open-label trials. This population is termed “all controlled studies (excluding open-label) safety population” (see Supplementary Table 1 for details).
In addition to being analyzed in the all studies (excluding open-label) safety population, confirmed hypoglycemic episodes (as defined in this article) were also assessed in monotherapy (monotherapy [excluding open-label] safety population), which was deemed more appropriate considering that the risk of hypoglycemia is influenced by antidiabetic background therapy. Confirmed hypoglycemia is thus not reflected under the most common AEs in the all studies (excluding open-label) safety population in . The monotherapy (excluding open-label) safety population includes 21 studies (see Supplementary Table 1 for details on the contributing studies).
Table 1 Adverse event (AE) summary and most common AEs (all studies [excluding open-label] safety population)
Assessments
All AEs were recorded and assessed by the investigator as to the severity and possible relationship to the study medication. This included laboratory abnormalities if considered an AE by the investigator. All laboratory assessments were performed by central laboratories (for details, see Ligueros-Saylan et alCitation4).
Confirmed hypoglycemia was defined as symptoms suggestive of low blood glucose confirmed by self-monitored blood glucose measurement <3.1 mmol/L plasma glucose equivalent.
Standardization of terms
AEs were encoded in all studies using the Medical Dictionary for Regulatory Activities (MedDRA, Version 12.1) system. This is a medically validated terminology database developed by the International Conference on Harmonization. Within the MedDRA, AEs are grouped by SOC, eg, “cardiac disorders” or “gastrointestinal disorders”. Within an SOC, specific AEs are identified by PT. The PTs included in the analysis of edema-related AEs are allergic edema, generalized edema, local swelling, localized edema, edema, peripheral edema, pitting edema, skin edema, skin swelling, and swelling.
Data analysis
For AEs, SAEs, discontinuations due to AEs, and deaths, incidences were calculated as number of patients with an event divided by the number of patients in the treatment group. For edema-related AEs (as defined previously), exposure-adjusted incidences were additionally calculated as number of patients having events per 100 subject-year exposure (SYE), defined as 100×(number of patients with an event divided by the total exposure time in years).
To further compare edema-related AEs between vildagliptin and comparators, for each trial, Peto OR and the corresponding 95% confidence interval (CI) were calculated. The pooled estimate was obtained using a fixed-effect model and presented in a forest plot. An OR below unity is indicative of a treatment effect favoring vildagliptin. Correction for continuity using the inverse of the opposite arm size was used when zero events occurred.Citation8 This correction causes less bias than the standard continuity correction of 0.5 when the sizes of the treatment arms are unbalanced.Citation9
Safety data of vildagliptin 50 mg bid (the highest approved and most commonly used dosage of the drug) are reported along with pooled safety data of all comparators (active or placebo) from the safety populations.
Ethics and good clinical practice
All study participants provided written informed consent. All protocols were approved by the independent ethics committee/institutional review board at each study site or country. All studies were conducted using good clinical practice and in accordance with the Declaration of Helsinki.
Results
Exposure and demography
As detailed in Ligueros-Saylan et al,Citation4 in the all studies (excluding open-label) safety population, 6116 patients received vildagliptin 50 mg bid (representing 7313.6 SYE) and 6210 patients received any comparator (representing 6512.7 SYE). The comparators group included placebo (23.7%), SUs (41.7%), metformin (18.8%), TZDs (12.3%), and acarbose (3.5%).
The mean duration of exposure was 62.4 weeks with vildagliptin 50 mg bid and 54.7 weeks with comparators (). This allows for direct comparisons between the two groups and provides a conservative estimate, as the slightly longer exposure with vildagliptin tends to favor the comparator group.
The demographic and baseline characteristics of patients in the all studies (excluding open-label) safety population have also been described previously.Citation4 In brief, the population studied was representative of a broad spectrum of T2DM patients, with a mean age, body mass index, glycosylated hemoglobin A, fasting plasma glucose, and duration of T2DM of approximately 56 years, 31.4 kg/m2, 8.1%, 9.8 mmol/L, and >4 years, respectively, and with nearly one-third of patients having some degree of renal insufficiency (glomerular filtration rate [Modification of Diet in Renal Disease] ≤80 mL/min per 1.73 m2).
Overall safety and tolerability
and report AE profiles for vildagliptin 50 mg bid and comparators in the all studies (excluding open-label) safety population.
Table 2 Adverse events (AEs) by system organ class (SOC) (all studies [excluding open-label] safety population)
Overall AEs, SAEs, discontinuations due to AEs, and deaths were all reported with a similar frequency in patients receiving vildagliptin (69.1%, 8.9%, 5.7%, and 0.4%, respectively) and patients receiving comparators (69.0%, 9.0%, 6.4%, and 0.4%, respectively), and drug-related AEs were seen with a lower frequency in vildagliptin-treated patients (15.7% vs 21.7% with comparators) ().
When the reported AEs were analyzed by SOC (), the incidences with vildagliptin and comparators were also similar overall. The four SOCs with the highest incidence of AEs were infections and infestations (35.3% for vildagliptin vs 32.4% for comparators), gastrointestinal disorders (23.5% vs 22.4%), musculoskeletal and connective tissue disorders (22.5% vs 21.1%), and nervous system disorders (21.6% vs 23.7%). Of note, there were no imbalances between vildagliptin and comparators in the overall reporting rates under the cardiac (6.1% with vildagliptin vs 6.0% with comparators), hepatobiliary (1.7% vs 1.6%), skin (12.6% vs 14.4%), and vascular (7.8% in both groups) SOCs. The most notable difference was observed in the metabolism/nutrition SOC, with incidences of 7.8% for vildagliptin and 11.4% for comparators, which were mainly due to hypoglycemia.
Overall, there were no appreciable trends in SAEs reported, and the majority of SAEs were scattered across many different SOCs. The primary SOC with the highest incidence of SAEs was cardiac disorders, with no imbalance between vildagliptin (1.7%) and comparators (1.9%). The only other SOCs with an incidence of SAEs ≥ 1% were infections and infestations (1.5% with vildagliptin 50 mg bid vs 1.4% with comparators); benign, malignant, and unspecified neoplasms (including cysts and polyps) (1.2% vs 1.1%); nervous system disorders (1.1% vs 1.0%); and gastrointestinal disorders (1.0% vs 0.9%).
There were no meaningful imbalances across the treatment groups in the incidence of AEs leading to discontinuation in any SOC. The SOC with the highest incidence of AEs leading to discontinuation was gastrointestinal disorders (1.2% with vildagliptin vs 1.4% with comparators).
A summary of the most commonly (≥3% in either group) reported specific AEs for vildagliptin 50 mg bid and comparators is also provided in . All of the individual AEs were reported with a low frequency of <10%. The most common AEs across treatment groups were nasopharyngitis (9.4% with vildagliptin vs 8.5% with comparators), dizziness (6.4% vs 7.4%), headache (7.0% vs 6.0%), and diarrhea (5.6% vs 6.7%). The incidences of the most commonly reported AEs were overall similar between vildagliptin and comparators. The most notable differences were lower incidences with vildagliptin of tremor (3.0% vs 7.6%) and hyperhidrosis (2.8% vs 6.8%). Furthermore, confirmed hypoglycemia was reported less frequently with vildagliptin (1.7%) than with comparators (5.8%). Hypoglycemia was additionally assessed in a pooled monotherapy population, which was deemed more appropriate than the assessment in the overall pooled data-set, considering that the risk of hypoglycemia is influenced by antidiabetic background therapy. In the pooled monotherapy safety population, confirmed hypoglycemic events were reported in 0.5% of patients treated with vildagliptin versus 0.3% treated with placebo and 0.6% treated with all comparators (the comparator group consisted of 38.3% metformin, 20.7% placebo, 17.9% SU, 7.2% acarbose, and 15.9% TZD).
Edema
As depicted in , there was no evidence of an increased risk of edema-related AEs with vildagliptin 50mg bid relative to comparators. The Peto OR for vildagliptin 50 mg bid was 0.72 (95% CI 0.59–0.88), indicating a statistically significant risk reduction versus the comparator group.
Figure 1 Incidences and Peto odds ratio (OR) for edema-related adverse events with vildagliptin 50 mg bid versus comparators (placebo and active comparators) in the all controlled studies (excluding open-label) safety population.
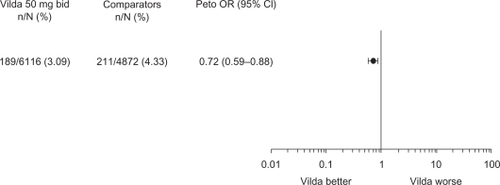
The overall incidence of any edema-related AE was low in both treatment groups (). The unadjusted and SYE-adjusted incidences of any edema-related AEs were lower for vildagliptin than for comparators (). The most commonly reported edema-related AE was peripheral edema, and for this AE the incidence was also lower with vildagliptin (2.9%, 2.46 events per 100 SYE) than with comparators (3.5%, 3.36 events per 100 SYE). For all other specific edema-related AEs, the SYE-adjusted incidences with vildagliptin were the same as or lower than with comparators.
Table 3 Edema-related adverse events (AEs) (all studies [excluding open-label] safety population)
Discussion
The present paper has evaluated in a large pooled database safety aspects of the DPP-4 inhibitor vildagliptin that were previously assessed only from individual study results. Although the latter approach represents generally a good approach to judging the efficacy of a drug, it has considerable limitations when assessing safety and tolerability and can lead to difficult-to-interpret or even misleading results. On the one hand, sample sizes of individual trials are often too small to reliably assess whether any imbalances observed in individual AEs reflect a true excess over the comparator treatment studied or rather a chance finding. On the other hand, the study duration, the safety surveillance measures, and, in particular, the comparator chosen for individual studies can influence the AE reporting rates in a specific study. Another complication arises if the results of an individual study are extrapolated as being representative for the overall safety of a drug, as happens in the literature, especially if pooled data are not available.
For vildagliptin, a higher incidence of peripheral edema, for example, was observed in a study that compared the drug with the TZD pioglitazone, for which edema is a known side effect.Citation7 In contrast, the new pooled analysis presented here did not confirm an increased incidence of edema-related events with vildagliptin but rather showed a statistically significant risk reduction versus the comparator group (OR = 0.72). Peripheral edema specifically occurred at an incidence rate of 2.46 events per 100 SYE with vildagliptin 50 mg bid versus 3.36 events per 100 SYE with comparators. Of note, only 12% of patients in the comparator group were treated with TZDs. Another study reported an imbalance with vildagliptin versus comparator treatment for the AE of hypertension.Citation10 In contrast, hypertension was well balanced when analyzed in the large pooled dataset (4.9% with vildagliptin vs 5.1% with comparators). These examples clearly highlight the value and importance of pooled safety analyses.
The safety of vildagliptin versus all comparators was previously assessed with regard to organs, systems, or tissues of particular interest in T2DM and areas of potential concern with DPP-4 inhibitors.Citation4 The meta-analyses indicated that vildagliptin was not associated with an increased risk of hepatic events or hepatic enzyme elevations indicative of drug-induced liver injury, pancreatitis, skin-related toxicity, or infections. In line with these results, the data presented here did not show any imbalances between vildagliptin and comparators for AEs in the SOCs of hepatobiliary disorders, skin and subcutaneous tissue disorders and infection and infestations.
The present pooled analysis further shows a general safety profile of vildagliptin 50 mg bid in clinical trials of up to >2 years in duration that was very similar to that of comparators regarding the overall incidences of AEs, SAEs, discontinuations due to AEs, and deaths. This also holds true when AEs were analyzed by SOCs or the most common AEs were evaluated. The only notable differences were for confirmed hypoglycemia and the likely hypoglycemia-related AEs of tremor and hyperhidrosis, for which lower incidences were observed with vildagliptin than with comparator treatment, mainly due to the use of SUs as a comparator in several studies (representing >40% of the comparator group). Because hypoglycemia incidences are largely influenced by antidiabetic background therapy, it is important to review hypoglycemia rates for specific treatment regimens. The overall safety population used for the present safety analyses consists of a broad range of studies with different treatment regimens, including add-on to insulin; thus, the frequency of confirmed hypoglycemia was also assessed in a pooled monotherapy safety population. Of the patients treated with vildagliptin monotherapy, 0.5% reported confirmed hypoglycemic episodes, which is very similar to the rate found with placebo (0.3%).
Taken together, the present pooled analysis provides a more comprehensive and reliable assessment of the general safety and tolerability of vildagliptin than can be obtained by extracting safety data from individual studies only.
Declaration
All authors are employees of Novartis.
Acknowledgements
The authors acknowledge the patients, investigators, and staff at participating sites for all the studies. This work was funded by Novartis Pharmaceuticals Corporation.
Supplementary material
Table S1 Vildagliptin studies contributing to safety analyses
References
- RisticSByiersSFoleyJHolmesDImproved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose responseDiabetes Obes Metab2005769269816219012
- PratleyREJauffret-KamelSGalbreathEHolmesDTwelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetesHorm Metab Res20063842342816823726
- DejagerSRazacSFoleyJESchweizerAVildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose studyHorm Metab Res20073921822317373638
- ScherbaumWASchweizerAMariAEfficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycemiaDiabetes Obes Metab20081067568218248490
- MariAScherbaumWANilssonPMCharacterization of the influence of vildagliptin on model-assessed {beta}-cell function in patients with type 2 diabetes and mild hyperglycemiaJ Clin Endocrinol Metab20089310310917925336
- ScherbaumWASchweizerAMariAEvidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemiaDiabetes Obes Metab2008101114112418355325
- SchweizerACouturierAFoleyJEDejagerSComparison between vildagliptin and metformin to sustain reductions in HbA1c over one year in drug-naïve patients with type 2 diabetesDiabet Med20072495596117509069
- GökeBHershonKKerrDEfficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naive patients with type 2 diabetes: comparison with metforminHorm Metab Res20084089289518726829
- FoleyJESreenanSEfficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetesHorm Metab Res20094190590919705345
- PanCYangWBaronaJPComparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trialDiabet Med20082543544118341596
- RosenstockJBaronMADejagerSComparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trialDiabetes Care20073021722317259484
- RosenstockJNiggliMMaldonado-LutomirskyMLong-term 2-year safety and efficacy of vildagliptin compared with rosiglitazone in drug-naive patients with type 2 diabetes mellitusDiab Obes Metab200911571578
- RosenstockJFoleyJERendellMEffects of the DPP-4 inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose toleranceDiabetes Care200831303517947341
- Pi-SunyerFXSchweizerAMillsDDejagerSEfficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetesDiabetes Res Clin Pract20077613213817223217
- SchweizerADejagerSBosiEComparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trialDiabetes Obes Metab20091180481219476473
- AhrénBGomisRStandlETwelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetesDiabetes Care2004272874288015562200
- BosiECamisascaRPColloberCEffects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metforminDiabetes Care20073089089317277036
- FerranniniEFonsecaVZinmanBFifty-two-week efficacy and safety of vildagliptin vs glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapyDiabetes Obes Metab20091115716619125777
- MatthewsDRDejagerSAhrenBVildagliptin add-on to metformin produces similar efficacy and reduced hypoglyceamic risk compared with glimepiride, with no weight gain: results from a 2-year studyDiabetes Obes Metab201012978078920649630
- FilozofCGautierJFA comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized studyDiab Med 201;273318326
- BolliGDottaFRochotteECohenSEEfficacy and tolerability of vildagliptin vs pioglitazone when added to metformin: a 24-week, randomized, double-blind studyDiabetes Obes Metab200810829018034842
- BolliGDottaFColinLComparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metforminDiabetes Obes Metab20091158959519515179
- GoodmanMThurstonHPenmanJEfficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapyHorm Metab Res20094136837319221978
- FilozofCSchwartzSFoleyJEEffect of metformin as add-on therapy to a low dose metforminWorld J Diabetes201011926
- BosiEDottaFJiaYGoodmanMVildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitusDiabetes Obes Metab20091150651519320662
- GarberAJSchweizerABaronMAVildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled studyDiabetes Obes Metab2007916617417300592
- RosenstockJBaronMACamisascaRPEfficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared to component monotherapy in patients with type 2 diabetesDiabetes Obes Metab2007917518517300593
- GarberAJFoleyJEBanerjiMAEffects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylureaDiabetes Obes Metab2008101047105618284434
- FonsecaVSchweizerAAlbrechtDAddition of vildagliptin to insulin improves glycaemic control in type 2 diabetesDiabetologia2007501148115517387446
- FonsecaVBaronMShaoQDejagerSSustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitusHorm Metab Res20084042743018401832
References
- KeatingGMVildagliptin: a review of its use in type 2 diabetes mellitusDrugs201070162089211220964454
- AhrénBSchweizerADejagerSVildagliptin improves islet glucose sensing in patients with type 2 diabetesJ Clin Endocrinol Metab2009941236124319174497
- SchweizerADejagerSFoleyJEAssessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large phase III type 2 diabetes populationDiabetes Obes Metab20101248549420518804
- Ligueros-SaylanMFoleyJESchweizerAAn assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trialsDiabetes Obes Metab20101249550920518805
- GerichJDPP-4 inhibitors: what may be the clinical differentiators?Diabetes Res Clin Pract20109013114020708812
- NeumillerJJWoodLCampbellRKDipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitusPharmacotherapy201030546348420411998
- BolliGDottaFRochotteECohenSEEfficacy and tolerability of vildagliptin vs pioglitazone when added to metformin: a 24-week, randomized, double-blind studyDiabetes Obes Metab200810829018034842
- CoxDRThe continuity correctionBiochmetrika197057217219
- SweetingMJSuttonAJLambertPCWhat to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse dataStat Med2004231351137515116347
- AhrénBGomesRStandlETwelve and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetesDiabetes Care20041228742880