Abstract
Vascular calcification (VC) is a life-threatening state in chronic kidney disease (CKD). High cardiovascular mortality and morbidity of CKD cases may root from medial VC promoted by hyperphosphatemia. Vascular calcification is an active, highly regulated, and complex biological process that is mediated by genetics, epigenetics, dysregulated form of matrix mineral metabolism, hormones, and the activation of cellular signaling pathways. Moreover, gut microbiome as a source of uremic toxins (eg, phosphate, advanced glycation end products and indoxyl-sulfate) can be regarded as a potential contributor to VC in CKD. Here, an update on different cellular and molecular processes involved in VC in CKD is discussed to elucidate the probable therapeutic pathways in the future.
Introduction
The growing burden of cardiovascular disease (CVD) in chronic kidney disease (CKD) patients and general population is presumably, at least in part, due to vascular calcification (VC).Citation1 VC is an active, complex, and extremely regulated procedure that involves cell-mediated processes and a complex interaction between the inhibitor and promoter factors.Citation2,Citation3 VC is a result of the pathological deposition of calcium phosphate mineral in soft tissues that decreases the blood vessels’ elasticity and elevates blood pressure.
Disturbed mineral homeostasis due to an impaired renal function, the uremic milieu and CKD provides a storm of risk factors for VC and the development of CVD. Dysregulated mineral metabolism and the elevated levels of circulating calcium (Ca) and phosphate (Pi) are key factors for the initiation and progression of VC in CKD since major resident cells in the media layer of blood vessels, vascular smooth muscle cells (VSMCs), are sensitive to these factors.Citation4,Citation5 VSMCs can undergo trans-differentiation to osteoblast-like cells and extrude matrix vesicles (MVs) that contain proteins similar to osteoblastic vesicles. When these proteins are secreted by VSMCs, the osteogenic environment is created and resulted in VC.Citation6,Citation7
Numerous pathological landscapes are associated with the development of VC. The impaired homeostasis of Ca/Pi and high levels of parathyroid hormone (PTH) cause Ca/Pi release by bone under severe hyperphosphatemia. The endothelial dysfunction, oxidative stress, chronic inflammation, VSMCs trans-differentiation, proliferation and apoptosis, loss of mineralization inhibitors, increased remodeling of extracellular matrix (ECM), and release of calcifying extracellular vesicles (cEVs) are the most important contributors to VC.Citation8 Moreover, the calciprotein particles (CPPs), the complexes of Pi, Ca, and proteins, are recognized to drive the calcification process.Citation9 It is reported that in CKD, uremic EVs and CPPs can modulate VSMCs' responses through an inflammation stress and trans-differentiation, causing an increase in mineral deposition. Hence, these circulating particles play an important role in the mechanisms of widespread calcification.Citation10 Additionally, several uremia-related factors contribute to the development of VC among patients with CKD. In addition to disrupted metabolism and other pathologies that promote VC, genetics, hereditary predisposition, and epigenetics are involved in VC development.Citation7,Citation11 In this review, we will review the recently updated state of knowledge on cellular and molecular mechanisms of VC in CKD. Unraveling the signaling pathways involved in VC in CKD patients will eventually offer novel therapies to limit the vicious effects of VC.
Vascular Calcification in CKD
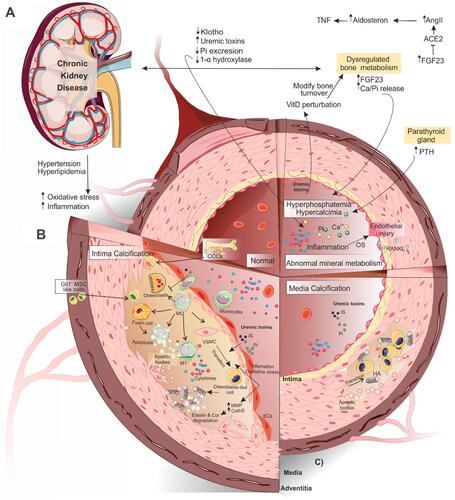
Under physiological circumstances, the active mineralization inhibitors including matrix Gla protein (MGP), pyrophosphate (PPi), fetuin-A, osteoprotegerin (OPG), adenosine, bone morphogenetic protein 7 (BMP-7) and osteopontin (OPN) protect blood vessels from the formation of stable hydroxyapatite crystals.Citation12,Citation13 A decline in these inhibitors along with elevated levels of active inducers of VC lead to a high prevalence of VC in the CKD population (). Although CKD patients can develop calcification in both media and intima layers of vessel wall ( and ), the media calcification is more common in these patients, especially in pediatrics.Citation14 It has been shown that all types of VC increase the mortality and morbidity rates in CKD patients.Citation15
Figure 1 Schematic view of vascular calcification in CKD. (A) As renal function continues to fall, normal defense mechanisms for Pi and Ca homeostasis (PTH, FGF-23, and klotho) become overwhelmed and the endocrine system of FGF23-klotho-VitaminD and RAAS is disturbed. As a result of nephron loss and higher levels of FGF-23, 1α-hydroxylase activity is diminished in the kidney, leading to elevated levels of inhibitor of this enzyme (FGF-23) and a decrease in 1,25(OH)2-vitamin D (calcitriol) productionCitation43 that, in turn, upregulates the production of renin in the kidney. Subsequently, the elevated levels of angiotensin II lead to kidney klotho loss, disruption of FGF-23 signaling, and the impairment of phosphaturia. Elevated levels of FGF-23 may activate the RAAS either by suppressing ACE-2 directlyCitation94 or decreasing calcitriol levels indirectly.Citation107 (B) Ca and Pi deposition in the VSMCs of medial layers may cause VC. (C) In the intimal calcification process, more diverse cells are involved including osteoclast-like cells, Gli1+-MSCs of the adventitia, and CCCs. The interaction of different factors and these cells causes atherosclerosis. Uremic toxins cause VSMCs trans-differentiation into osteoblast-like cells. In the process of calcification, macrophage differentiation into osteoclast-like cells is inhibited. In turn, macrophages increase apoptosis and accumulation of apoptotic bodies through transition into foam cells. A pro-inflammatory form of circulating monocytes (M1 macrophages) promotes the initial calcium deposition within the necrotic core of the lesions. All the above factors together cause atherosclerosis. For more details, see the full text.
Abbreviations: CKD, chronic kidney disease; FGF-23, fibroblast growth factor-23; PTH, parathyroid hormone; VC, vascular calcification; MMPs, matrix metalloproteinases; DH-VitD, 1, 25-dihydroxyvitamin D. Gli1+-MSCs, Gli1+ mesenchymal stem cells; CCCs, calcifying circulating cells; ACE-2, angiotensin-converting enzyme-2; RAAS, renin-angiotensin-aldosterone system; HA, hydroxyapatite crystal; ECs, endothelial cells; MQ, macrophage; IS, indoxyl-sulfate; VSMC, vascular smooth muscle cell; OS, oxidative stress.
Beyond a high incidence of the traditional risk factors in CKD patients, for instance, age, family history, sex, diabetes, hypertension, dyslipidemia, and tobacco use, VC in this population is connected with numerous other factors. Oxidative stress, inflammation, the CKD-related disorder of mineral metabolism, and bone are the most important non-traditional risk factors that accelerate VC in CKD patients.Citation16 The accumulation of uremic toxins [ie, Pi, advanced glycation end products (AGEs), and indoxyl-sulfate (IS)] and uremia-related factors (ie, malnutrition, hyperhomocysteinemia, and anemia) may also directly enhance the VC in CKD patients. As a source of uremic toxins, the gut microbiome is a potential contributor to CVD in CKD. In CKD, p-cresyl sulfate (PCS) and IS stimulate toxin-induced VC directly through the activation of coagulation and inflammation pathways in the arterial wall.Citation17 Furthermore, CKD risk factors including the history of dialysis, phosphate retention, high doses of vitamin D therapy, extra calcium, and uremic hyperparathyroidism can promote the VC development in patients with CKD.Citation18 It is also reported that the dysregulated mineral metabolism derives oxidative DNA injury and premature senescence to stimulate inflammation and VC in children on dialysis.Citation19 Different cells and factors can regulate the VC process in CKD patients. Epigenetics (microRNAs), the formation and release of extracellular vesicles, elastin degradation, and CPPs continue to disclose the involved mechanisms in the initiation and development of VC in CKD. Autophagy, mitochondrial dysfunction, microtubule destabilization, and endoplasmic reticulum stress are also involved in the pathogenesis of VC in CKD and restoring their functions can be effective therapeutic targets.Citation20–Citation25 Hortells et al (2017) clarified the expression patterns of factors contributed to the pathogenesis of VC in uremic rats in detail.Citation26 In the following sections, we highlight the most common factors in the development of VC.
Cells Involved in Vascular Calcification
In addition to the fact that osteogenic transition of VSMCs is the main cause of VC, other cells are involved in this process. Among them, osteoclast-like cells, endothelial progenitor cells (EPCs), Gli1+ mesenchymal stem cells (Gli1+-MSCs) of the adventitia, and calcifying circulating cells (CCCs) can be mentioned.
Vascular Smooth Muscle Cells
In CKD, the oxidative stress, chronic inflammation, and uremic toxins may influence the VSMCs’ physiological functions directly. Under these circumstances, the cellular environment is capable to stimulate a VSMC trans-differentiation from a contractile cell to an osteoblastic/chondroblastic-like cell and undergoes irregular senescence, proliferation, apoptosis, migration, and calcification ().Citation27
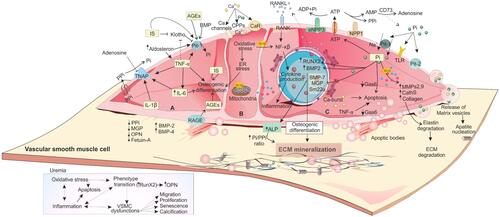
Figure 2 The impact of uremic toxins on CKD-induced VSMC dysfunction and VC. Due to hyperphosphatemia, hypercalcemia, elevated oxidative stress, and inflammation,Citation132 VSMCs manifest dysregulated functions and phenotype. Uremic toxins including Pi, IS, AGEs, IL-1β, IL-6, and TNF-α are involved in CV. (A) IL-1β, IL-6, and TNF-α induce osteoblast-like trans-differentiation of VSMCs through different mechanisms.Citation16 Interaction of AGEs with their receptor (RAGE) induces the expression of Pit-1 via ROS productionCitation49 and leads to osteogenic transition. It also causes apoptosis through NAD(P)H oxidase-derived oxidative stress.Citation133 (B) In CKD, normal Ca homeostasis is also dysregulated. This homeostasis is mediated by klotho, PTH, active vitamin D metabolites, and calcitonin. In VSMCs, Ca signaling is mediated by Ca channels, CaR, and pumps that maintain Ca concentrations in these cells.Citation134 Higher level of extracellular Ca is associated with the release of MVs and cell death promotion and release of apoptotic bodies.Citation43 (C) Extracellular Pi, as a signaling molecule, can trigger numerous changes in VSMCs through regulating different molecular pathways. NPP1 is responsible for extracellular ATP degradation to AMP and PPi, CD73 degrades AMP to adenosine and Pi and TNAP breaks PPi into phosphate and adenosine.Citation15 Higher Pi level simultaneously upregulates the expression of osteo/chondrogenic genes (Runx2, ALP, OPN, and osterix) and downregulates VSMCs genes (SM22α and αSMA). ALP controls vascular matrix mineralization by degradation and inactivation of the VC inhibitors (PPi and P-OPN) to allow uncontrolled tissue mineralization and simultaneously releasing free Pi.Citation43 These osteo-/chondroblast-like cells actively induce apoptosis and vesicle release, a reduction in calcification inhibitors, elastin degradation, increased ECM remodeling, and a pro-inflammatory state. Moreover, under high levels of Pi, VSMCs synthesize collagen at high amount and provide a collagen-enriched ECM. Downregulation of Gas6 and Bcl2 may be the basic mechanism of VSMCs apoptosis. The released apoptotic bodies provide a further nidus for deposition of Pi and Ca. For more details, see the full text.
Abbreviations: Ca, calcium; Pi, phosphate; PPi, pyrophosphate; ECM, extracellular matrix; MMP, matrix metalloproteinases; Gas6, growth arrest-specific gene 6; ALP, alkaline phosphatase; ROS, reactive oxygen species; SM, α-smooth muscle actin; CPPs, calciprotein particles; CaR, Ca sensing receptor; MVs, matrix vesicles; AGEs, advanced glycation end products; RAGE, receptor for advanced glycation end products.
A switch to the osteogenic- and/or chondrogenic-like phenotype is characterized by the expression of Runx2 also known as core-binding factor a-1 (CBFA1), SRY-Box 9 (SOX9), Msh Homeobox 2 (MSX2), and Osterix that are important transcription factors for both intimal and medial VC.Citation28 The VSMCs trans-differentiation into osteo-/chondrogenic-like cells is coordinated by a complex network of intracellular signaling pathways like nuclear factor kappa beta (NF-κB), receptor activator of NF-κB (RANK)/RANK ligand (RANKL), Wnt-β-catenin, mitogen-activated protein kinase (MAPK) p38, calcium-induced signaling, and nitric oxide–plasminogen activator inhibitor-1 (PAI-1) pathways.Citation29–Citation32 Moreover, trans-differentiation can be mediated by extracellular signal-regulated kinases 1/2 (ERK1/2) pathways.Citation31 The downstream impacts of ERK1/2 during VC are not still completely understood.Citation3
Primary CPPs are amorphic and solid-phase Ca-Pi that bound to serum fetuin-A protein and calcium-regulatory proteins and disperse as colloids in the circulation to eliminate mineral crystal formation and guard against the ectopic calcification. This defense mechanism maintains blood mineral homeostasis and inhibits calcification. In the pathology of VC, the balance between the primary CPP formation and absorption is dysregulated and these CPPs may undergo a transition to the crystalline (secondary CPPs) phase. Secondary CPPs are stretched particles that contain predominantly hydroxyapatite surrounding proteins. Clinical studies have showed that the level of serum CPP was elevated with the decline of kidney function and connected with inflammation and VC in CKD patients.Citation13,Citation33 The CPPs of CKD patients had the features of secondary CPPs; a reduced level of fetuin-A and GRP (Gla-rich protein).Citation10 The secondary CPPs may directly stimulate VC through the induction of VSMCs trans-differentiation.Citation10 This event is mediated by uptake of the CPPs, increasing the intracellular levels of Ca2+, initiation of oxidative stress and inflammatory responses (TNF-α) in VSMCs to promote mineralization via its receptor (TNFR1).Citation34 This event may be involved in VC but no sufficient proof is available to underline that this is the only pathway.
In CKD patients, the elevated levels of Ca and Pi induce the discharge of membrane-bound MVs from VSMCs. These MVs contain lipids, microRNAs, and proteins that are essential to induce the calcification cascade. Proteins for import of Pi and Ca into the MVs and proteins-related to cellular stress, cytoskeleton, and extracellular mineralization along with other intracellular proteins can be found in MVs. These MVs also contain tissue nonspecific alkaline phosphatase (TNAP) to degrade PPi for making a local calcifying microenvironment.Citation35 The apoptotic bodies and Ca/Pi-loaded MVs (released from VSMCs and macrophages) are different types of extracellular vesicles that eventually form hydroxyapatite crystals and deposit on a collagen matrix in the vessel wall and provide a bed for nucleation and VC in the media layer.Citation8,Citation27
Additionally, macrophage-released calcifying MVs are directly associated with arterial medial early intimal and calcification in the CKD patients.Citation36 A pathological calcification-inflammation cycle exists between VSMCs and macrophages. The presence of Ca/Pi mineral in ECM, cEVs, and secondary CPPs of blood vessels can trigger pro-inflammatory responses in VSMCs and immune cells. Osteogenic-like VSMCs cause ECM calcification by releasing cEVs and increasing pro-inflammatory responses in macrophage. This inflammatory response in macrophages contributes to the elevated VC via releasing cEVs and inducing VSMCs osteogenic trans-differentiation.Citation37
Elastin degradation is facilitated by proteases such as Cathepsin-S and matrix metalloproteinases (MMP-2 and −9) that are upregulated in CKD. Elastin disruption in the aortic wall causes an increase in the expression level of transforming growth factor (TGF-β) involved in osteoblast differentiation and increases arterial stiffness in CKD.Citation38,Citation39 After the VSMCs phenotypical alteration toward osteoblast-like phenotype, the deposition of mineral crystals (biomineralization) happens. This regulated process requires MVs release to concentrate the Ca and Pi and support the nucleation of mineral crystals via the matrix proteins.Citation18 Kapustin et al proposed a model for depicting the possible mechanism of MVs calcification. Phosphate is taken up by Pi transporters, while Ca passes through the MV membrane by voltage-dependent anion-selective channel protein 1 (VDAC1). The nucleation complexes are formed via the binding of mineral ions (Pi and Ca) with phosphatidylserine and annexin A6 on the inner and outer MVs surfaces. The formation of these complexes stimulates the growth of crystal apatite. Moreover, MMP-2 degrades elastin and stimulates calcification on the MV surface.Citation40
It should be noted that the VSMCs are believed to take up chondrogenic properties. The VSMCs chondrogenic-like transformation underlies the cartilaginous metaplasia formation that is associated with VC in animal models and humans. TGFβ-Wnt16-Notch signaling is involved in this process.Citation41
Sensing and Transduction of Osteogenic Signals in CKD
Sensing and the transduction of osteogenic stimuli in CKD are mediated through different signaling molecules, receptors, and channels that modulate the osteogenic response in the VSMC. Some harmful effects of Pi are triggered by its excessive entry into the VSMCs through sodium-dependent phosphate transporters (PiT-1 and −2). Moreover, in response to elevated Pi levels, PiT-1 (but not Pi that is taken through PiT-1) is necessary for ERK1/2 phosphorylation. Therefore, for the calcification process of VSMC, both Pi transport-dependent and -independent effects of PiT-1 are important.Citation42 The phosphate-induced calcification is mediated by reactive oxygen species (ROS) production and oxidative stress, osteochondrogenic differentiation, apoptosis of VSMCs and the release and instability of extracellular vesicles.Citation43–Citation45 Under uremic conditions, PiT-2 is up-regulated along with PiT-1 in the vasculature; however, it defends against VC by unidentified mechanisms.Citation46 Toll-like receptors (TLRs) may be also involved in Pi-sensing; the activation of TLR4/NF-κB signaling in VSMCs directly induces VC in CKD.Citation47 The NF-κB signaling activation, at least, through the serum- and glucocorticoid-inducible kinase 1 (SGK1) can also promote VC in CKD.Citation48
The endocytosis of Ca/Pi particles in lysosomes causes calcium release and apoptosis in VSMCs. It is also reported that VSMCs can be stimulated through AGE products and their receptors (RAGE). RAGE ligands mediate ROS production in VSMC that is involved in the up-regulation of Pit-1 and Runx2.Citation49 Different channels, pumps, and exchangers are involved in the sensing, entrance of Ca into the VSMCs, and preserving of Ca concentrations in the cytosol and sarcoplasmic reticulum. Changes in intra- and extracellular pools of Ca affect VSMC function and phenotype and the regulation of Ca is dependent on the phenotypic state of VSMC.
Circulating Calcifying Cells
Circulating calcifying cells (CCCs), which originate from the bone marrow (BM), play a role in the intima calcification processes. CCCs have an osteogenic phenotype and express bone alkaline phosphatases and osteocalcin (OCN).Citation50 Recent studies have demonstrated that the pool of CCCs contains calcifying endothelial progenitor cells (EPCs), MSC–derived circulating osteoprogenitors and myeloid calcifying cells (a group of circulating monocytes) in CKD patients.Citation51
The endothelium integrity presents a crucial role in the establishment of VC and EPCs support it during endothelial injury. In response to cellular apoptosis or activation, mature endothelial cells secret soluble microparticles (MPs) to regulate procalcificant activity in VSMCs and to differentiate EPCs. In CKD cases with VC, higher endothelial MPs are connected with a lower percentage of EPCs. This data proposesan inequality between the repair procedures and endothelial in patients who suffer from CKD. By the expression of osteogenic factor, OCN, EPCs could contribute to the VC procedure directly. Moreover, the OCN gene is expressed in the fibroblast and VSMCs of CKD patient as a result of MPs action.Citation52,Citation53 Moreover, EPCs undertake an endothelial to procalcific shift in CKD-MBD and trigger VC.Citation54
Bone morphogenetic protein 2 (BMP-2), which increases in uremic patients, promotes the migration of MSCs from BM to other tissues.Citation55–Citation57 Cianciolo et al have proven the relationship between a particular type of EPC subset (CD34+/KDR+/CD133–/CD45– cells) and an increased VC in CKD patients.Citation58 Calcifying myeloid cells in the bloodstream can cause VC but their exact role in CKD patients has not been established.Citation59 Altogether, the issue of CCCs is a new topic in recognizing the pathophysiology of calcification in CKD patients and needs further investigation.Citation2,Citation50
Gli1+ Mesenchymal Stem Cells
Gli1+ MSC-like cells are located in the vascular adventitious layer and play a role in the process of vascular repair and neointima formation.Citation60 These cells are important in maintaining kidney homeostasis, angiogenesis, and vascular stability.Citation61 Gli1+ cells affect arterio- and athero-sclerosis in ApoE−/− mice by migrating to the media and neointima layers.Citation62 These cells are a key source of osteoblast-like cells during VC in the intima and media.Citation62 The interference of Gli1+ in osteogenic differentiation is controlled by the Sonic Hedgehog (SHH) pathway.Citation2 It can be concluded that during the uremic calcification, Gli1+-MSCs are a chief reservoir of osteoblast-like cells that can be therapeutically targeted to inhibit CV in CKD.Citation2
Microbiota
The human intestinal tract homes to a collection of symbiotic, commensal, and pathogenic micro-organisms in a local ecologic community called microbiome.Citation63 The gut microbiome as a “second human genome” has a significant role in both human health and the pathogenesis of kidney diseases.Citation64,Citation65 Recent studies demonstrate dysbiosis, a shift in the bacterial populations, in patients with CKD and end-stage renal disease (ESRD).Citation66,Citation67 The administration of antibiotics and phosphate binders, dietary restriction, and CKD itself may contribute to dysbiosis in kidney disease.Citation68–Citation71 Gut dysbiosis may elevate the production of microbial byproducts that are absorbed from the intestinal lumen. The increased absorption along with a decreased kidney clearance lead to a rise in gut-derived toxin levels in circulation.Citation63 In CKD, the influx of urea and other toxins causes an alteration in the gut microbiome. A diminished number of beneficial bacteria are associated with an increase in uremic toxin-producing bacteria. Because of the degradation of cellular tight junctions and inflammation in intestinal, gut-derived uremic toxins including phenylacetylglutamine, indole-3 acetic acid, IS, trimethylamine-N-oxide (TMAO) and p-cresyl sulfate (PCS), translocate into the bloodstream and cause an extensive oxidative stress damage to the kidney, cardiovascular system, bone-mineral, erythropoiesis, and endocrine systems.Citation72
Recent evidence indicates that different gut-derived byproducts are associated with VC, CVD, and adverse cardiovascular outcomes and mortality in CKD.Citation63 In patients with CKD, the serum levels of IS have an inverse association with renal function and a direct correlation with aortic calcification and cardiovascular mortality.Citation73,Citation74 It is also reported that both PCS and IS can directly stimulate VC in the peripheral arteries and aorta of CKD rat through the stimulation of insulin resistance and hyperglycemia that activate the coagulation pathways and the acute-phase response signaling in the arterial wall.Citation17 In patients on hemodialysis, the serum levels of IS were connected with coronary artery calcium, an independent predictor of cardiovascular events.Citation75
Uremic toxins endorse the proliferation and transformation of VSMCs into osteoblast-like cells, leading to vascular wall thickening and calcification. The effect of IS on VSMCs is mediated by organic anion transporter 3 (OAT3). Moreover, the stimulation of VSMC proliferation is mediated by MAPK activation in vitro. This may be one of the mechanisms which leads to the development of atherosclerotic lesions in ESRD patients.Citation76 Furthermore, IS stimulates the expression of (Pro) renin receptor (PRR) and renin/prorenin in aorta by ROS production, OAT3-mediated uptake as well as aryl hydrocarbon receptor (AhR) and NF-κB p65 activation in VSMCs. The activation of PRR by IS stimulates the proliferation and expression of tissue factor in VSMCs.Citation77
Epigenetics
During the hyperphosphatemia, different epigenetic modifications including DNA methylation, histone modifications, and microRNAs (miRNAs, miRs) dysregulation contribute to the osteo-induced cellular signaling.Citation78,Citation79 It is indicated that through the hypermethylation of Klotho gene, IS can suppress vascular Klotho gene expression and contribute to pathological mechanism of CV in CKD.Citation80 Likewise, the methylation of the SM22α promoter region induces VC at a higher level of Pi.Citation81 It is also reported that through a reduction in the ALP promoter region methylation and an increase in the ALP expression, DNA methyltransferases inhibitors ease the Pi-induced VC.Citation78 Changes in the chromatin conformation, histone modification (histone tail methylation), hypermethylation of calcification inhibitory genes, activation of osteoblast-differentiation genes, or the deregulation of histone deacetylase members may predispose VSMCs to calcification. Furthermore, there is a cross-talk between different epigenetic mechanisms in VC; microRNAs may be upstream regulators of histone deacetylase that can modulate the severity of the calcification.Citation82
Over the last decade, the roles of microRNAs have been identified in the course of CKD, pathogenesis of VC, and atherosclerosis.Citation83,Citation84 microRNAs are small non-coding RNAs that negatively regulate the gene expression at both transcription and translation levels. In a systematic review, the agonistic and antagonistic miRNAs that positively and negatively regulate VC are reviewed comprehensively.Citation82 The protective effect of miR-30b against VC is mediated by stimulating autophagy and mitochondrial membrane potential.Citation85 Increased levels of miR-29b and decreased levels of miR-133b and miR-211 that are correlated with lower and higher expression of inhibitors and RUNX2 of osteoblastic differentiation, respectively, are reported in uremic rats.Citation86 It is also reported that the down-regulation of miR-29b and activation of Wnt/β-catenin signaling may be involved in IS-induced VC in CKD.Citation87 Likewise, down-regulated levels of miR-125-b accelerate trans-differentiation of VSMCs and calcification by targeting Ets1.Citation88 Moreover, miR-142-3p prevents VC in both humans and mice with ESRD.Citation89 It has been proven that miR-223 and miR-126 that are expressed in VSMCs interfere with the trans-differentiation of these cells to an osteoblastic phenotype that increases the vascular wall stiffness.Citation90 There were higher levels of miR-29a/b and miR-223 expression in hemodialysis patients with VC and the calcification intensity was associated with the miR-29a level.Citation91
Inducers and Inhibitors of VC
An imbalance between the inducers and inhibitors of VC happens in CKD and chronic hemodialysis patients.Citation92 In this section, we summarize some of these factors ().
Table 1 The Impact of Other Factors on VC in CKD
Fetuin-A
Fetuin-A (α2 Heremans-Schmid glycoprotein) is a glycoprotein that is secreted into the circulatory system by adipose tissue and liver.Citation93,Citation94 It has been revealed that fetuin-A acts as a prominent protective factor in preventing VC in patients with CKD and ESRD.Citation93 Decreased fetuin-A increases the morbidity and mortality rate in ESRD cases.Citation95 It has been shown that fetuin-A is involved in preventing aortic calcification.Citation95 This is explained by the fact that despite the general similarity of risk factors for aortic and coronary calcification, these factors are specific to each site in the general population.Citation95 Therefore, the pathophysiology involved in the calcification of these two sites is somewhat different.Citation95 Some studies have shown that fetuin-A is also effective in preventing the calcification of the heart valves, some others have not shown a link between them.Citation96 The ability of fetuin-A to prevent the mineralization is mediated by the CPPs formation.Citation97,Citation98
Magnesium
Higher levels of Pi, amorphous Ca2+-Pi particle (ACP) formation, and reduced levels of VC inhibitors in the circulation start the VSMC trans-differentiation that is enhanced by the osteogenic genes expression and amplified by the release of apoptotic bodies and exosomes.Citation99 At different levels, magnesium hinders these processes of VC. Osteogenic differentiation and VC are negatively regulated by magnesium via increasing the activity of its transporter, transient receptor potential melastatin 7 (TRPM7) and its entry into the cell that leads to the expression of anti-calcifying proteins (BMP-7, OPN, and MGP).Citation100 Moreover, by reduction in microRNAs expression (miR-133a, miR-30b, and miR-143a), magnesium influences the expression of osteogenesis (Runx2, Smad1, and Osterix).Citation101 Anti-calcifying impact of magnesium is also mediated by the inhibition of Wnt/β-catenin pathway.Citation102 As an antagonist of Ca-channel, magnesium hinders the Ca entry into the VSMCs, loss of inhibitors, and osteogenic differentiation.Citation103 Moreover, it prevents hydroxyapatite formation in the extracellular space, thereby, avoids VSMCs calcification.Citation104 Although several recent studies propose that the advantageous role of magnesium on VC can be elucidated via a delayed formation of secondary CPP,Citation9 the definitive proof to support this hypothesis is missing.
Hormones
Vascular autocrine and paracrine factors participate in keeping circulatory homeostasis. However, under pathological circumstances, these factors may modulate the pathogenesis of VC and CVD. The constant activation of the RAAS (renin-angiotensin-aldosterone system) has a foremost role in cardiovascular remodeling and CKD progression.Citation105 Angiotensin II (Ang II), an active factor of the RAS, is participated in the control of cardiovascular function and kidney homeostasis through acting on different cells, mainly VSMCs.Citation106 As renal function continues to fall, normal defense mechanisms, as an endocrine axis for Pi and Ca metabolism, become overwhelmed and a disruption happens in this axis. Low kidney klotho, high levels of fibroblast growth factor 23 (FGF-23), vitamin D deficiency, and RAAS activation are associated with adverse kidney outcome in CKD.Citation107 Klotho is a transmembrane protein that acts as a co-receptor of FGF23. Klotho is expressed in kidney, choroid plexus, and parathyroid glands and mediates the functional role of the FGF-23 in regulating the Pi and Ca levels; hence, Klotho deficiency causes hyperphosphatemia. In klotho-hypomorphic mice, deficiency of klotho leads to an uncontrolled formation of calcitriol that increases the reabsorption of Pi in kidney and intestine, increasing phosphate levels.Citation28
In CKD, the impaired kidney elimination of Pi leads to hyperphosphatemia that promotes the FGF-23 secretion, a regulator of serum Pi level, from bone osteocytes. Due to nephron loss and higher levels of FGF-23, the 1α-hydroxylase activity, an enzyme for the production of active vitamin D (1,25(OH)2 vitamin D, calcitriol) is reduced; leading to a reduction in calcitriol level that in turn increases renin production in kidney. The activation of RAAS reduces the expression of renal klotho, a critical factor for accurate FGF-23 signaling.
Phosphate may directly induce aldosterone synthase expression in adrenal glands and vascular tissue that may have an important effect on how FGF-23 mediates the activation of RAAS. On the whole, an increased level of FGF-23 activates the RAAS by two possible mechanisms: (a) indirectly by reducing calcitriol levels and (b) directly by suppressing the activity of angiotensin-converting enzyme 2 (ACE-2) that inhibits the conversion of Ang II into Ang (1–7).Citation108 As a result, the increased levels of Ang II increase the aldosterone production that activates Pit-1; resulting in Pi entrance into VSMCs. Moreover, aldosterone fosters the inflammatory processes by induction of TNF-α.Citation109 It is also reported that Ang II is able to prevent Pi-induced VSMCs calcification by increasing the influx of Mg that is mediated by stimulating the TRPM7 activity as well as prohibition of the canonical Wnt/β-catenin and the activation of the ERK1/2 intracellular signaling pathways.Citation106 Moreover, the activation of angiotensin II type 2 (AT2) receptor could mediate an endogenous protective pathway for VC in CKD since it may decrease the adverse cardiovascular events.Citation110 The secreted FGF-23 from osteogenic cells in the calcified vessel may further increase the serum levels FGF-23 (reviewed in Ref. Citation109).
The deregulated levels of Ca and vitamin D ease the osteogenic differentiation and mineralization of VSMC; leading to deleterious VC. As discussed, there is a complex relationship between vitamin D, Klotho, and FGF-23 on the vasculature (). Collectively, the perturbation of vitamin D activity, including its turnover and systemic levels along with vitamin D receptor signaling activity are contributed in VC that may ultimately be anti- or pro-calcifying. On the other hand, evidence suggests that vitamin D exerts biphasic impact on the vasculature; both hypo- and hypervitaminosis D can contribute to the VC development through several mechanisms [reviewed comprehensibly in RefCitation111].
Diagnosis and Treatment of VC
Non-invasive imaging techniques plain X-rays, two-dimensional ultrasound, echocardiography, and computed tomography (CT) are accessible to screen the existence of VC. The multi-detector CT (MDCT) is a highly sensitive method for accurately and quantitatively assessing VC, especially coronary artery calcification (CAC).Citation112 Currently, no definite therapy can reverse VC and available therapeutic modalities can reduce the progression of VC. Most candidate drugs such as phosphate-binders, bisphosphonates, magnesium, and vitamin K are currently under clinical investigation that can correct the imbalance of inhibitors and promoters (ie, hyperphosphatemia) of calcification in VC-affected patients.Citation113,Citation114 The therapeutic potential of antioxidant compounds that can target different pathways in VC pathology is also reportedCitation115 ().
Table 2 Therapeutic Strategies for Vascular Calcification
Conclusion
Overall, a plethora of several contributing factors are associated with VC in CKD. Different conditions (uremia, hyperglycemia, hyperphosphatemia, hyperlipidemia, inflammation, oxidative stress, and hypertension) might coincide with VC pathogenesis. The histological location of VC, the anatomical site of the calcified artery, and many other factors affect the commencement and progression of VC. VSMCs along with CCCs are active members of the calcification process. A range of pathogenic mechanisms are involved in the intracellular molecular mechanisms of VC. Although different therapeutically opportunities have been studied in VC, no study has strongly established that these modulations alter the patient outcome. Thus, future research might focus on the exact demonstration of VC in CKD to develop and assess tailored interventions in the organized clinical trials.
Data Sharing Statement
Research data were not shared. Data sharing is not applicable to this article as no new data were created or analyzed in this study and this is a review article.
Acknowledgment
The authors gratefully acknowledge Fatemeh Zununi Vahed for her kind support.
Disclosure
The authors declare that they have no conflict of interest regarding this work.
Additional information
Funding
References
- Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24(2):179–189. doi:10.1681/ASN.2011121191
- Hénaut L, Chillon J-M, Kamel S, Massy ZA,. Updates on the mechanisms and the care of cardiovascular calcification in chronic kidney disease. Semin Nephrol. 2018;38(3):233–250. doi:10.1016/j.semnephrol.2018.02.004
- Voelkl J, Lang F, Eckardt KU, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. 2019;76(11):2077–2091. doi:10.1007/s00018-019-03054-z
- Azpiazu D, Gonzalo S, Gonzalez-Parra E, Egido J, Villa-Bellosta R. Role of pyrophosphate in vascular calcification in chronic kidney disease. Nefrologia. 2018;38(3):250–257. doi:10.1016/j.nefro.2017.07.005
- Shroff R. Phosphate is a vascular toxin. Pediatr Nephrol. 2013;28(4):583–593. doi:10.1007/s00467-012-2347-x
- Pérez-Hernández N, Aptilon-Duque G, Blachman-Braun R, et al. Vascular calcification: current genetics underlying this complex phenomenon. Chin Med J (Engl). 2017;130(9):1113–1121. doi:10.4103/0366-6999.204931
- Bowman MAH, McNally EM. Genetic pathways of vascular calcification. Trends Cardiovasc Med. 2012;22(4):93–98. doi:10.1016/j.tcm.2012.07.002
- Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25(4):267–274. doi:10.1016/j.tcm.2014.10.021
- Zeper LW, de Baaij JHF. Magnesium and calciprotein particles in vascular calcification: the good cop and the bad cop. Curr Opin Nephrol Hypertens. 2019;28(4):368–374. doi:10.1097/MNH.0000000000000509
- Viegas CSB, Santos L, Macedo AL, et al. Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification: a role for GRP (Gla-Rich Protein). Arterioscler Thromb Vasc Biol. 2018;38(3):575–587. doi:10.1161/ATVBAHA.117.310578
- Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109(5):578–592. doi:10.1161/CIRCRESAHA.111.247965
- Schurgers LJ, Barreto DV, Barreto FC, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5(4):568–575. doi:10.2215/CJN.07081009
- Smith ER, Ford ML, Tomlinson LA, Rajkumar C, McMahon LP, Holt SG. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant. 2012;27(5):1957–1966. doi:10.1093/ndt/gfr609
- Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118(17):1748–1757. doi:10.1161/CIRCULATIONAHA.108.783738
- Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone. 2017;100:87–93. doi:10.1016/j.bone.2016.11.012
- Henaut L, Mary A, Chillon JM, Kamel S, Massy ZA. The Impact of Uremic Toxins on Vascular Smooth Muscle Cell Function. Toxins (Basel). 2018;10(6):218. doi:10.3390/toxins10060218
- Opdebeeck B, Maudsley S, Azmi A, et al. Indoxyl sulfate and p-Cresyl sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol. 2019;30(5):751–766. doi:10.1681/ASN.2018060609
- Disthabanchong S. Vascular calcification in chronic kidney disease: pathogenesis and clinical implication. World J Nephrol. 2012;1(2):43–53. doi:10.5527/wjn.v1.i2.43
- Sanchis P, Ho CY, Liu Y, et al. Arterial “inflammaging” drives vascular calcification in children on dialysis. Kidney Int. 2019;95(4):958–972. doi:10.1016/j.kint.2018.12.014
- Frauscher B, Kirsch AH, Schabhuttl C, et al. Autophagy protects from uremic vascular media calcification. Front Immunol. 2018;9:1866. doi:10.3389/fimmu.2018.01866
- Lee K, Kim H, Jeong D. Microtubule stabilization attenuates vascular calcification through the inhibition of osteogenic signaling and matrix vesicle release. Biochem Biophys Res Commun. 2014;451(3):436–441. doi:10.1016/j.bbrc.2014.08.007
- Ma WQ, Sun XJ, Wang Y, Zhu Y, Han XQ, Liu NF. Restoring mitochondrial biogenesis with metformin attenuates beta-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis. Mol Cell Endocrinol. 2019;479:39–53. doi:10.1016/j.mce.2018.08.012
- Yao L, Wang J, Tian BY, Xu TH, Sheng ZT. Activation of the Nrf2-ARE signaling pathway prevents hyperphosphatemia-induced vascular calcification by inducing autophagy in renal vascular smooth muscle cells. J Cell Biochem. 2017;118(12):4708–4715. doi:10.1002/jcb.26137
- Panda DK, Bai X, Sabbagh Y, et al. Defective interplay between mTORC1 activity and endoplasmic reticulum stress-unfolded protein response in uremic vascular calcification. Am J Physiol Renal Physiol. 2018;314(6):F1046–f61. doi:10.1152/ajprenal.00350.2017
- Lee K, Kim H, Jeong D. Protein kinase C regulates vascular calcification via cytoskeleton reorganization and osteogenic signaling. Biochem Biophys Res Commun. 2014;453(4):793–797. doi:10.1016/j.bbrc.2014.10.026
- Hortells L, Sosa C, Guillen N, Lucea S, Millan A, Sorribas V. Identifying early pathogenic events during vascular calcification in uremic rats. Kidney Int. 2017;92(6):1384–1394. doi:10.1016/j.kint.2017.06.019
- Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. 2014;307(8):F891–F900. doi:10.1152/ajprenal.00163.2014
- Lang F, Leibrock C, Pelzl L, et al. Therapeutic interference with vascular calcification-lessons from klotho-hypomorphic mice and beyond. Front Endocrinol (Lausanne). 2018;9:207. doi:10.3389/fendo.2018.00207
- Kanno Y, Into T, Lowenstein CJ, Matsushita K. Nitric oxide regulates vascular calcification by interfering with TGF- signalling. Cardiovasc Res. 2008;77(1):221–230. doi:10.1093/cvr/cvm049
- Cai T, Sun D, Duan Y, et al. WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res. 2016;345(2):206–217. doi:10.1016/j.yexcr.2016.06.007
- Lee GL, Yeh CC, Wu JY, et al. TLR2 promotes vascular smooth muscle cell chondrogenic differentiation and consequent calcification via the concerted actions of osteoprotegerin suppression and IL-6-Mediated RANKL induction. Arterioscler Thromb Vasc Biol. 2019;39(3):432–445. doi:10.1161/ATVBAHA.118.311874
- Osako MK, Nakagami H, Shimamura M, et al. Cross-talk of receptor activator of nuclear Factor-κB ligand signaling with renin–angiotensin system in vascular calcification. Arterioscler Thromb Vasc Biol. 2013;33(6):1287–1296. doi:10.1161/ATVBAHA.112.301099
- Hamano T, Matsui I, Mikami S, et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol. 2010;21(11):1998–2007. doi:10.1681/ASN.2009090944
- Aghagolzadeh P, Bachtler M, Bijarnia R, et al. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis. 2016;251:404–414. doi:10.1016/j.atherosclerosis.2016.05.044
- New SE, Aikawa E. Role of extracellular vesicles in de novo mineralization: an additional novel mechanism of cardiovascular calcification. Arterioscler Thromb Vasc Biol. 2013;33(8):1753–1758. doi:10.1161/ATVBAHA.112.300128
- New SE, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113(1):72–77. doi:10.1161/CIRCRESAHA.113.301036
- Viegas C, Araujo N, Marreiros C, Simes D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): challenging old concepts with new facts. Aging (Albany NY). 2019;11(12):4274–4299. doi:10.18632/aging.102046
- Gauthier-Bastien A, Ung RV, Lariviere R, Mac-Way F, Lebel M, Agharazii M. Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin Exp Hypertens. 2014;36(3):173–180. doi:10.3109/10641963.2013.804541
- Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010;21(10):1637–1640. doi:10.1681/ASN.2010040349
- Kapustin AN, Davies JD, Reynolds JL, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109(1):e1–e12. doi:10.1161/CIRCRESAHA.110.238808
- Beazley KE, Nurminsky D, Lima F, Gandhi C, Nurminskaya MV. Wnt16 attenuates TGFbeta-induced chondrogenic transformation in vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2015;35(3):573–579. doi:10.1161/ATVBAHA.114.304393
- Chavkin NW, Chia JJ, Crouthamel MH, Giachelli CM. Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp Cell Res. 2015;333(1):39–48. doi:10.1016/j.yexcr.2015.02.002
- Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109(6):697–711. doi:10.1161/CIRCRESAHA.110.234914
- Ciceri P, Galassi A, Alfieri C, Messa P, Cozzolino M. Uremic patients with increased vascular calcification score have serum with high calcific potential: role of vascular smooth muscle cell osteoblastic differentiation and apoptosis. Blood Purif. 2019;48(2):142–149. doi:10.1159/000497229
- Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52(10):843–850. doi:10.1016/j.jacc.2008.05.043
- Yamada S, Leaf EM, Chia JJ, Cox TC, Speer MY, Giachelli CM. PiT-2, a type III sodium-dependent phosphate transporter, protects against vascular calcification in mice with chronic kidney disease fed a high-phosphate diet. Kidney Int. 2018;94(4):716–727. doi:10.1016/j.kint.2018.05.015
- Zhang D, Bi X, Liu Y, et al. High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-kappab signaling pathway. Kidney Blood Press Res. 2017;42(6):1205–1215. doi:10.1159/000485874
- Voelkl J, Luong TT, Tuffaha R, et al. SGK1 induces vascular smooth muscle cell calcification through NF-kappaB signaling. J Clin Invest. 2018;128(7):3024–3040. doi:10.1172/JCI96477
- Belmokhtar K, Ortillon J, Jaisson S, et al. Receptor for advanced glycation end products: a key molecule in the genesis of chronic kidney disease vascular calcification and a potential modulator of sodium phosphate co-transporter PIT-1 expression. Nephrol Dial Transplant. 2019;34(12):2018–2030. doi:10.1093/ndt/gfz012
- Cianciolo G, Capelli I, Cappuccilli M, Schillaci R, Cozzolino M, La Manna G. Calcifying circulating cells: an uncharted area in the setting of vascular calcification in CKD patients. Clin Kidney J. 2016;9(2):280–286. doi:10.1093/ckj/sfv145
- Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S. Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation. 2012;125(22):2772–2781. doi:10.1161/CIRCULATIONAHA.112.090860
- Soriano S, Carmona A, Trivino F, et al. Endothelial damage and vascular calcification in patients with chronic kidney disease. Am J Physiol Renal Physiol. 2014;307(11):F1302–11. doi:10.1152/ajprenal.00114.2014
- Shin V, Zebboudj AF, Bostrom K. Endothelial cells modulate osteogenesis in calcifying vascular cells. J Vasc Res. 2004;41(2):193–201. doi:10.1159/000077394
- Cianciolo G, Capelli I, Cappuccilli M, et al. Is chronic kidney disease-mineral and bone disorder associated with the presence of endothelial progenitor cells with a calcifying phenotype? Clin Kidney J. 2017;10(3):389–396. doi:10.1093/ckj/sfw145
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res Ther. 2000;2(6):477–488. doi:10.1186/ar130
- Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354(2):453–458. doi:10.1016/j.bbrc.2006.12.226
- Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow‐derived osteoblast progenitor cells are recruited to the bone‐forming site by the CXCR4/stromal cell‐derived factor‐1 pathway. Stem Cells. 2008;26(1):223–234. doi:10.1634/stemcells.2007-0515
- Cianciolo G, La Manna G, Della Bella E, et al. Effect of vitamin D receptor activator therapy on vitamin D receptor and osteocalcin expression in circulating endothelial progenitor cells of hemodialysis patients. Blood Purif. 2013;35(1–3):187–195. doi:10.1159/000347102
- Fadini GP, Albiero M, Menegazzo L, et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res. 2011;108(9):1112–1121. doi:10.1161/CIRCRESAHA.110.234088
- Bardeesi ASA, Gao J, Zhang K, et al. A novel role of cellular interactions in vascular calcification. J Transl Med. 2017;15(1):95. doi:10.1186/s12967-017-1190-z
- El Agha E, Kramann R, Schneider RK, et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21(2):166–177. doi:10.1016/j.stem.2017.07.011
- Kramann R, Goettsch C, Wongboonsin J, et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19(5):628–642. doi:10.1016/j.stem.2016.08.001
- Jovanovich A, Isakova T, Stubbs J. Microbiome and Cardiovascular Disease in CKD. Clin J Am Soc Nephrol. 2018;13(10):1598–1604. doi:10.2215/CJN.12691117
- Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: a Potential Therapeutic Target. Am J Kidney Dis. 2016;67(3):483–498. doi:10.1053/j.ajkd.2015.09.027
- Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S. The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother. 2017;93:412–419. doi:10.1016/j.biopha.2017.06.066
- Xu KY, Xia GH, Lu JQ, et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep. 2017;7(1):1445. doi:10.1038/s41598-017-01387-y
- Stadlbauer V, Horvath A, Ribitsch W, et al. Author Correction: structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci Rep. 2019;9(1):8522. doi:10.1038/s41598-019-43263-x
- Nazzal L, Roberts J, Singh P, et al. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol Dial Transplant. 2017;32(11):1809–1817. doi:10.1093/ndt/gfx029
- Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670. doi:10.1681/ASN.2013080905
- Riccio E, Sabbatini M, Bruzzese D, et al. Plasma p-cresol lowering effect of sevelamer in non-dialysis CKD patients: evidence from a randomized controlled trial. Clin Exp Nephrol. 2018;22(3):529–538. doi:10.1007/s10157-017-1504-8
- Andersen K, Kesper MS, Marschner JA, et al. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol. 2017;28(1):76–83. doi:10.1681/ASN.2015111285
- Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond). 2018;132(5):509–522. doi:10.1042/CS20171107
- Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. doi:10.2215/CJN.03980609
- Lin CJ, Liu HL, Pan CF, et al. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res. 2012;43(6):451–456. doi:10.1016/j.arcmed.2012.08.002
- Asami M, Tanabe K, Ito S, et al. Impact of indoxyl sulfate on coronary plaques in patients on hemodialysis. Int Heart J. 2018;59(3):489–496. doi:10.1536/ihj.17-351
- Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69(10):1780–1785. doi:10.1038/sj.ki.5000340
- Yisireyili M, Saito S, Abudureyimu S, et al. Indoxyl sulfate-induced activation of (pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS One. 2014;9(10):e109268. doi:10.1371/journal.pone.0109268
- Azechi T, Sato F, Sudo R, Wachi H. 5-aza-2ʹ-Deoxycytidine, a DNA methyltransferase inhibitor, facilitates the inorganic phosphorus-induced mineralization of vascular smooth muscle cells. J Atheroscler Thromb. 2014;21(5):463–476. doi:10.5551/jat.20818
- Xie SA, Zhang T, Wang J, et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: role of DNA methyltransferase 1. Biomaterials. 2018;155:203–216. doi:10.1016/j.biomaterials.2017.11.033
- Chen J, Zhang X, Zhang H, et al. Indoxyl sulfate enhance the hypermethylation of klotho and promote the process of vascular calcification in chronic kidney disease. Int J Biol Sci. 2016;12(10):1236–1246. doi:10.7150/ijbs.15195
- Montes de Oca A, Madueno JA, Martinez-Moreno JM, et al. High-phosphate-induced calcification is related to SM22alpha promoter methylation in vascular smooth muscle cells. J Bone Miner Res. 2010;25(9):1996–2005. doi:10.1002/jbmr.93
- Hou YC, Lu CL, Yuan TH, Liao MT, Chao CT, Lu KC. The epigenetic landscape of vascular calcification: an integrative perspective. Int J Mol Sci. 2020;21(3):980. doi:10.3390/ijms21030980
- Fakhry M, Skafi N, Fayyad-Kazan M, et al. Characterization and assessment of potential microRNAs involved in phosphate-induced aortic calcification. J Cell Physiol. 2018;233(5):4056–4067. doi:10.1002/jcp.26121
- Massy ZA, Metzinger-le Meuth V, Metzinger L. MicroRNAs are associated with uremic toxicity, cardiovascular calcification, and disease. Contrib Nephrol. 2017;189:160–168.
- Xu TH, Qiu XB, Sheng ZT, et al. Restoration of microRNA-30b expression alleviates vascular calcification through the mTOR signaling pathway and autophagy. J Cell Physiol. 2019;234(8):14306–14318. doi:10.1002/jcp.28130
- Panizo S, Naves-Diaz M, Carrillo-Lopez N, et al. MicroRNAs 29b, 133b, and 211 regulate vascular smooth muscle calcification mediated by high phosphorus. J Am Soc Nephrol. 2016;27(3):824–834. doi:10.1681/ASN.2014050520
- Zhang H, Chen J, Shen Z, et al. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/beta-catenin signaling. Toxicol Lett. 2018;284:29–36. doi:10.1016/j.toxlet.2017.11.033
- Wen P, Cao H, Fang L, et al. miR-125b/Ets1 axis regulates transdifferentiation and calcification of vascular smooth muscle cells in a high-phosphate environment. Exp Cell Res. 2014;322(2):302–312. doi:10.1016/j.yexcr.2014.01.025
- Kétszeri M, Kirsch A, Frauscher B, et al. MicroRNA-142-3p improves vascular relaxation in uremia. Atherosclerosis. 2019;280:28–36. doi:10.1016/j.atherosclerosis.2018.11.024
- Fourdinier O, Schepers E, Metzinger-Le Meuth V, et al. Serum levels of miR-126 and miR-223 and outcomes in chronic kidney disease patients. Sci Rep. 2019;9(1):4477. doi:10.1038/s41598-019-41101-8
- Lee CT, Lee YT, Tain YL, Ng HY, Kuo WH. Circulating microRNAs and vascular calcification in hemodialysis patients. J Int Med Res. 2019;47(7):2929–2939. doi:10.1177/0300060519848949
- Wang CL, Lin KP, Hsu GW, Liu KL, Guo CH. Altered mineral metabolism and disequilibrium between calcification promoters and inhibitors in chronic hemodialysis patients. Biol Trace Elem Res. 2019.
- Akbari M, Nayeri H, Nasri H. Association of fetuin-A with kidney disease; a review on current concepts and new data. J Nephropharmacol. 2019;8(2).
- Dai L, Qureshi AR, Witasp A, Lindholm B, Stenvinkel P. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J. 2019;17:721–729. doi:10.1016/j.csbj.2019.06.015
- Maréchal C, Schlieper G, Nguyen P, et al. Serum fetuin-A levels are associated with vascular calcifications and predict cardiovascular events in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(5):974–985. doi:10.2215/CJN.06150710
- Koca N, Ersoy A, Şensoy B, et al. The association between cardiac valvular calcification and fetuin-A levels in kidney transplant recipients. Clin Exp Nephrol. 2019;23(10):1250–1256. doi:10.1007/s10157-019-01761-2
- Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278(24):22144–22152. doi:10.1074/jbc.M300744200
- Holt SG, Smith ER. Fetuin-A-containing calciprotein particles in mineral trafficking and vascular disease. Nephrol Dial Transplant. 2016;31(10):1583–1587. doi:10.1093/ndt/gfw048
- Ter Braake AD, Shanahan CM, de Baaij JHF. Magnesium counteracts vascular calcification: passive interference or active modulation? Arterioscler Thromb Vasc Biol. 2017;37(8):1431–1445. doi:10.1161/ATVBAHA.117.309182
- Montezano AC, Zimmerman D, Yusuf H, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56(3):453–462. doi:10.1161/HYPERTENSIONAHA.110.152058
- Louvet L, Metzinger L, Buchel J, Steppan S, Massy ZA. Magnesium attenuates phosphate-induced deregulation of a microRNA signature and prevents modulation of Smad1 and osterix during the course of vascular calcification. Biomed Res Int. 2016;2016:7419524. doi:10.1155/2016/7419524
- Montes de Oca A, Guerrero F, Martinez-Moreno JM, et al. Magnesium inhibits Wnt/beta-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One. 2014;9(2):e89525. doi:10.1371/journal.pone.0089525
- Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol. 1987;65(4):729–745. doi:10.1139/y87-120
- Ter Braake AD, Tinnemans PT, Shanahan CM, Hoenderop JGJ, de Baaij JHF. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep. 2018;8(1):2069. doi:10.1038/s41598-018-20241-3
- Briet M, Burns KD. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci (Lond). 2012;123(7):399–416. doi:10.1042/CS20120074
- Herencia C, Rodriguez-Ortiz ME, Munoz-Castaneda JR, et al. Angiotensin II prevents calcification in vascular smooth muscle cells by enhancing magnesium influx. Eur J Clin Invest. 2015;45(11):1129–1144. doi:10.1111/eci.12517
- de Borst MH, Vervloet MG. Ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603–1609. doi:10.1681/ASN.2010121251
- Goldfarb S, Martin K. Disorders of divalent ions, renal bone disease and nephrolithiasis. Nephrol Self Assess Program. 2018;17(3):205–209.
- Lu KC, Wu CC, Yen JF, Liu WC. Vascular calcification and renal bone disorders. Sci World J. 2014;2014:637065. doi:10.1155/2014/637065
- Kukida M, Mogi M, Kan-No H, et al. AT2 receptor stimulation inhibits phosphate-induced vascular calcification. Kidney Int. 2019;95(1):138–148. doi:10.1016/j.kint.2018.07.028
- Wang J, Zhou JJ, Robertson GR, Lee VW. Vitamin D in vascular calcification: a double-edged sword? Nutrients. 2018;10(5):652. doi:10.3390/nu10050652
- Nitta K, Ogawa T, Hanafusa N, Tsuchiya K. Recent advances in the management of vascular calcification in patients with end-stage renal disease. Contrib Nephrol. 2019;198:62–72.
- Schantl AE, Verhulst A, Neven E, et al. Inhibition of vascular calcification by inositol phosphates derivatized with ethylene glycol oligomers. Nat Commun. 2020;11(1):721. doi:10.1038/s41467-019-14091-4
- Schantl AE, Ivarsson ME, Leroux JCJAT. Investigational pharmacological treatments for vascular calcification. Adv Ther. 2019;2(1):1800094. doi:10.1002/adtp.201800094
- Chao CT, Yeh HY, Tsai YT, et al. Natural and non-natural antioxidative compounds: potential candidates for treatment of vascular calcification. Cell Death Discov. 2019;5:145. doi:10.1038/s41420-019-0225-z
- Henze LA, Luong TTD, Boehme B, et al. Impact of C-reactive protein on osteo-/chondrogenic transdifferentiation and calcification of vascular smooth muscle cells. Aging (Albany NY). 2019;11(15):5445–5462. doi:10.18632/aging.102130
- Chen B, Zhao Y, Han D, et al. Wnt1 inhibits vascular smooth muscle cell calcification by promoting ANKH expression. J Mol Cell Cardiol. 2019;135:10–21. doi:10.1016/j.yjmcc.2019.07.008
- Wei R, Enaka M, Muragaki Y. Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Sci Rep. 2019;9(1):10366. doi:10.1038/s41598-019-46824-2
- Li Z, Wu J, Zhang X, et al. CDC42 promotes vascular calcification in chronic kidney disease. J Pathol. 2019;249(4):461–471. doi:10.1002/path.5334
- Paloian NJ, Leaf EM, Giachelli CM. Osteopontin protects against high phosphate-induced nephrocalcinosis and vascular calcification. Kidney Int. 2016;89(5):1027–1036. doi:10.1016/j.kint.2015.12.046
- Kuo TH, Lin WH, Chao JY, et al. Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: a cross-sectional study. BMC Nephrol. 2019;20(1):266. doi:10.1186/s12882-019-1452-5
- Carracedo M, Witasp A, Qureshi AR, et al. Chemerin inhibits vascular calcification through ChemR23 and is associated with lower coronary calcium in chronic kidney disease. J Intern Med. 2019;286(4):449–457. doi:10.1111/joim.12940
- Hawkins CL. Protein carbamylation: a key driver of vascular calcification during chronic kidney disease. Kidney Int. 2018;94(1):12–14. doi:10.1016/j.kint.2018.03.022
- Mori D, Matsui I, Shimomura A, et al. Protein carbamylation exacerbates vascular calcification. Kidney Int. 2018;94(1):72–90. doi:10.1016/j.kint.2018.01.033
- Kaesler N, Goettsch C, Weis D, et al. Magnesium but not nicotinamide prevents vascular calcification in experimental uraemia. Nephrol Dial Transplant. 2019. doi:10.1093/ndt/gfy410
- Nagy A, Petho D, Gall T, et al. Zinc Inhibits HIF-Prolyl hydroxylase inhibitor-aggravated VSMC calcification induced by high phosphate. Front Physiol. 2019;10:1584. doi:10.3389/fphys.2019.01584
- Shin MY, Kwun IS. Zinc restored the decreased vascular smooth muscle cell viability under atherosclerotic calcification conditions. Prev Nutr Food Sci. 2014;19(4):363–366. doi:10.3746/pnf.2014.19.4.363
- Voelkl J, Tuffaha R, Luong TTD, et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-kappaB. J Am Soc Nephrol. 2018;29(6):1636–1648. doi:10.1681/ASN.2017050492
- Liu H, Zhang X, Zhong X, et al. Puerarin inhibits vascular calcification of uremic rats. Eur J Pharmacol. 2019;855:235–243. doi:10.1016/j.ejphar.2019.05.023
- Gueiros APS, Gueiros JEB, Nobrega KT, et al. Effect of spironolactone on the progression of coronary calcification in peritoneal dialysis patients: a pilot study. J Bras Nefrol. 2019;41(3):345–355. doi:10.1590/2175-8239-jbn-2019-0009
- Wang P, Quan Z, Luo D, Chen W, Peng D. Spironolactone dose-dependently alleviates the calcification of aortic rings cultured in hyperphosphatemic medium with or without hyperglycemia by suppressing phenotypic transition of VSMCs through downregulation of Pit1. Mol Med Rep. 2019;19(5):3622–3632. doi:10.3892/mmr.2019.10039
- Agharazii M, St-Louis R, Gautier-Bastien A, et al. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens. 2015;28(6):746–755. doi:10.1093/ajh/hpu225
- Koike S, Yano S, Tanaka S, Sheikh AM, Nagai A, Sugimoto T. Advanced Glycation End-Products Induce Apoptosis of Vascular Smooth Muscle Cells: A Mechanism for Vascular Calcification. Int J Mol Scip. 2016;17(5):pii: E1567. doi:10.3390/ijms17091567
- Bisson SK, Ung RV, Picard S, et al. High calcium, phosphate and calcitriol supplementation leads to an osteocyte-like phenotype in calcified vessels and bone mineralisation defect in uremic rats. J Bone Miner Metab. 2019;37(2):212–223. doi:10.1007/s00774-018-0919-y
