Abstract
African Americans (AA) tend to have heightened systemic inflammation and endothelial dysfunction. Endothelial microparticles (EMP) are released from activated/apoptotic endothelial cells (EC) when stimulated by inflammation. The purpose of our study was to assess EMP responses to inflammatory cytokine (TNF-α) and antioxidant (superoxide dismutase, SOD) conditions in human umbilical vein ECs (HUVECs) obtained from AA and Caucasians. EMPs were measured under four conditions: control (basal), TNF-α, SOD, and TNF-α + SOD. Culture supernatant was collected for EMP analysis by flow cytometry and IL-6 assay by ELISA. IL-6 protein expression was assessed by Western blot. AA HUVECs had greater EMP levels under the TNF-α condition compared to the Caucasian HUVECs (6.8 ± 1.1 vs 4.7% ± 0.4%, P = 0.04). The EMP level increased by 89% from basal levels in the AA HUVECs under the TNF-α condition (P = 0.01) compared to an 8% increase in the Caucasian HUVECs (P = 0.70). Compared to the EMP level under the TNF-α condition, the EMP level in the AA HUVECs was lower under the SOD only condition (2.9% ± 0.3%, P = 0.005) and under the TNF-α + SOD condition (2.1% ± 0.4%, P = 0.001). Basal IL-6 concentrations were 56.1 ± 8.8 pg/mL/μg in the AA and 30.9 ± 14.9 pg/mL/μg in the Caucasian HUVECs (P = 0.17), while basal IL-6 protein expression was significantly greater (P < 0.05) in the AA HUVECs. These preliminary observational results suggest that AA HUVECs may be more susceptible to the injurious effects of the proinflammatory cytokine, TNF-α.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Endothelial dysfunction precedes hypertension and atherosclerosisCitation1,Citation2 and is a prognostic indicator of future cardiovascular events. In response to sensing hormonal, biochemical, and mechanical stimuli, the endothelium releases mediators of vascular function, initiates inflammatory processes, and influences homeostasis. In the US, African Americans experience higher mortality from cardiovascular disease (CVD) including hypertension compared to Caucasians or Mexican Americans.Citation3
An impaired endothelium is conventionally thought to be analogous to diminished nitric oxide (NO)-mediated vasodilation in response to endothelial NO synthase (eNOS) agonists such as acetylcholine or bradykinin. Currently, there is an expanded appreciation of endothelial dysfunction which includes a proinflammatory, pro-oxidant, and prothrombic EC state. Several studies have shown that African-Americans have impaired endothelial function as determined by flow-mediated dilation (FMD)Citation4–Citation6 and other measures of endothelial-dependent vasodilation.Citation7–Citation12
The mechanisms underlying racial disparities in CVD and endothelial dysfunction are multifactorial, but could be due, in part, to differences in endothelial cell (EC) responses to stimuli. However, a major problem with research on the racial disparity in endothelial dysfunction is that it is conducted at the organ or clinical levels and not at the cellular level. This lack of knowledge about the EC biology of African Americans severely impedes advances in finding optimal preventive and treatment strategies.
In response to proinflammatory cytokine stimulation, ECs undergo functional changes that include increased expression/production of IL-6 and adhesion molecules, and induction of procoagulant activity.Citation13–Citation15 African Americans have also been shown to have higher levels of inflammatory markers compared to Caucasians and this also may contribute to the greater CVD-related morbidity and mortality in this population.Citation16–Citation21 Studies have linked inflammation to EC activation, an early step in endothelial dysfunction, and loss of NO bioactivity.Citation22 In a cell culture study, Kalinowski et al showed that human umbilical ECs (HUVECs) from African Americans had reduced NO bioavailability compared to Caucasian HUVECs primarily due to increased superoxide (O2−) production and eNOS uncoupling.Citation23
Endothelial microparticles (EMP) are submicroscopic membranous particles that are released from activated or apoptotic parent EC when stimulated by proinflammatory cytokines, oxidative stress, or infectious agents.Citation24 They carry with them a subset of membrane proteins and phospholipids, or markers, of their parent EC including those induced by activation, apoptosis or oxidative stress. Recent evidence indicates that EMPs provide valuable information about the biological status of the endothelium. Previous studies have shown that EMPs expressing constitutive surface markers such as CD31 (platelet endothelial cell adhesion molecule; PECAM) are increased in injury and/or apoptosis.Citation25 Results from both clinical and laboratory studies demonstrate that higher EMP levels are related to diminished endothelium-dependent vasodilation. The level of EMP appears to correlate with the degree of endothelial dysfunction in patients with chronic renal failureCitation26 or coronary artery disease.Citation27 Thus, the level of EMPs is emerging as a novel direct marker of EC impairment mediated by activation and apoptosisCitation28,Citation29 and may provide new insight into the mechanisms of racial disparities in endothelial dysfunction. However, EMPs have never been used to assess potential racial differences in endothelial function.
Due to the hypothesized mechanisms leading to endothelial dysfunction and EC impairment in African Americans, the purpose of our study was to observe EMP responses to inflammatory cytokine (TNF-α) and antioxidant (superoxide dismutase, SOD) conditions in HUVECs obtained from African Americans and Caucasians.
Methods
Human umbilical vein endothelial cells
HUVECs were purchased from Lonza Inc (Walkersville, MD), who harvested ECs from umbilical cord donors (AA, n = 3; Caucasian, n = 3). Cryopreserved HUVECs were then shipped by Lonza as frozen primaries. The HUVECs were cultured in parallel, in EGM medium supplemented with 2% fetal bovine serum (FBS) and growth factors (Lonza, Inc) at 37°C in a 95% air −5% CO2 atmosphere, following methods by Lonza. All Lonza HUVECs are characterized by morphological observation through serial passaging, positive test for von Willebrand Factor VIII and acetylated low density lipoprotein (LDL) uptake, and negative test for α-smooth muscle actin.
Experimental procedures
Human recombinant TNF-α was purchased from Sigma (St Louis, MO) and SOD was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Parallel HUVEC cultures were tested under four separate conditions: Control, TNF-α (100 U/mL) for 4 hours, SOD (100 U/mL) for 24 hours, and TNF-α + SOD. 100 U/mL of TNF-α was selected because it is a typical level used to stimulate ECs and falls approximately midway between the highest and lowest levels used to stimulate ECs in similar studies.Citation30–Citation35 Culture media samples (4 mL) obtained from 106 ECs were collected and immediately frozen at −80°C for subsequent EMP analysis.Citation25,Citation36 Remaining culture supernatant was collected for IL-6 assay.
Cell lysate was collected by cellular fractionation for total SOD activity measurements. Briefly, cells were washed once with ice-cold phosphate buffer solution, and resuspended in 2 mL cold 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (Lonza) using a rubber scraper. Cells were centrifuged at 600g for 10 minutes at 4°C. The cell pellet was resuspended in 500 μL cold HEPES buffer and then transferred to a Teflon glass homogenizer. The cell solution was homogenized at 1600 rpm for 30 strokes while on ice, and then immediately centrifuged at 1500g for 5 minutes at 4°C. Protein concentration was measured using the Bradford method.
For all procedures, AA and Caucasian HUVECs at passage 4 were treated identically. For assay, experimental samples were tested in duplicate and control samples of culture media were tested along with the cell samples in order to eliminate potential interference from culture media in measurements. Absorbance was read using a SpectraMax Microplate Reader (Molecular Devices, Sunnyvale, CA).
EMP immunolabeling
Microparticles express several different cell surface markers which can be quantified. The preferred method is the 2-color combination of phycoerythrin (PE)-labeled anti-CD31 with fluorescein isothiocyanate (FITC)-labeled anti-CD42. Because CD31 is also found on platelets, platelet-specific CD42 allows counting the platelet microparticles population (CD31+, CD42+) distinct from EMP (CD31+, CD42−), giving both counts in a single run. This pair of markers has the advantage of being very bright and therefore sensitive.Citation29 EMP samples were prepared as previously described.Citation37,Citation38
To remove unwanted cellular fragments, thawed culture media (1.5 mL) was centrifuged for 5 minutes at 4300g (20°C). Supernatant was removed and transferred into a new tube and centrifuged for 90 minutes at 3152g (20°C). 100 μL of the supernatant was transferred to a new tube and incubated with 20 μL of anti-human CD31+ (PE) and 20 μL of anti CD42 (FITC) in the dark at room temperature (30 minutes), then fixed by adding 93 μL of 10% formaldehyde. The mixture was protected from light and incubated while being gently mixed for 20 minutes using a shaker. Samples were diluted in 500 μL of double-filtered (0.22 μm) PBS for a total sample volume of 733 μL. Two additional tubes were prepared to serve as a negative control and as a calibration. For the negative control tube, 733 μL of PBS was added to one tube. To prepare the calibrator sample, two drops of 0.9 μm standard precision NIST traceable polystyrene particle beads (Polysciences Inc, Warrington PA) were added to PBS according to the manufacturer’s instructions. All samples were then immediately analyzed by flow cytometry.
Flow cytometry
Labeled EMP produced by 106 ECs were analyzed using an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with BD FACSDiva software (v 1.2.6; BD Biosciences). There is no consensus on the threshold level setting which determines the smallest size microparticle analyzed, therefore we set the threshold levels based on the number of background events per second when double-filtered PBS was passed through the flow cytometer as reported by Orozco and Lewis.Citation39 The upper limit of gate was determined by 0.9 μm standard beads. CD31+/ C42− events included in this gate were identified in forward and side scatter intensity dot representation and plotted on 2-color fluorescence histograms and were considered EMP.Citation5,Citation40,Citation41–Citation43 From each 50 μL sample, the percentage of EMP from 50,000 total events was recorded.Citation40,Citation41 Before every run, the machine was kept running until the background events fell to baseline levels. The final EMP values were then expressed as % total eventsCitation40 normalized to protein content (μg/μL). The inter- and intra-assay CVs were 8% and 6%, respectively.
Western blot for IL-6 protein
HUVECs were washed twice in ice-cold Hanks buffered saline solution and lysed in Radio-Immunoprecipitation Assay Buffer with Roche protease inhibitor (RIPA-Pi). Phenylmethylsulfonyl fluoride protease inhibitor was also added to the RIPA-Pi to eliminate interference. At confluence, cells were collected, centrifuged at 16,000g for 10 minutes at 4°C. Quantification of protein content was measured by Bradford assay. 20 μg of protein was separated by electrophoresis through 10% SDS-polyacrylamide gel. Proteins were then transferred to nitrocellulose filter membranes. Membranes were blocked with non-fat dry milk in Tris-buffered saline and incubated overnight with primary antibodies at 4°C. Subsequently, the membranes were washed and then incubated with secondary antibody conjugated with horseradish peroxidase. Immunoreactive proteins were detected by chemiluminescence with Thermo Scientific SuperSignal substrate systems (Pierce Biotechnology). Anti-IL-6 (Abcam, Inc, MA). Actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the internal control. Band densitometry analysis was performed using National Institutes of Health ImageJ software.
Assay for superoxide dismutase (SOD) activity
Total SOD activity was measured in order to have an assessment of the cell’s potential for superoxide quenching. Total SOD activity in cell lysate samples by assay (Cayman Chemical, Ann Arbor, MI), as previously reported.Citation42 Concentrations were normalized to protein content (μg/μL). Inter-assay and intra-assay CVs were 5.9% and 12.4%, respectively.
Assay for IL-6
IL-6 proteins released into media were measured in cell culture supernatants by ELISA (Thermo Scientific-Pierce Biotechnology, Rockford, IL), according to manufacturer’s instructions. Concentrations were normalized to protein content (μg/μL). The intra- and inter-assay CVs were 9% and 10%, respectively.
Statistical analysis
All values were presented as means ± SE. Two-way (ethnicity by condition) and one-way (within ethnicity and within experimental condition) ANOVA followed by Fisher’s protected least significant difference (PLSD) were performed for statistical comparisons. An independent t-test was used to compare band densities for IL-6 expression between AA and Caucasian HUVECs. A P-value <0.05 was considered statistically significant.
Results
HUVEC morphology under all experimental conditions
Representative images of AA and Caucasian HUVECs at confluency under all experimental conditions are shown in . HUVECs displayed a cobblestone-like shape. There were no morphological differences between the AA and Caucasian HUVECs.
EMP levels under basal conditions
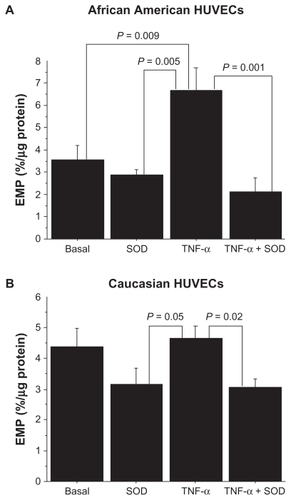
shows comparisons of EMP levels between AA and Caucasian HUVECs under all experimental conditions. There were no significant differences in EMP levels under basal conditions between AA (3.6% ± 0.7%) and Caucasian (4.4% ± 0.6%) HUVECs (P = 0.38).
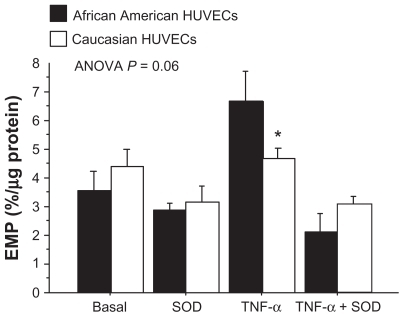
Figure 2 Comparison of EMP levels between the African American and Caucasian HUVEC groups under each of the experimental conditions.
Abbreviations: HUVEC, human umbilical vein endothelial cells; SOD, superoxide dismutase, EMP, endothelial microparticles; ANOVA, analysis of variance.
Effects of TNF-α stimulation and SOD on EMP generation
TNF-α is a potent inducer of inflammatory reactions and oxidative stress in EC. When the levels of EMPs were analyzed by experimental condition (AA and Caucasian HUVEC responses combined), TNF-α significantly increased EMP from the basal level of 3.9% ± 0.4% to 5.7% ± 0.6% (P = 0.01). Compared to the basal EMP level, SOD decreased the EMP level to 3.0% ± 0.5% (P = 0.19). Compared to the TNF-α condition, the addition of SOD significantly reduced the EMP level to 3.0 ± 0.3 (P < 0.0001). The EMP levels under the SOD only condition (3.0 ± 0.3) and the TNF-α + SOD condition (2.6 ± 0.3) were not different.
The interactive effect of HUVEC race and experimental condition was borderline significant (P = 0.06, ). Post hoc analyses showed that AA HUVECs had a significantly greater level of EMP under the TNF-α condition compared to the Caucasian HUVECs (6.8 ± 1.1 vs 4.7% ± 0.4%, P = 0.04). Within the AA HUVECs, the EMP level increased by 89% from a basal level of 3.5 ± 0.7 to 6.8% ± 1.1% under the TNF-α condition (P = 0.01) (). Compared to the EMP level under the TNF-α condition, the EMP level was significantly lower under the SOD only condition (2.9% ± 0.3%, P = 0.005) and under the TNF-α + SOD condition (2.1% ± 0.4%, P = 0.001). Within the Caucasian HUVECs, there was only an 8% increase in the EMP level from the basal condition to the TNF-α condition (P = 0.70) (). The only significant difference in EMP levels in the Caucasian HUVECs was between the TNF-α (4.7% ± 0.4%) and the TNF-α + SOD condition (3.0% ± 0.3%, P = 0.02). The difference in the level of EMP between the TNF-α condition (4.7 ± 0.4) and the SOD condition (3.2 ± 1.0) was borderline significant (P = 0.05).
Total SOD activity under basal and TNF-α conditions
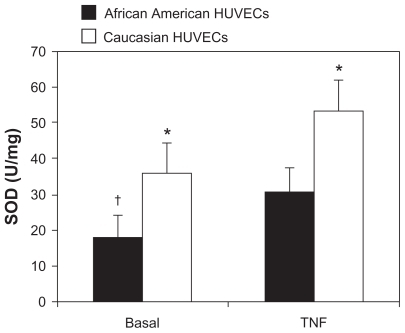
Total SOD activity in AA and Caucasian HUVECs under basal and TNF-α conditions are shown in . Basal SOD activity levels were (17.2 ± 12.0 U/mg) in the AA HUVECs and (35.8 ± 17.2 U/mg) the Caucasian HUVECs (P = 0.03). Compared to the basal levels, TNF-α stimulation caused a significant increase in SOD activity in the AA HUVECs (P = 0.04), while no significant change occurred with TNF-α stimulation in Caucasian HUVECs (P = 0.09).
Figure 4 Total SOD activity under basal and TNF-α conditions in African American and Caucasian HUVECs.
Abbreviations: HUVEC, human umbilical vein endothelial cells; SOD, superoxide dismutase; TNF-α, tissue necrosis factor alpha.
IL-6 concentrations under basal conditions
IL-6 concentrations in AA and Caucasian HUVECs under all experimental conditions are shown in . Basal IL-6 concentrations were (56.1 ± 8.8 pg/mL/μg) in the AA HUVECs and (30.9 ± 14.9 pg/mL/μg) the Caucasian HUVECs (P = 0.17). Given the nearly two-fold higher basal IL-6 concentration in the AA HUVECs, we wanted to confirm whether IL-6 protein expression was greater. Representative Western blots for IL-6 protein under basal conditions are shown in . Basal IL-6 protein expression was significantly greater in the AA HUVECs compared to the Caucasian HUVECs. This confirmed the greater basal IL-6 concentrations in the culture media of AA HUVECs.
Figure 5 IL-6 concentrations under each of the experimental conditions in African American and Caucasian HUVECs.
Abbreviations: HUVEC, human umbilical vein endothelial cells; SOD, superoxide dismutase; TNF-α, tissue necrosis factor alpha; ANOVA, analysis of variance.
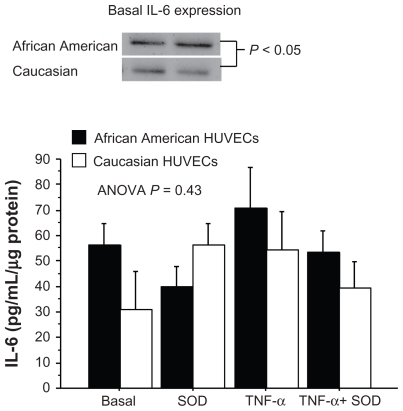
Effects of TNF-α stimulation and SOD on IL-6 concentrations and protein expression
The binding of TNF-α to its receptor on EC induces down-stream gene expression of IL-6, which can influence EC function and cause increased adhesion and chemokine expression and apoptosis. When the levels of IL-6 were analyzed by experimental condition (AA and Caucasian HUVEC responses combined), IL-6 concentration increased from 44.9 ± 8.8 pg/mL/μg protein under basal conditions to 62.6 ± 10.6 pg/mL/μg protein under TNF-α stimulation, but the difference was not statistically significant (P = 0.14). There were no significant differences in IL-6 concentrations between any of the other conditions. The interactive effect of HUVEC race and condition was not statistically significant (P = 0.43) (). Within the AA HUVECs, the IL-6 concentration under the SOD condition tended to lower compared to the TNF-α condition (40.0 ± 8.0 vs 70.8 ± 15.7 pg/mL/μg, P = 0.07) (). Within the Caucasian HUVECs, there were no significant differences in IL-6 concentrations between any of the experimental conditions ().
Discussion
The most important finding of this preliminary observational study was that for the first time we showed that TNF-α-stimulated EMP generation was different between African American and Caucasian HUVECs. AA HUVECs demonstrated an 89% increase in EMP generation as a result of TNF-α stimulation compared to an 8% increase in EMP generation in the Caucasian HUVECS. This finding is important because it is generally thought that for any given level of BP, African Americans have a greater degree of endothelial dysfunction compared to Caucasians and because the measurement of the EMP expression of marker CD31 is linked to EC activation/apoptosis. Therefore, these observations suggest that AA HUVECs have greater EC damage from TNF-α stimulation.
Microparticles are not released randomly into the circulation. Citation43 Activation or injury of the endothelium leads to various inflammatory-related processes including the release of microparticles. Combes et al were the first to demonstrate that HUVECs release EMP when stimulated with TNF-α.Citation24 Other proinflammatory, prothrombotic, proapoptotic, or oxidative substances also induce the release of EMP.Citation28,Citation44 EMP express adhesion molecules specific for mature ECs such as CD54 (ICAM-1), CD62E (E-selectin), CD62P (P-selectin), or CD31 (PECAM).Citation28,Citation29 CD31 is also expressed by platelet-derived microparticles, therefore EMP specificity is ensured by the CD31+/CD41− phenotype with CD41 being the platelet integrin GPIIbIIIa.Citation28,Citation29 Studies have demonstrated that EMP can be used as a novel biomarker of endothelial injury that directly reflects the homeostatic state of the endothelium.Citation29,Citation45–Citation49 To our knowledge, no study has used EMPs as an index of endothelial cell status to assess potential racial differences in EC function.
TNF-α stimulation caused a significant 89% increase in EMP level in AA HUVECs and a nonsignificant 8% increase in EMP level in the Caucasian HUVECs. The EMP levels under TNF-α stimulation were significantly different between the HUVEC groups. Based on these results, it appears that AA HUVECs were more susceptible to the damaging effects of TNF-α. Plasma TNF-α concentrations have been associated with early atherosclerosis.Citation50 Moreover, there are reports that African Americans have higher systemic levels of inflammatory biomarkersCitation16–Citation21 which suggests that at least a portion of their endothelial dysfunction may be secondary to cytokine-induced endothelial activation/ apoptosis.
The endothelium has several important functions related to cardiovascular health. Functional changes of the activated endothelium include disturbance of the regulation of vessel tone and the maintenance of a vascular environment that favors coagulation, inflammation, and atherosclerosis. EMP levels are increased in hypertension and a significant correlation was shown between EMP and blood pressure.Citation46 It is thought that African Americans have lower endothelial NO bioavailability than Caucasians based on findings of attenuated flow-mediated arterial vasodilatation.Citation4–Citation6 Other investigations have also documented lower endothelial-dependent vasodilation in African Americans.Citation8–Citation11,Citation51,Citation52
TNF-α can induce endothelial dysfunction through both inflammatory and oxidative stress mechanisms. TNF-α activates the p38 MAPK pathwayCitation53 and the transcription factor, NF-κB, which regulates the expression of genes involved in inflammation, oxidative stress, and endothelial dysfunction.Citation54–Citation56 Inflammatory cytokines induce the expression of adhesion molecules and chemoattractants by ECs. Gertzberg et al and Yamagishi et al showed that in response to TNF-α, EC NADPH oxidase activity increased, leading to increased O2− production.Citation57,Citation58 Under normal physiological conditions, the dismutation of O2− by SOD yields H2O2. In the case of heightened NADPH oxidase activity and O2− overproduction, O2− reacts with NO to produce ONOO− thus reducing NO bioavailability.Citation59 In order to assess the potential for O2− dismutation in the present study, total SOD activity was measured in cell lysate from both basal and TNF-α stimulated AA and Caucasian HUVECs. We observed significantly higher SOD activity in the Caucasian HUVECs under both conditions. The AA HUVECs had a 79% increase in SOD activity compared to a 50% increase in Caucasian HUVECs. This could suggest a higher oxidative stress response to the TNF-α stimulation which is also evidenced by the heightened EMP production with TNF-α stimulation in the African American HUVECs.
In the present study, we also measured EMP levels after incubation of the HUVECs with SOD alone and after incubation with TNF-α + SOD. The response to SOD alone was similar in both groups of HUVECs. Though both the AA and Caucasian HUVECs showed a significant decrease in EMP levels when the TNF-α + SOD condition was compared to the TNF-α alone condition, the AA HUVECs showed a 68% decrease in EMP levels whereas the Caucasian HUVECs showed a 35% decrease in EMP level. These results suggest that TNF-α-induced oxidative stress played a greater role in EMP generation in AA compared to Caucasian HUVECs because the effect of SOD was nearly twice that of the Caucasian HUVECs.
The proinflammatory cytokine TNF-α also induces EC gene expression and production of IL-6 via the NF-κB pathway. Citation58 IL-6 is a secondary inflammatory cytokine that mediates the regulation of the acute-phase response to injury and infection. IL-6 induces the increase of plasma concentrations of fibrinogen, PAI-1, and CRP. Evidence indicates that the elevation of CRP predicts future cardiovascular events.Citation60
In the present study, we observed that the AA HUVECs had nearly a two-fold greater basal IL-6 concentration compared to the Caucasian HUVECs. Western blot analysis of basal IL-6 expression showed that the AA HUVECs had significantly greater IL-6 protein expression. The IL-6 response to SOD was also different between the HUVEC groups. SOD decreased IL-6 concentration in the AA HUVECs but increased IL-6 concentration in the Caucasian HUVECs. The reasons for the disparate IL-6 response to SOD are not clear. Incubating the HUVECs with TNF-α + SOD similarly decreased IL-6 concentrations compared to incubation with TNF-α alone. These changes were similar in both HUVEC groups. In healthy men, elevated levels of IL-6 were associated with increased future CVD risk, endothelial dysfunction, and hypertension.Citation61–Citation63 Epidemiologic data support the existence of an association between different inflammatory markers and high blood pressure.Citation64,Citation65
The normal functional integrity of ECs is maintained by continuous cell regeneration and the incorporation of endothelial progenitor cells. Under these normal quiescent conditions, EC activation tends to be localized, low-grade, and reversible. The level of circulating EMP is very low when ECs are quiescent.Citation66 The exposure of the endothelium to cytokines causes vascular inflammation by inducing EC activation and apoptosis. There is a tendency for African Americans to have heightened systemic inflammation as measured by various inflammatory biomarkers.Citation16–Citation21 Long-term exposure of the endothelium to proinflammatory cytokines leads to increased oxidative stress, inflammation, and apoptosis, and promotes leukocyte infiltration and thrombosis. All of these changes to ECs cause vascular dysfunction and support an EC environment favoring hypertension and atherosclerosis.
The aim of this observational study was to assess ECs that have not been exposed to chronic diseases in order to assess their fundamental response to stimuli. The HUVECs were purchased from Lonza Inc in which only the racial origin is known, due to The Health Insurance Portability and Accountability Act, which protects the privacy of individually identifiable health information. HUVECs are derived from fetal tissue which makes them better suited for measuring fundamental responses to stimuli because adult ECs may have been subjected to prolonged exposure to cytokines, hormones, and other stimuli, potentially resulting in an altered phenotype.Citation67 An EC obtained from a newborn has already developed to the point where it is suitable for its particular function. One study found that out of the potentially confounding variables such as the interval between delivery and cell isolation, sex and weight of newborn, type of delivery, age, smoking habits, diabetes, or hypertension of the mother, only the short time between delivery and cell isolation and the mother’s smoking habits lead to alterations in cell culture success rateCitation68 and morphology.Citation69 Thus while we do not know the specific characteristics of the mothers, we believe that the HUVECs were relatively unaffected by the mother.
There are several limitations of the study that are worth noting. First, we did not directly measure the levels of the superoxide radical. The measurement of total SOD activity provides insight into the cell’s potential for superoxide quenching. Second, we only incubated the cells with TNF-α for 4 hours. This was done in order to avoid significant apoptosis. Lastly, the present observational study cannot provide insight into potential mechanisms for heightened EC susceptibility to TNF-α in AA HUVECs but it may lie in differences in TNF-α receptor sensitivity and/or post-receptor signaling mechanisms.
The preliminary results of our study suggest that AA HUVECs are more susceptible to the injurious effects of the proinflammatory cytokine, TNF-α. In addition, the results suggest that TNF-α-induced oxidative stress plays a greater role in EMP generation in AA compared to Caucasian HUVECs. If African Americans tend to have a subclinical heightened state of chronic systemic low-grade inflammation, then the manner in which their ECs respond to inflammatory cytokines could make them more susceptible to long-term endothelial dysfunction and its sequelae such as hypertension and atherosclerosis. This warrants the importance of aggressive preventive lifestyle modification strategies in African Americans in early subclinical stages of hypertension development to reduce the inflammation and oxidative stress burdens.
Disclosure
The authors report no conflicts of interest in this work.
References
- TaddeiSVirdisAMatteiPGhiadoniLSudanoISalvettiADefective L-arginine-nitric oxide pathway in offspring of essential hypertensive patientsCirculation1996946129813038822983
- RossRAtherosclerosis – an inflammatory diseaseN Engl J Med199934021151269887164
- Lloyd-JonesDAdamsRJBrownTMHeart disease and stroke statistics – 2010 update: a report from the American Heart AssociationCirculation20101217e46e21520019324
- PerregauxDChaudhuriARaoSBrachial vascular reactivity in blacksHypertension200036586687111082158
- CampiaUChoucairWKBryantMBWaclawiwMACardilloCPanzaJAReduced endothelium-dependent and -independent dilation of conductance arteries in African AmericansJ Am Coll Cardiol200240475476012204507
- JonesDSAndrawisNSAbernethyDRImpaired endothelial-dependent forearm vascular relaxation in black AmericansClin Pharmacol Ther199965440841210223778
- CardilloCKilcoyneCMCannonRO3rdPanzaJARacial differences in nitric oxide-mediated vasodilator response to mental stress in the forearm circulationHypertension1998316123512399622135
- LangCCSteinCMBrownRMAttenuation of isoproterenol-mediated vasodilatation in blacksN Engl J Med199533331551607791817
- RosenbaumDAPretoriusMGainerJVEthnicity affects vasodilation, but not endothelial tissue plasminogen activator release, in response to bradykininArterioscler Thromb Vasc Biol20022261023102812067915
- GainerJVSteinCMNealTVaughanDEBrownNJInteractive effect of ethnicity and ACE insertion/deletion polymorphism on vascular reactivityHypertension2001371465111208755
- KahnDFDuffySJTomasianDEffects of black race on forearm resistance vessel functionHypertension200240219520112154113
- SteinCMLangCCNelsonRBrownMWoodAJVasodilation in black Americans: attenuated nitric oxide-mediated responsesClin Pharmacol Ther19976244364439357395
- Vanden BergheWVermeulenLDe WildeGDe BosscherKBooneEHaegemanGSignal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6Biochem Pharmacol20006081185119511007957
- MackayFLoetscherHStueberDGehrGLesslauerWTumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55J Exp Med19931775127712868386742
- NawrothPPSternDMModulation of endothelial cell hemostatic properties by tumor necrosis factorJ Exp Med198616337407453753996
- LampertRIckovicsJHorwitzRLeeFDepressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomesAm Heart J2005150115316016084163
- HarenMTMalmstromTKMillerDKHigher C-reactive protein and soluble tumor necrosis factor receptor levels are associated with poor physical function and disability: a cross-sectional analysis of a cohort of late middle-aged African AmericansJ Gerontol A Biol Sci Med Sci201065327428119812256
- FoxERBenjaminEJSarpongDFEpidemiology, heritability, and genetic linkage of C-reactive protein in African Americans (from the Jackson Heart Study)Am J Cardiol2008102783584118805107
- DoumateyAPLashleyKSHuangHRelationships among obesity, inflammation, and insulin resistance in African Americans and West AfricansObesity (Silver Spring)201018359860319798069
- SlopenNLewisTTGruenewaldTLEarly life adversity and inflammation in African Americans and whites in the midlife in the United States surveyPsychosom Med201072769470120595419
- LaiSFishmanEKLaiHPannuHDetrickBSerum IL-6 levels are associated with significant coronary stenosis in cardiovascularly asymptomatic inner-city black adults in the USInflamm Res2009581152119130178
- HuangALVitaJAEffects of systemic inflammation on endothelium-dependent vasodilationTrends Cardiovasc Med2006161152016387625
- KalinowskiLDobruckiITMalinskiTRace-specific differences in endothelial function: predisposition of African Americans to vascular diseasesCirculation2004109212511251715159296
- CombesVSimonACGrauGEIn vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulantJ Clin Invest199910419310210393703
- FengBChenYLuoYChenMLiXNiYCirculating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitusAtherosclerosis2010208126426919674745
- AmabileNGuerinAPLeroyerACirculating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failureJ Am Soc Nephrol200516113381338816192427
- WernerNWassmannSAhlersPKosiolSNickenigGCirculating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery diseaseArterioscler Thromb Vasc Biol200626111211616239600
- ChironiGNBoulangerCMSimonADignat-GeorgeFFreyssinetJMTedguiAEndothelial microparticles in diseasesCell Tissue Res2009335114315118989704
- HorstmanLLJyWJimenezJJAhnYSEndothelial microparticles as markers of endothelial dysfunctionFront Biosci200491118113514977533
- LaiPFMohamedFMongeJCStewartDJDownregulation of eNOS mRNA expression by TNF-alpha: identification and functional characterization of RNA-protein interactions in the 3′UTRCardiovasc Res200359116016812829187
- LiJMMullenAMYunSEssential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alphaCirc Res200290214315011834706
- LiJMFanLMChristieMRShahAMAcute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4Mol Cell Biol20052562320233015743827
- KataokaHMurakamiRNumaguchiYOkumuraKMuroharaTAngiotensin II type 1 receptor blockers prevent tumor necrosis factor-alpha-mediated endothelial nitric oxide synthase reduction and superoxide production in human umbilical vein endothelial cellsEur J Pharmacol201063613364120361959
- FranzenBDuvefeltKJonssonCGene and protein expression profiling of human cerebral endothelial cells activated with tumor necrosis factor-alphaBrain Res Mol Brain Res2003115213014612877984
- De KeulenaerGWUshio-FukaiMYinQConvergence of redox-sensitive and mitogen-activated protein kinase signaling pathways in tumor necrosis factor-alpha-mediated monocyte chemoattractant protein-1 induction in vascular smooth muscle cellsArterioscler Thromb Vasc Biol200020238539110669634
- HeissCAmabileNLeeACBrief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide productionJ Am Coll Cardiol200851181760177118452782
- PiccinAMurphyWGSmithOPCirculating microparticles: pathophysiology and clinical implicationsBlood Rev200721315717117118501
- WangJMWangYHuangJYC-Reactive protein-induced endothelial microparticle generation in HUVECs is related to BH4-dependent NO formationJ Vasc Res200744324124817351328
- OrozcoAFLewisDEFlow cytometric analysis of circulating microparticles in plasmaCytometry A201077650251420235276
- MaceyMGEnniksNBevanSFlow cytometric analysis of microparticle phenotype and their role in thrombin generationCytometry B Clin Cytom2011801576320632415
- GeldermanMPSimakJFlow cytometric analysis of cell membrane microparticlesMethods Mol Biol2008484799318592174
- FeairhellerDLSturgeonKMDiazKMPrehypertensive African-American women have preserved nitric oxide and renal function but high cardiovascular riskKidney Blood Press Res201033428229020628261
- BlannAShantsilaEShantsilaAMicroparticles and arterial diseaseSemin Thromb Hemost200935548849619739039
- LeroyerASAnfossoFLacroixREndothelial-derived microparticles: biological conveyors at the crossroad of inflammation, thrombosis and angiogenesisThromb Haemost2010104345646320664896
- PrestonRAJyWJimenezJJEffects of severe hypertension on endothelial and platelet microparticlesHypertension200341221121712574084
- KogaHSugiyamaSKugiyamaKElevated levels of VEcadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery diseaseJ Am Coll Cardiol200545101622163015893178
- Bernal-MizrachiLJyWJimenezJJHigh levels of circulating endothelial microparticles in patients with acute coronary syndromesAm Heart J2003145696297012796750
- HeloireFWeillBWeberSBatteuxFAggregates of endothelial microparticles and platelets circulate in peripheral blood. Variations during stable coronary disease and acute myocardial infarctionThromb Res2003110417318014512078
- JimenezJJJyWMauroLMHorstmanLLAhnYSElevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active diseaseBr J Haematol20011121819011167788
- SkoogTDichtlWBoquistSPlasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged menEur Heart J200223537638311846495
- CardilloCKilcoyneCMCannonROPanzaJAAttenuation of cyclic nucleotide-mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator functionCirculation199999190959884384
- SteinbergHOBayazeedBHookGJohnsonACroninJBaronADEndothelial dysfunction is associated with cholesterol levels in the high normal rangeCirculation19979610328732939396418
- CurtisAMWilkinsonPFGuiMGalesTLHuEEdelbergJMp38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticlesJ Thromb Haemost20097470170919192109
- dela PazNGSimeonidisSLeoCRoseDWCollinsTRegulation of NF-kappaB-dependent gene expression by the POU domain transcription factor Oct-1J Biol Chem2007282118424843417192276
- RimbachGValacchiGCanaliRVirgiliFMacrophages stimulated with IFN-gamma activate NF-kappa B and induce MCP-1 gene expression in primary human endothelial cellsMol Cell Biol Res Commun20003423824210891398
- KumarATakadaYBoriekAMAggarwalBBNuclear factor-kappaB: its role in health and diseaseJ Mol Med200482743444815175863
- GertzbergNNeumannPRizzoVJohnsonANAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alphaAm J Physiol Lung Cell Mol Physiol20042861L374812807699
- YamagishiSInagakiYNakamuraKPigment epithelium-derived factor inhibits TNF-alpha-induced interleukin-6 expression in endothelial cells by suppressing NADPH oxidase-mediated reactive oxygen species generationJ Mol Cell Cardiol200437249750615276019
- Darley-UsmarVWhiteRDisruption of vascular signalling by the reaction of nitric oxide with superoxide: implications for cardiovascular diseaseExp Physiol19978223053169129945
- RidkerPMRifaiNStampferMJHennekensCHPlasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy menCirculation2000101151767177210769275
- RidkerPMRifaiNPfefferMSacksFLepageSBraunwaldEElevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarctionCirculation2000101182149215310801754
- BrevettiGSilvestroASchianoVChiarielloMEndothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure indexCirculation2003108172093209814530195
- RifaiNJoubranRYuHAsmiMJoumaMInflammatory markers in men with angiographically documented coronary heart diseaseClin Chem199945111967197310545067
- ChaeCULeeRTRifaiNRidkerPMBlood pressure and inflammation in apparently healthy menHypertension200138339940311566912
- SessoHDBuringJERifaiNBlakeGJGazianoJMRidkerPMC-reactive protein and the risk of developing hypertensionJAMA2003290222945295114665655
- LeroyerASIsobeHLesecheGCellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaquesJ Am Coll Cardiol200749777277717306706
- TanPHChanCXueSAPhenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cellsAtherosclerosis2004173217118315064090
- BalconiGPietraABusaccaMde GaetanoGDejanaESuccess rate of primary human endothelial cell culture from umbilical cords is influenced by maternal and fetal factors and interval from deliveryIn Vitro198319118078106654377
- AsmussenIKjeldsenKIntimal ultrastructure of human umbilical arteries. Observations on arteries from newborn children of smoking and nonsmoking mothersCirc Res19753655795891122569