Abstract
Purpose
The aim of this study was to determine the long-term safety of drug-eluting stent (DES) versus bare metal stent (BMS) implantation in a “real-world” setting.
Patients and methods
A total of 1809 patients who were treated with implantation of either BMS or DES were assessed. Kaplan-Meier and multivariate Cox regression analyses concerning primary endpoint of cardiac mortality were performed.
Results
A total of 609 patients received DES. Mean age was 66.2 ± 11.3 years, 69.4% were male, and 1517 (83.8%) were treated for acute coronary syndrome (unstable angina 510 [28.2%], non-ST-elevation myocardial infarction [NSTEMI] 506 [28.0%], and ST-elevation myocardial infarction [STEMI] 501 [27.7%]). Mean follow-up was 34 ± 15 months. During follow-up, 268 patients died of cardiac causes (DES 42 [7.3%]; BMS 226 [19.6%]; P < 0.001). Univariate Kaplan-Meier analysis showed an advantage of DES over BMS concerning the primary end-point (P < 0.001). When adjusting for classic risk factors and additional factors that affect the progression of coronary heart disease (CHD), DES was not found to be superior to BMS (hazard ratio 0.996, 95% confidence interval 0.455–2.182, P = 0.993). Severely impaired renal function was an independent predictor for cardiac mortality after stent implantation.
Conclusion
Treatment with DES is safe in the long term, also in patients presenting with STEMI. However, in multivariate analyses it is not superior to BMS treatment.
Introduction
Stent implantation has been established as a safe and effective method of treating coronary heart disease (CHD).Citation1,Citation2 Several clinical trials investigating the safety and efficacy of drug-eluting stents (DES) versus bare metal stents (BMS) showed an advantage of DES over BMS concerning restenosis.Citation3–Citation5 Since being introduced into clinical practice in 2003, DES have been increasingly used in patients with stable and unstable CHD.Citation6
Late stent thrombosis as a fatal event 12 or more months after DES implantation is associated with a mortality of up to 45%Citation7,Citation8 and represents a major concern. Therefore, the long-term safety of DES has been extensively discussed in the past several years. Various authors reported an increasing incidence of cardiac death related to DES implantation.Citation9–Citation11 One of the main reasons for the increase in cardiac and cardiovascular mortality after DES treatment seems to be the fact that patient populations in these trials are highly selected and, thus, applying the findings to “real-world” use has limitations.
The aim of this study, therefore, was to determine the safety of DES versus BMS implantation in a “real-world” setting.
Patients and methods
Patients, demographic, clinical, and angiographic data
A total of 2056 CHD patients in whom a percutaneous coronary intervention (PCI) was performed with implantation of a BMS or DES at the Department of Medicine III, Martin Luther-University Halle-Wittenberg between 2004 and 2006 were retrospectively assessed. Inclusion criterion was the implantation of a DES or BMS. Exclusion criteria included balloon dilatation without stent implantation or unsuccessful PCI and the decision for solely medical treatment. The choice of stent type was up to the interventional cardiologist based on national and international guidelines and recommendations. Thus, DES were more frequently used in patients with stable or unstable angina in native vessels, whereas BMS were more frequently implanted in patients presenting with acute myocardial infarction and more complicated lesions (ie, bifurcational or ostial lesions, unprotected left main).Citation7 In case a patient received both stent types during the hospital stay, she/he was assigned to the drug-eluting stent cohort. PCI during a prior hospital stay was not considered in this analysis.
Data were collected concerning admission diagnosis (stable angina [SAP], unstable angina [UAP], non-ST-elevation myocardial infarction [NSTEMI], ST-elevation myocardial infarction [STEMI]), bodyweight, height, medical history, classic risk factors (family history of CHD, diabetes mellitus, hyperlipidemia, hypertension, and current smoking), history of transient ischemic attacks (TIA) or stroke, current medication, duration of hospital stay, laboratory test results, and angiographic data. Left ventricular ejection fraction was measured by routinely performed echocardiography.
In-hospital adverse events included cardiac and noncardiac death, myocardial infarction, stroke/TIA, re-PCI, operative revascularization, and acute renal failure. Bleeding complications were recorded as puncture site bleeding, hematoma, aneurysm, arteriovenous fistula, gastrointestinal bleeding, intracranial bleeding, and minor bleeding complications (not related to puncture site, eg, epistaxis).
Primary endpoint was death from cardiac causes. This information was (as described in the following) obtained from electronic patient files, physicians, relatives, and civil registration offices.
Follow-up
For acquiring follow-up data a standardized questionnaire was sent out, which included questions concerning adverse events such as hospital admission, re-PCI or surgical coronary revascularization, bleeding complications, and thromboembolic events. If the patients did not send back the questionnaires, a telephone interview was conducted with the patient or his/her relatives or the patient’s physician was contacted. If this information could not be obtained from these persons, civil registration offices were contacted and information was requested about current address or date of death. The study was approved by the ethics committee of the Martin Luther-University Halle-Wittenberg.
Statistical analyses
Continuous variables were described as mean and standard deviation; skewed variables as median and 25% and 75% quartiles. Categorical variables were documented as a percentage. For comparison of metric, normally distributed variables, t-test was used. Mann-Whitney U-test was used to compare skewed variables. For normally distributed, categorical variables, the chi-square test was employed. Survival analyses included Kaplan-Meier analyses with log-rank test and multivariate Cox regression analyses. Multivariate Cox regression was applied to analyze the influence of DES on the primary endpoint, which included the classic risk factors (gender, body mass index, current smoking, diabetes mellitus, hypertension, hyperlipidemia, and family history of CHD) and additional factors affecting prognosis after stent implantation (age, hemoglobin, C-reactive protein, low density lipoprotein [LDL] cholesterol, glomerular filtration rate, and left ventricular ejection fraction).
P-values <0.05 were considered significant. Statistical analyses were performed using SPSS Statistics (SPSS Inc, Chicago, IL) software.
Results
Patient characteristics
Of the 2056 patients, 1809 individuals met the inclusion criteria: 609 patients received DES, and 1200 received BMS. At the time of admission to the hospital, the mean age was 66.2 ± 11.3 years; patients treated with DES were significantly younger than patients treated with BMS (60.9 ± 11.4 versus 68.9 ± 10.2 years, P < 0.001). Of the patients, 69.4% were male (73.6% in the DES group versus 67.2% in the BMS group (P = 0.007) ().
Table 1 Baseline characteristics, admission diagnosis, and number of diseased vessels
The two groups also differed significantly regarding other cardiovascular risk factors: the prevalence of diabetes mellitus (27.6% versus 38.6%, P < 0.001) and hypertension (69.3% versus 74.2%, P = 0.020) was significantly higher in patients treated with BMS, whereas a positive family history of CHD (30.0% versus 17.4%, P < 0.001), current smoking (27.8% versus 20.2%, P < 0.001), and hyperlipidemia (40.9% versus 34.0%, P = 0.005) was found more often in patients treated with DES ().
The mean left ventricular ejection fraction was .50% in both groups; however, it was significantly lower in the BMS group (52% ± 16% BMS versus 56% ± 15% DES, P < 0.001) ().
Interestingly, the two groups did not differ significantly regarding the medication at admission to the hospital and at discharge, except for the use of diuretic agents, which were used more frequently in the BMS group.
Indication for hospital admission
A total of 258 (14.3%) patients suffered from stable angina, and 1517 (83.9%) suffered from acute coronary syndrome (unstable angina 510 [28.2%], NSTEMI 506 [28.0%], and STEMI 501 [27.7%]). In the DES group, the rate of stable angina was significantly higher than in the BMS group (19.9% versus 11.4%, P < 0.001). Unstable angina and STEMI were found more often in the BMS group, whereas NSTEMI did not differ between the two groups ().
Laboratory results
Markers of inflammation (C-reactive protein and white blood cell count) were slightly, but significantly different in the two groups (). Further prognostically relevant parameters,Citation12,Citation13 ie, hemoglobin and LDL cholesterol, were within normal range in the group as a whole. Nevertheless, the mean value of these parameters was slightly higher in the DES group than in the BMS group. The glomerular filtration rate, calculated via the Cockroft-Gault formula, was significantly decreased in the BMS group. shows the details for these markers.
In-hospital adverse events
The median duration of hospital stay was 7 (4/11) days (DES 6 [3/9] versus BMS 8 [6/12], P = 0.042). In-hospital cardiac (5.7% versus 0.5%, P = 0.035) and in-hospital noncardiac death (1.1% versus 0.2%, P < 0.001) occurred more frequently in the BMS than in the DES group. The results were similar regarding incidence of myocardial infarctions (DES 0.3% versus BMS 1.3%, P = 0.042). Bleeding complications were observed more frequently in the BMS cohort than in the DES cohort, especially gastrointestinal and minor bleedings.
Follow-up and survival analyses
Follow-up data concerning the primary endpoint of cardiac death were obtained for 1730 patients (95.6%). The mean follow-up was 34 ± 15 months. During follow-up, 376 patients died of any causes (59 in the DES group [10.2%] and 317 in the BMS group [27.5%], P < 0.001). The incidence of death of cardiac causes was 7.3% in the DES and 19.6% in the BMS group (P < 0.001).
In addition, the incidence of stroke (3.1% versus 5.5%, P = 0.037) and coronary artery bypass surgery (DES 1.9% versus BMS 5.3%, P = 0.002) was lower in the DES group. The incidence of other adverse events during follow-up did not differ between the two groups ().
Table 2 Adverse events during follow-up
Kaplan-Meier analysis showed an increased cardiac mortality in the BMS group () (P < 0.001 via log-rank test). According to the multivariate model, including the classic risk factors and additional risk factors with prognostic relevance for progression of CHD, DES was not superior to BMS (hazard ratio [HR] 0.996, 95% confidence interval [CI] 0.455–2.182, P = 0.993) (). In this “fully adjusted model,” only glomerular filtration rate (GFR) was an independent predictor for cardiac mortality after PCI with stent implantation. Compared to a GFR > 90 mL/min, a GFR between 15 and 30 mL/min increased the HR to 6.788 (95% CI 1.406–32.764, P = 0.017) and one of <15 mL/min to 7.011 (95% CI 1.228–40.024, P < 0.028), respectively (see ).
Figure 1 Kaplan-Meier analysis for cardiac death, DES versus BMS.
Abbreviations: DES, drug-eluting stent; BMS, bare-metal stent.
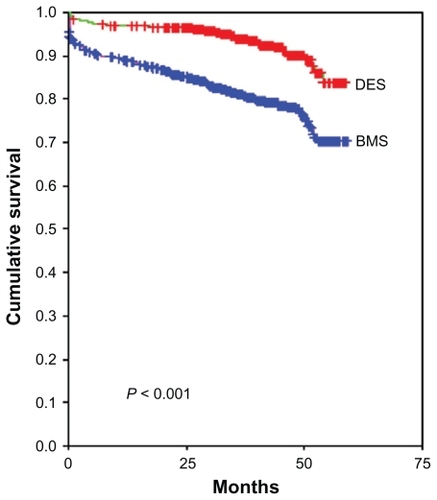
Table 3 Multivariate Cox regression analysis
Discussion
After being introduced into daily clinical practice, the use of DES in PCI increased until several papers reported concerns about the long-term safety of these stents.Citation9,Citation10,Citation14 Findings from various registries and meta-analyses showed an increase in all-cause mortality and the rate of major cardiovascular eventsCitation9,Citation15 after DES implantation as compared with BMS-treated patients. The aim of the present study was to compare mortality in an unselected patient population undergoing PCI with DES versus BMS implantation.
As described in the results section, the two groups in part significantly differed concerning the prevalence of cardiovascular risk factors (current smoking, diabetes mellitus, hypertension, hyperlipidemia, and positive family history). Also regarding the number of diseased vessels and the indication for PCI (ie, SAP, UAP, NSTEMI, and STEMI), significant differences can be observed. Patients presenting with SAP or UAP or 1-vessel-disease more often received DES, whereas patients suffering from STEMI or multivessel disease more often received BMS. These differences can be explained by the indications given in the revascularization guidelines.Citation7 Meanwhile, current guidelines give a wider range of indications for the use of DES in more complex lesions and patient subsets.Citation16
According to univariate analysis, DES are associated with an improved survival compared with BMS (P < 0.001) (). However, in multivariate Cox regression analyses that include classic risk factors and further prognostic variables, use of DES completely lost its prognostic value. Also hemoglobin level, age, diabetes mellitus, and left ventricular ejection fraction, which are well known predictors for worse outcome of CHD patients, were not independently associated with the primary endpoint. In this model, the only independent predictor for cardiac mortality after PCI with stent implantation was impaired renal function. The authors conclude therefore that in a routine setting DES and BMS implantation are equally safe in terms of the occurrence of death of cardiac causes in the long term.
The findings of this present study are partly in line with recently published data on the safety of DES in the long and short term. Randomized studies taking diabetes mellitus, myocardial infarction, and multivessel disease as inclusion criteriaCitation17–Citation21 show encouraging results for the use of DES in these patient populations.
In patients with impaired renal function, systemic changes such as chronic inflammation, oxidative stress, anemia, a procoagulative milieu and endothelial dysfunction promote the progression of atherosclerotic changes and therefore also count as cardiovascular risk factors.Citation22–Citation24 Studies dealing with the impact of DES compared to BMS on the outcome in patients with renal impairment showed that the benefit of DES implantation depends on the degree of renal impairment. Whereas patients with normal renal function or mildly impaired renal function (ie, creatinine clearance >60 mL/min and 40[30]–60 mL/min, respectively) profit from DES implantation,Citation25–Citation28 this advantage can no longer be seen in patients with moderate or severe renal impairment (CrCl 15–30 mL/min and <15 mL/min, respectively). The advantages for DES are mainly driven by a lower rate of revascularization (target vessel revascularization and target lesion revascularization), whereas no advantage concerning the occurrence of major adverse cardiac events, including cardiac death or death from any cause, was found. The fact that in this present study, impaired renal function was the only independent predictor in the multivariate model for worse outcome in patients after PCI with stent implantation emphasizes the influence of renal insufficiency on the progression of CHD (see ).
Patients who suffer myocardial infarction (STEMI) comprise a high-mortality population. The use of stents is considered standard treatment in these patients. Nevertheless, the use of DES under these circumstances is the subject of controversial discussion. Recently published results of randomized controlled trials included 300–700 patients and evaluated angiographic and clinical events 9 months and 12 months after PCI, respectively.Citation19,Citation20,Citation29,Citation30 Most of them concluded that DES is safe in STEMI and decreases the rate of re-interventions, but they did not find any advantage for DES concerning death or recurrent myocardial infarction. In contrast to these findings the results of observational studies which covered a follow-up period of 30 days up to 2 years in “real-world” settings varied. They ranged from a significantly increased risk-adjusted mortality in patients treated with DESCitation31 over comparable outcomes regarding long-term mortalityCitation32 to a lower risk-adjusted mortality for DES.Citation33,Citation34
Among the patients in this present study, 501 presented with acute myocardial infarction with ST-segment elevation. Of them, 126 were treated with DES and 375 with BMS. When applying univariate Kaplan-Meier analysis with log-rank test, DES was an independent factor concerning cardiac mortality (P = 0.005). In multivariate analysis including the abovementioned prognostically relevant factors, DES was no longer an independent factor (HR 0.114, 95% CI 0.007–1.805, P = 0.123; data not shown in the results section). However, the use of DES in the “real-world” patient collective was not linked to poorer survival.
Conclusion
Treatment of patients with DES is safe in the long term, also in the subgroup of patients presenting with STEMI; however, it is not superior to BMS treatment.
Limitation
As this study represents the results of “real-life” practice, the choice of stent type was up to the interventional cardiologist based on national and international guidelines and recommendations. Thus, a limitation of this study is the smaller sample size of patients treated with DES in comparison to patients treated with BMS.
Disclosure
The authors report no conflicts of interest in this work.
References
- SerruysPde JaegerePKiemeneijFA comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study GroupN Engl J Med199433184894958041413
- FischmanDLeonMBaimDA randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery diseaseN Engl J Med19943314965018041414
- MoriceMSerruysPSousaJA randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularizationN Eng J Med20023462317731780
- MosesPLeonMPopmaJSirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary arteryN Engl J Med2003349141315132314523139
- GrubeESilberSHauptmannKSix- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions (TAXUS I)Circulation20031073812515740
- SerruysPWKutrykMJOngATCoronary-artery stentsN Engl J Med200635448349516452560
- SilberSAlbertssonPAvilésFThe task force for percutaneous coronary interventions of the European Society of CardiologyEur Heart J200526880484715769784
- GrinesCBonowRCaseyDJPrevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of PhysiciansCirculation2007115681381817224480
- LagerquistBJamesSStenestrandULindbäckJNilssonTWallentinLLong-term outcomes with drug-eluting stents versus bare-metal stents in SwedenN Engl J Med2007356101009101917296822
- CamenzindEStegGWijnsWStent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concernCirculation20071151440145517344324
- PfistererMBrunner-LaRoccaHBuserPLate clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stentsJ Am Coll Cardiol200648122584259117174201
- NikolskyEAymongEHalkinAImpact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) TrialJ Am Coll Cardiol200444354755315358018
- HalkinASinghMNikolslyEPrediction of Mortality After Primary Percutaneous Coronary Intervention for Acute Myocardial Infarction: The CADILLAC risk scoreJ Am Coll Cardiol20054591397140515862409
- DaemenJWenaweserPTsuchidaKEarly and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort studyLancet2007369956266767817321312
- NordmannABrielMBucherHMortality in randomized controlled trials comparing drug-eluting vs bare metal stents in coronary artery disease: a meta-analysisEur Heart J200627232784281417020889
- WijnsWKolhPDanchinNGuidelines on myocardial revascularizationEur Heart J2010312501255520802248
- MarestaAVaraniEBalducelliMComparison of effectiveness and safety of sirolimus-eluting stents versus bare-metal stents in patients with diabetes mellitus (from the Italian Multicenter Randomized DESSERT Study)Am J Cardiol2008101111560156618489933
- BaumgartDKlaussVHartmannFOne-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patientsJ Am Coll Cardiol200750171627163417950142
- SpauldingCHenryPTeigerESirolimus-eluting versus uncoated stents in acute myocardial infarctionN Engl J Med2006355111093110416971716
- LaarmanGSuttorpMJDirksenMTPaclitaxel-eluting versus uncoated stents in primary percutaneous coronary interventionN Engl J Med2006355111105111316971717
- ChechiTVittoriGBiondi ZoccaiGSingle-center randomized evaluation of paclitaxel-eluting versus conventional stent in acute myocardial infarction (SELECTION)J Interv Cardiol200720428229117680858
- SarnakMLeveyASchoolwerthAKidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and PreventionCirculation20081082154216914581387
- LuftFRenal disease as a risk factor for cardiovascular diseaseBasic Res Cardiol200095Suppl 1I727611192357
- GuptaRBirnbaumYUretskyBThe renal patient with coronary artery disease: current concepts and dilemmasJ Am Coll Cardiol20044471343135315464310
- KimBOhSJeonDYangJLong-term clinical outcomes and stent thrombosis of sirolimus-eluting versus bare metal stents in patients with end-stage renal disease: results of Korean multicenter angioplasty team (KOMATE) RegistryJ Interv Cardiol20092241141919702679
- HalkinAMehranRCaseyCImpact of moderate renal insufficiency on restenosis and adverse clinical events after paclitaxel-eluting and bare metal stent implantation: results from the TAXUS-IV TrialAm Heart J20051501163117016338253
- AoyamaTIshiiHToriyamaTSirolimus-eluting stents vs bare metal stents for coronary intervention in Japanese patients with renal failure on hemodialysisCirc J200872566018159100
- ShawJAndrianopoulosNDuffySRenal impairment is an independent predictor of adverse events post coronary intervention in patients with and without drug-eluting stentsCardiovasc Revasc Med20089421822318928945
- van der HoevenBLiemSJukemaJSirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION! Intervention StudyJ Am Coll Cardiol20085161862618261680
- ValgimigliMPercocoGMalaguttiPTirofiban and sirolimuseluting stent vs abciximab and bare-metal stent for acute myocardial infarction: a randomized trialJAMA20052932109211715870414
- StegPFoxKEagleKMortality following placement of drugeluting and bare-metal stents for ST-segment elevation acute myocardial infarction in the Global Registry of Acute Coronary EventsEur Heart J20093032132919147604
- LemosPSaiaFHofmaSShort- and long-term clinical benefit of sirolimus-eluting stents compared to conventional bare stents for patients with acute myocardial infarctionJ Am Coll Cardiol20044370470814975486
- MauriLSilbaughTGargPDrug-eluting or bare-metal stents for acute myocardial infarctionN Engl J Med20083591330134218815397
- SlottowTSteinbergDRoyPDrug-eluting stents are associated with similar cardiovascular outcomes when compared to bare metal stents in the setting of acute myocardial infarctionCardiovasc Revasc Med20089242818206634