Abstract
Azilsartan–chlorthalidone fixed combination is a new drug in the management of hypertension. Azilsartan has been shown to have greater blood pressure-lowering effects than other angiotensin-receptor blockers (ARBs), and the debate regarding the superiority of chlorthalidone over hydrochlorothiazide has been ongoing for years. The combination is unique because it is the first to partner an ARB with this, possibly more effective, diuretic. This review will address trials involving both components of this drug, as well as phase III trials involving the fixed-combination product. The article will also discuss the benefit of combination therapy in the treatment of hypertension.
Introduction
Despite the knowledge gained from large clinical trials, cardiovascular disease remains one of the leading causes of death, and hypertension remains a significant cause of cardiovascular morbidity and mortality. According to the National Heart, Lung, and Blood Institute, over one-third of the United States was diagnosed with hypertension during 2005–2008. Fortunately, over 70% of these patients were being treated for their hypertension, but disappointingly only 47.7% had controlled blood pressure (BP).Citation1 This lack of medical success is one reason why new antihypertensive agents continue to be developed and also explains the interest in fixed-combination antihypertensive agents, such as the new angiotensin-receptor blocker/thiazide-type combination of azilsartan–chlorthalidone.
The current guidelines recommend administering more than one drug as initial therapy for BP >20 mmHg systolic or >10 mmHg diastolic above goal, with one of these drugs being a thiazide-type diuretic.Citation2 Angiotensin-receptor blockers (ARBs) as initial therapy have also fallen into favor due to their cardiovascular morbidity and tolerability benefits over beta blockers.Citation3 Several trials have shown that combination therapy increases achievement of BP control at lower doses which also decreases side effects.Citation4–Citation6 When using combination therapy, fixed-combination agents have the benefit of also increasing efficacy by increasing compliance.Citation4
Several fixed-combination antihypertensive agents exist, and seven of them are prescribed enough to be on the top 200 drugs list.Citation7 Azilsartan–chlorthalidone is unique in that it is the only combination drug that contains an ARB with the long-acting thiazide-type diuretic, chlorthalidone.Citation8 This article will discuss the clinical utility of fixed-combination azilsartan–chlorthalidone in the management of hypertension.
Azilsartan: a new ARB
Angiotensin II (AngII) is an integral hormone in the regulation of BP. AngII causes most of its hypertensive effects by stimulating the angiotensin type I receptor (AT1), making this receptor the primary target of ARBs. When activated, AT1 results in hypertension from direct vasoconstriction and secretion of aldosterone, and central AngII has also been shown to affect regulation of the sympathetic nervous system.Citation9 ARBs have shown their value in BP therapy by being as efficacious as angiotensin-converting enzyme (ACE) inhibitors, while causing fewer side effects than other drug classes, including idiosyncratic cough and angioedema associated with ACE inhibitors.Citation10 Within the ARB class, there are differences in pharmacokinetic and pharmacologic properties that may translate into different therapeutic effects, including BP-lowering.
The newer ARB, azilsartan, is commercially available as 40 mg and 80 mg oral tablets. The bioavailability of the drug is estimated at 60%, with peak plasma concentrations obtained at 1.5 to 3 hours. Azilsartan demonstrated a volume of distribution of approximately 16 L and is highly protein-bound (>99%). The half-life has been measured at 11 hours, and the major enzyme responsible for its metabolism is CYP2C9.Citation11
Azilsartan, a prodrug, is a selective and insurmountable AT1 antagonist with evidence suggesting it not only has high binding affinity, but also has slow dissociation from the target receptor.Citation12 Azilsartan has been shown in vitro to have a higher percentage of inhibition at human AT1 than olmesartan, telmisartan, valsartan, and irbesartan. In the same study, azilsartan also showed the greatest receptor binding after washout when compared to the other ARBs.Citation12 These data suggest that azilsartan may bind more tightly and dissociate more slowly from the selective target AT1 than the comparator ARBs. Clinically, azilsartan’s binding characteristics may allow for this drug to have greater BP-lowering effects and a longer duration of action compared to other ARBs.
The insurmountable, or tight-binding behavior, may be attributable to the presence of a carboxyl group as part of the ARB chemical structure. The ARBs with a carboxyl moiety and insurmountable binding include olmesartan, candesartan, and azilsartan.Citation12 The carboxyl group is thought to be responsible for interaction with the amino acid lysine and provide tight binding to AT1. The carboxyl group may also be related to the beneficial decrease in hypertrophy exhibited by these ARBs. In addition to a carboxyl group, azilsartan also has a distinctive oxadiazolone group in place of the tetrazole ring that exists as part of the structure on other currently available ARBs. It is possible that the oxadiazolone structure in azilsartan allows azilsartan to bind more strongly to essential residues of the AT1. Tighter receptor binding, as has been shown with azilsartan, may be an advantageous characteristic since this binding would allow azilsartan to maintain its effects even when AngII levels increase.Citation12 When ARBs are administered to patients, AngII levels are known to increase as a compensatory mechanism caused by AT1 inhibition during ARB treatment. Elevated AngII levels may theoretically displace ARBs from their receptor sites. ARBs with tighter binding characteristics may be less likely to be displaced resulting in greater therapeutic efficacy as compared to ARBs with less tight binding characteristics. Azilsartan’s unique structure and pharmacological properties may provide advantages in the treatment of hypertension compared to other ARBs.
Azilsartan versus other ARBs
As a monotherapy, azilsartan 80 mg once daily has been shown to be more effective in lowering systolic and trough BP than other ARBs at their highest approved doses. One trial compared increasing doses of azilsartan (20, 40, and 80 mg) to olmesartan 40 mg and placebo.Citation13 The primary endpoint in this trial was a change in 24-hour mean ambulatory BP after 6 weeks of treatment in 1260 randomized essential hypertension patients. Clinic BP (measured 24 hours after previous dose) and percentage of responding patients were evaluated in this study. A “responder” was defined as a patient achieving systolic BP (SBP) of <140 mmHg or a decrease of ≥20 mmHg. All BP was measured with an automated device. The trial excluded patients with estimated glomerular filtration rate (eGFR) < 30 mL/min and type I or poorly controlled type II diabetes. The 40 mg dose of azilsartan was shown to be noninferior to olmesartan. The difference of change in 24-hour mean SBP was −2.1 mmHg (95% confidence interval [CI]: −4.0–0.1; P = 0.038) greater with azilsartan 80 mg compared to olmesartan 40 mg. The results also showed a −2.7 mmHg greater decrease (95% CI: −5.3–0.1; P = 0.043) in clinical SBP with administration of azilsartan 80 mg compared to olmesartan. However, there was no significant difference in the amount of patients that achieved clinical response (57% azilsartan and 53% olmesartan; P = 0.402) between these two arms. Although not statistically significant, a subgroup analysis regarding patients with a body mass index (BMI) ≥ 30 kg/m2 showed a treatment difference between azilsartan 80 mg and olmesartan 40 mg of −2.7 mmHg (95% CI: −5.8–0.32) compared to a −1.7 mmHg difference in patients with a BMI < 30 (95% CI: −4.2–0.9). There was no difference in adverse effects between all treatment groups. In conclusion, azilsartan showed a statistically significant decrease in SBP based on 24-hour ambulatory BP and trough (clinical) BP.
A trial similar in patient population, timeline, and primary outcome to the previously discussed trial above, compared azilsartan 40 mg and 80 mg to placebo, olmesartan 40 mg, or valsartan 320 mg in 1175 randomized hypertensive patients.Citation13,Citation14 This study assessed the same endpoints of mean 24-hour BP, clinical BP, and percentage of responding patients. All BP measurements were obtained using automated devices. Comparable to the previous trial, azilsartan 40 mg was shown to be noninferior to olmesartan, while azilsartan 80 mg showed superiority to both of the highest doses approved for olmesartan and valsartan (difference in reduction of mean 24-hour SBP −2.5 mmHg, 95% CI: −4.4–0.6; −4.3 mmHg, 95% CI: −6.3–2.4, respectively). Azilsartan 40 mg and 80 mg also had a greater reduction in clinical BP compared to olmesartan 40 mg and valsartan 320 mg (−14.6, −14.9, −11.4, and −9.5 mmHg, respectively; all P < 0.05). Unlike the previous trial, this trial found a significantly larger amount of “responders” in the azilsartan arm (58%) than the valsartan (49%) or olmesartan (49%) arms (P < 0.05). No difference was seen when comparing obese to nonobese patients, and all treatment arms showed similar adverse events.
The above trials demonstrated that azilsartan has greater efficacy in decreasing overall SBP and greater BP effects at the end of the dosing interval. The extent of azilsartan’s effects on SBP, although marginal, may prove to be clinically significant as current guidelines discuss a decrease of cardiovascular events with SBP reductions of 2 mmHg.Citation2 In addition, it should be emphasized that azilsartan actually showed superiority to agents in the same class as opposed to merely demonstrating noninferiority. These findings suggest azilsartan could be an innovative ARB that has distinct advantages over the other ARBs and these advantages may be related to its pharmacological profile.
Azilsartan–chlorthalidone combination
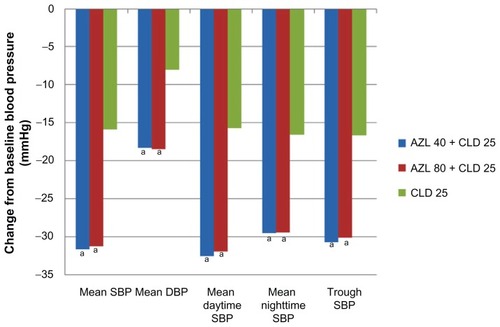
The combination of azilsartan–chlorthalidone is a novel combination agent because it is the first to combine an ARB with the long-acting diuretic, chlorthalidone. This fixed-dose combination (Edarbyclor®; Takeda Pharmaceutical, Osaka, Japan) was recently approved by the US Food and Drug Administration (FDA) on December 20, 2011 as 40/12.5 mg and 40/25 mg dosages. The first phase III trial to evaluate this combination was a randomized, double-blind, multicenter, 6-week treatment study comparing two different doses of azilsartan (40 mg or 80 mg) combined with 25 mg chlorthalidone to 25 mg chlorthalidone monotherapy in essential hypertension patients.Citation15 Baseline characteristics were not reported in the study. The results showed a statistical decrease in 24-hour mean SBP in both the azilsartan–chlorthalidone 40/25 mg and 80/25 mg arms (−31.72 and −31.3 mmHg, respectively; P < 0.001) when compared to chlorthalidone alone (−15.85 mmHg). Similar results were seen when comparing mean diastolic pressure, mean daytime systolic pressure, and mean nighttime systolic pressure (). Another secondary outcome examined the difference between each treatment arm regarding trough SBP, defined as systolic measurement recorded 22 to 24 hours after dosing. The difference in trough SBP was statistically greater for both azilsartan combination arms compared to the chlorthalidone arm (40 mg: −14.04 mmHg, 95% CI: −17.13–10.94; 80 mg: −13.48 mmHg, 95% CI: −16.58–10.37; P < 0.001, respectively). This trial displays that the combination of azilsartan–chlorthalidone is more effective at decreasing BP than chlorthalidone monotherapy. This study also demonstrates azilsartan–chlorthalidone’s long-acting benefit by assessing the continued decrease in BP 22 to 24 hours after dosing. This trial did not show any statistical difference in BP effects between the different dosing regimens of azilsartan 40 or 80 mg plus chlorthalidone.
Figure 1 Change from baseline blood pressure measures at 6 weeks.
Abbreviations: AZL, azilsartan; CLD, chlorthalidone; DBP, diastolic blood pressure; SBP, systolic blood pressure.
In another trial only published in abstract form, several different fixed-dose azilsartan–chlorthalidone combinations were compared to different doses of both of the monotherapies. This was an 8-week, randomized, double-blind trial in 1714 hypertensive patients with a primary endpoint of change in trough SBP.Citation16 Patients received either azilsartan placebo, 20, 40, or 80 mg and chlorthalidone placebo, 12.5, or 25 mg either as monotherapy or in combination. There was no double-placebo arm. Baseline characteristics provided were mean age 57 years, 47% men, and 20% black patients, with no differences reported among the treatment groups. All six of the combination therapy arms resulted in a significantly greater decrease in trough SBP when compared to their comparative monotherapy components (). As seen in , there was no greater benefit seen when comparing azilsartan–chlorthalidone 40/25 mg to 80/25 mg. There was a dose-dependent increase in therapy discontinuation and serum creatinine seen with azilsartan–chlorthalidone. Interestingly, it was noted in the study that the addition of azilsartan seemed to attenuate the chlorthalidone-related hypokalemia, but no data was provided in the abstract.Citation16
Table 1 Change in trough SBP of combinations when compared to the monotherapy components at week 8
Another published abstract examined the fixed-dose combination of azilsartan–chlorthalidone 20/12.5 mg titrated to 40/25 mg (AZL40-CLD25), 40/12.5 mg titrated to 80/25 mg (AZL80-CLD25), and olmesartan–hydrochlorothiazide 20/12.5 mg titrated to 40/25 mg (OLM40-HCTZ25) in 1085 patients with hypertension.Citation17 Patients were only titrated to the higher dose if their SBP was not at goal by week 4. This was an 8-week, randomized, double-blind trial. Baseline characteristics included 52% men, 26.7% blacks, 17.3% diabetics, a mean age of 56 years, and a BMI of 31.8 kg/m2. Both azilsartan–chlorthalidone combinations decreased clinical BP (seated trough BP) significantly more than olmesartan/hydrochlorothiazide (AZL40-CLD25 = −33.0 and AZL80-CLD25 = −34.1 vs OLM40-HCTZ25 = −26.9 mmHg; P < 0.001). A greater reduction in 24-hour mean SBP was also seen with both azilsartan–chlorthalidone combinations (−26.4 and −27.9 versus −20.7 mmHg; P < 0.001) Similarly, there was also less titration to higher doses in the azilsartan–chlorthalidone arms (38.4% and 34.7%) than in the olmesartan/hydrochlorothiazide group (51.7%). Adverse events causing either temporary or permanent discontinuation of therapy occurred in 6.2% and 9.5% of the azilsartan–chlorthalidone arms and 3.1% of the olmesartan–hydrochlorothiazide group (no P values were provided in the abstract).Citation17 These trials demonstrate a greater decrease in mean and trough SBP with the azilsartan–chlorthalidone combination.
Chlorthalidone versus hydrochlorothiazide
Current guidelines suggest thiazide-type diuretics as first-line treatment in hypertension and list several options, but do not distinguish preference between any agents.Citation2 Generally chlorthalidone and hydrochlorothiazide are viewed as interchangeable, but their effects on cardiovascular events have never been compared in a prospective trial. Most of the major clinical trials involving a thiazide-type diuretic have included either chlorthalidone or hydrochlorothiazide, yet hydrochlorothiazide is much more commonly prescribed and included in combination products.Citation18 The debate regarding possible differences in clinical benefit between the two agents has been an increasing topic of interest.
Though several differences between the drugs are known, it has not been determined which, or if any, could cause a change in patient outcomes. Structural differences exist between chlorthalidone and hydrochlorothiazide. Hydrochlorothiazide belongs to the drug class benzothiadiazines, simply known as thiazides. Drugs in this class all share a similar dual-ring structure to the thiazide diuretic prototype, chlorothiazide. Chlorthalidone is commonly referred to as a thiazide, but is more properly addressed as a thiazide-type diuretic. Though commonalities exist, chlorthalidone is molecularly unique in structure.Citation18 It is also known that chlorthalidone has a longer half-life (40 hours) compared to hydrochlorothiazide (6–9 hours). Some studies found that chlorthalidone concentrates in erythrocytes. The slow release of the drug from these erythrocytes is one explanation for chlorthalidone’s long half-life.Citation18 A review of available studies suggests that chlorthalidone 25 mg daily is roughly equivalent to hydrochlorothiazide 50 mg daily. This ratio has been found in other analyses as well.Citation18
Although no prospective, randomized trials assessing cardiovascular outcomes between the two have been conducted, evidence indicates that chlorthalidone may be superior to hydrochlorothiazide.Citation19 The Multiple Risk Factor Intervention Trial (MRFIT) was first to propose a clinical benefit with chlorthalidone compared to hydrochlorothiazide.Citation20 MRFIT was a large randomized primary prevention trial assessing the efficacy of a multifactor intervention program, which included dietary advice, smoking cessation counseling, and hypertension treatment compared to usual care (UC). Patients in the study could receive chlorthalidone or hydrochlorothiazide for hypertensive therapy. Approximately 5 years after randomization, the diuretic treatment protocol was changed to replace treatment with hydrochlorothiazide to chlorthalidone due to a 44.1% higher coronary heart disease (CHD) mortality in clinics predominantly prescribing hydrocholorothiazide compared to the UC group (P = 0.23), whereas the clinics predominantly prescribing chlorthalidone had a −58.2% (reduction) in CHD mortality compared to UC.Citation21 After the change was instituted, the rate of CHD mortality in the previously predominant hydrochlorothiazide clinics decreased to –7.9% when compared to UC.Citation21
A retrospective, cohort analysis of the MRFIT data was completed to evaluate the cardiovascular endpoints between patients taking the two different drugs.Citation22 The results showed a 21% lower risk of cardiovascular events in patients taking chlorthalidone compared to hydrochlorothiazide (hazard ratio [HR] 0.79; 95% CI: 0.68–0.92; P = 0.0016). Secondary outcome measures showed that patients prescribed chlorthalidone had a significantly lower SBP, total cholesterol, and low-density lipoprotein (LDL) compared with hydrochlorothiazide. In contrast to these optimistic results, the study also revealed significantly lower serum potassium and higher uric acid with chlorthalidone, but these did not seem to increase events in this group. A small, randomized, prospective study evaluated the efficacy of chlorthalidone to hydrochlorothiazide on ambulatory and trough BP.Citation23 This was a force titration study that compared chlorthalidone 25 mg daily to hydrochlorothiazide 50 mg daily, doses which have been shown in other literature to be relatively equivalent.Citation18 Though underpowered, the results revealed a trend towards greater reduction in mean SBP with chlorthalidone than with hydrochlorothiazide after 8 weeks (−12.4 versus −7.4 mmHg, P = 0.054). The difference was attributed to chlorthalidone’s greater effect on nighttime SBP (−13.5 versus −6.4 mmHg; P = 0.009). No significant differences in serum potassium were noted between the two groups (−0.5 versus −0.4 mEq/L; P = 0.76).Citation23 These trials support the idea that chlorthalidone is a more potent and longer lasting antihypertensive medication compared to hydrochlorothiazide. The MRFIT cohort analysis also supplies additional information regarding its effects on the metabolic profile, which may reveal the mechanism of cardiovascular benefit when compared to hydrochlorothiazide.
Combination therapy versus monotherapy
Initial combination therapy is recommended for many hypertensive patients requiring a >20 mmHg decrease in SBP to reach goal.Citation2 As seen in the ALLHAT trial, 63% of the study population was eventually on two or more drugs; and at the end of 5 years only 66% had achieved controlled BP.Citation8 Numerous newer trials have also shown high rates of patients requiring more than one drug for BP management; such as INVEST (80%), ASCOT-BPLA (78%), and LIFE (88%).Citation3,Citation24,Citation25 Since a large number of hypertensive patients requires more than one drug to control their hypertension, many trials have examined the benefit of combinations of antihypertensive medications as well as the benefit of combination therapy as initial treatment.
Suggested benefits of combination therapy compared to monotherapy include increased BP control by different mechanisms of action, lower dosing requirements, and fewer adverse events. In a study comparing losartan 50 mg plus barnidipine and losartan 100 mg monotherapy, no significant difference in amount of BP decrease was seen; however there was a higher rate of BP goal obtainment with the combination arm (82.1% versus 56.0%; P = 0.044).Citation26 Both treatment arms were well tolerated. The ACCELERATE trial, examined initial combination therapy (aliskiren–amlodipine) compared to initial monotherapy of each component followed by subsequent combination therapy in all arms.Citation27 The initial combination therapy had a greater decrease in SBP (−7.4 mmHg versus aliskiren and −5.5 mmHg versus amlodipine; P < 0.0001) than either monotherapy. After 24 weeks and all patients advanced to combination, the initial monotherapy groups’ SBPs decreased and there ceased to be a significant difference between the three arms. This trial also demonstrated balanced adverse events between all groups.Citation27
The prompter achievement of BP goal may also be a benefit of initial combination therapy opposed to initial monotherapy later progressing to combination therapy. Results from the VALUE trial, comparing a valsartan-based BP regimen to a amlodipine-based BP regimen, suggest that obtaining BP control within the first month of treatment is correlated to a lower rate of cardiovascular events.Citation28 The previously discussed ACCELERATE trial showed cessation of a significant difference between all combination therapy arms at the end of the study period (24 weeks), yet the SBP in the initial combination treatment arm remained lower than either of the progression groups.Citation27 A large, case-control study showed an 11% decrease in cardiovascular events when patients receiving antihypertensive medications were initiated on combination therapy instead of monotherapy.Citation29 An important finding in this study was that no difference in events was seen between continuous monotherapy and when monotherapy was switched to combination therapy (). The only scenario that showed significant decrease in cardiovascular events was the continuous combination therapy arm (odds ratio [OR] 0.74; 95% CI: 0.65–0.85). These data suggest that the best outcomes may be achieved when combination therapy is initially started in hypertensive patients.
Table 2 Effects of complete blood pressure therapy (initial + follow-up) on the risk of cardiovascular events
Another advantage to consider with combination therapy is the added benefit of fixed combination drugs. Fixed combination drugs have the ability to simplify complex medication regimens, increase adherence, and be more cost effective than their separate parts. Most patients require combination therapy for treatment of hypertension, and initiation with a fixed combination drug, such as azilsartan–chlorthalidone, can be clinically beneficial and more convenient.
Conclusion
Azilsartan–chlorthalidone is a unique combination drug and does have a place in the treatment of hypertension. As the use of ARBs is becoming more popular, azilsartan sets itself apart from the class by demonstrating greater SBP-lowering and longer-lasting hypertensive effects. The chlorthalidone component of the drug also makes this fixed combination distinctive. Chlorthalidone’s suggested superiority over hydrochlorothiazide could add to the clinical benefit of this regimen. Additionally a fixed-combination drug can offer the benefit of increasing the amount of patients initiated on combination therapy, leading to patients obtaining their BP goals faster. The effects of azilsartan–chlorthalidone fixed combination have yet to be tested in large, morbidity and mortality trials, but the current data are promising.
Disclosure
The authors report no conflicts of interest in this work.
References
- RogerVGoALloyd-JonesDHeart disease and stroke statistics- 2011 update: a report from the american heart associationCirculation2011123e18e20921160056
- ChobanianAVBakrisGLBlackHRThe seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 reportJAMA2003289192560257212748199
- DahlöfBDevereuxRBKjeldsenSELIFE Study GroupCardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet20023599311995100311937178
- CheungBMLauCPKumanaCRCombination therapy for hypertensionHong Kong Med J20039322422612777663
- GradmanAHCutlerNRDavisPJRobbinsJAWeissRJWoodBCCombined enalapril and felodipine extended release (ER) for systemic hypertension. Enalapril-felodipine ER factorial study groupAm J Cardiol19977944314359052345
- LangtryHDMcClellanKJValsartan/hydrochlorothiazideDrugs1999575751755 discussion 756–75810353300
- Drugs.comTop 200 drugs for 2010 Available from: http://www.drugs.com/top200_units.htmlAccessed December 13, 2011
- CushmanWFordCCutlerJSuccess and predictors of blood pressure control in diverse north american settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT)J Clin Hypertens20024393404
- GoncalvesAFontesMKlussmannESpinophilin regulates central angiotensin II-mediated effect on blood pressureJ Mol Med2011891219122921818582
- ChalmersJArimaHManagement of hypertension: evidence from the blood pressure lowering treatment trialists’ collaboration and from major clinical trialsPol Arch Med Wewn2009119637338019694219
- Edarbi(azilsartan medoxomil) [Package Insert]Deerfield, ILTakeda Pharmaceuticals America, Inc2011
- OjimaMIgataHTanakaMIn vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studiesJPET2011336801808
- BakrisGSicaDWeberMThe comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressureJ Clin Hypertens2011138188
- WhiteWWeberMSicaDEffects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertensionHypertension20115741342021282560
- Clinicaltrials.govEfficacy and safety of azilsartan medoxomil co- administered with chlorthalidone in participants with essential hypertension. Study NCT00591773 Available from: http://clinicaltrials.gov/ct2/show/NCT00591773?term=NCT00591773&rank=1Accessed December 13, 2011
- American Society of HypertensionTwenty-Sixth Annual Scientific Meeting and Exposition [featured posters, poster 182]J Clin Hypertens201113S1A12A12163 Available from: http://onlinelibrary.wiley.com//10.1111/j.1751-7176.201100459.x/abstractAccessed January 2011
- American Society of HypertensionTwenty-Sixth Annual Scientific Meeting and Exposition [featured posters, poster 162]J Clin Hypertens201113S1A12A163 Available from: http://onlinelibrary.wiley.com//10.1111/j.1751-7176.201100459.x/abstractAccessed January 2011
- CarterBErnstMCohenJHydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeabilityHypertension2004434914638621
- FlackJSicaDNesbittSChlorthalidone versus hydrochlorothiazide as a preferred diuretic: is there a verdict yet?Hypertension20115766566621383308
- Five-year findings of the hypertension detection and follow-up program. I. reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative GroupJAMA19792422325622571490882
- Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention trialCirculation1990825161616282225366
- DorschMGillespieBEriksonSChlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysisHypertension20115768969421383313
- ErnstMCarterBGoerdtCComparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressureHypertension20064735235816432050
- PepineCJHandbergEMCooper-DeHoffRMA calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. the international verapamil-trandolapril study (INVEST): a randomized controlled trialJAMA2003290212805281614657064
- DahlofBSeverPPoulterNASCOT InvestigatorsPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiaizde as required in the Ango-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet200536689590616154016
- ParatiGGiglioALonatiLEffectiveness of barnidipine 10 or 20 mg plus losartan 50-mg combination versus losartan 100-mg monotherapy in patients with essential hypertension not controlled by losartan 50-mg monotherapy: a 12-week, multicenter, randomized, open-label, parallel-group studyClin Ther2010321270128420678675
- BrownMMcInnesGPapstCAliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomized, parallel-group trialLancet201137731232021236483
- JuliusAKjeldsenSWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet20043632022203115207952
- CorraoGNicotraFParodiACardiovascular protection by initial and subsequent combination of antihypertensive drugs in daily life practiceHypertension201158456657221825231