Abstract
Cardiovascular diseases (CVDs) are the leading cause of death worldwide. The use of stem cells to improve recovery of the injured heart after myocardial infarction (MI) is an important emerging therapeutic strategy. However, recent reviews of clinical trials of stem cell therapy for MI and ischemic heart disease recovery report that less than half of the trials found only small improvements in cardiac function. In clinical trials, bone marrow, peripheral blood, or umbilical cord blood cells were used as the source of stem cells delivered by intracoronary infusion. Some trials administered only a stem cell mobilizing agent that recruits endogenous sources of stem cells. Important challenges to improve the effectiveness of stem cell therapy for CVD include: (1) improved identification, recruitment, and expansion of autologous stem cells; (2) identification of mobilizing and homing agents that increase recruitment; and (3) development of strategies to improve stem cell survival and engraftment of both endogenous and exogenous sources of stem cells. This review is an overview of stem cell therapy for CVD and discusses the challenges these three areas present for maximum optimization of the efficacy of stem cell therapy for heart disease, and new strategies in progress.
Introduction
The recovery of function after a myocardial infarction (MI) is dependent on increasing blood flow and regeneration of tissue. Stem cells (SCs) can provide cellular precursors for cardiomyocyte differentiation, endothelial and supporting cells, as well as signals for activation of cells and prevention of apoptosis. The results of clinical trials have been encouraging, however either no change or only small increments in recovery were found. Recent reviews of completed clinical trials (2002–2010) for SC therapy report improvements of 10% or less in about half of the studies.Citation1–Citation4 In the review by George,Citation1 13 studies of SC therapy for acute MI were described. In the eight randomized controlled studies, bone-marrow (BM) cells were administered by intracoronary injection and left ventricular ejection fraction (LVEF) measured 3–6 months following the MI. In five of the randomized controlled trials, there was only an average increase of 6% (3%–12%) in cardiac function. Mozid et alCitation2 reported two additional studies of BM SC therapy for acute MI,Citation5,Citation6 and only one study showed improvement (5%) of LVEF function. Mozid et alCitation2 also described eight clinical trials of SC therapy for chronic ischemic heart failure. There was improvement in LVEF in three of the four studies in patients treated with BM SCs and improvement in two of the four studies in patients transplanted with autologous skeletal myoblasts. Wen et alCitation4 performed a meta-analysis of eight randomized controlled trials and concluded that BM cell therapy provided only moderate (6%–10%) but definite improvements in LVEF. SC therapy has the potential to provide gains not only for MI, but also for chronic ischemia and heart failure. Currently, there are 33 ongoing clinical trials described on the ClinicalTrials.gov WebsiteCitation7 (see ). While autologous BM cells are still the major source of SCs in the ongoing studies, new SC sources are rigorously being investigated. SC therapy for cardiovascular disease (CVD) is an intensive area of research, and collective improvements in the source and number of SCs, and better mobilizing and homing agents, are needed to increase the effectiveness of this emerging therapy.
Table 1 Ongoing clinical trials of stem-cell therapy for heart diseases
Challenges for SC therapy
Improved identification and expansion of autologous SCs and their role in cardiac recovery
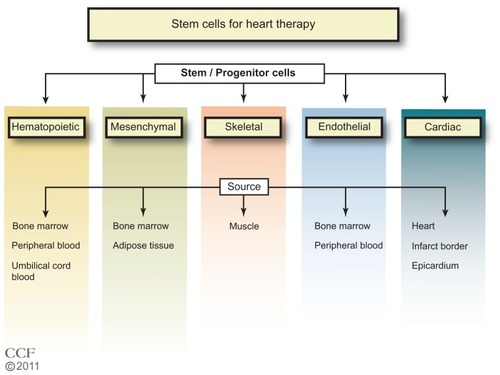
In the 1960s, Till et al,Citation8 while studying the components responsible for regenerating blood cells, defined two required properties of SCs: (1) self-renewal – the ability to go through numerous cycles of cell division while maintaining the undifferentiated state; and (2) potency – the capacity to differentiate into specialized cell types. SCs are identified by their capacity to form colonies in culture and by cell surface markers that are cell specific. The majority of clinical trials of SC therapy for heart disease have used BM cells, particularly the mononuclear cells (MNCs) (). In the ongoing trials listed in , other types of SCs are being tested, including specific BM, CD34+ or CD133+, and mesenchymal cells. One study tests adipose tissue-derived SCs, and three trials are testing cardiac progenitor/stem cells.
Skeletal myoblasts
Skeletal myoblasts isolated from muscle biopsies were the first cells used for the SC therapy for cardiac recovery.Citation9 In a comparison of rats with chronic MI, treated with human skeletal myoblasts or BM-derived CD133+ progenitors, improvements in cardiac function were similar with the two cell types.Citation10,Citation11 In trials of skeletal myoblast treatmentCitation3 in patients with chronic ischemic heart failure, there were improvements in LVEF in two of four studies (SEISMIC, TOPCARD-CHD).Citation3 While the initial evaluation in clinical studies of skeletal myoblast treatment showed there was improved function, the effect was not sustained, and the cells were not electrically integrated into the heart.Citation12 Enthusiasm for this approach has waned. However, second-generation products are now being developed.Citation9,Citation13 Six trials of skeletal myoblast therapy have been discontinued, but currently there are two active trials with skeletal myoblasts () for patients with an old MI (PERCUTANEO) or congestive heart failure (MARVEL).
Hematopoietic progenitor/stem cells (HPSCs)
In clinical trials for MI or ischemic heart disease, BM, peripheral blood (PB), or umbilical cord blood (UCB) have been used as the source of SCs.Citation1,Citation3 Autologous BM and PB have an advantage over UCB cells since UCB cells may be at risk for immunological rejection. However, the UCB have a high proliferation potential.Citation12 Autologous BM cells from aging individuals may have reduced transplant efficiency, and UCB cells would be advantageous.Citation14,Citation15 A limitation of the PB is the low yield of SCs. BM is the major source of adult SCs and the best characterized. The BM cells have long been used in therapeutic BM replacement for blood diseases.Citation16–Citation18 BM SCs provide the myeloid and lymphoid lineages that give rise to blood cells.Citation19 The cell surface markers that identify hematopoietic SCs (HSCs) for humans include: CD34+, CD59+, Thy1/CD90+, CD38lo/−, c-kit/CD117+, and lin−. There are differences in mouse HSC markers; namely, CD34lo/−, Sca-1+, Thy1.1+/lo, and CD38+, but with c-kit+ and lin− as common markers. The lineage negative designation includes the absence of 13–14 cell surface markers found on mature cells. BM has been the major source of SCs for reported and ongoing clinical trials. Currently, studies are underway that isolate subsets of the BM cells such as CD34+, and CD133+ for use in therapy. Whether these subsets of SCs will have an advantage in heart disease recovery remains to be seen.
Endothelial SCs
Stages of lineage development of endothelial SCs and their sites of origin are less well defined than those for the hematopoietic lineage.Citation20 The endothelial progenitor cells (EPCs) found in the PB are thought to originate in the BM from a subset of SCs or from the myeloid precursors. There is considerable controversy with regard to the identification of the EPCs.Citation21 Some investigators have identified the EPCs as CD34+ cells and/or CD133+ cells,Citation22 while others view these cells as HPSCs.Citation23,Citation24 Recently,Citation25,Citation26 a consensus definition of EPC markers was suggested for cross-study comparisons and with the cell surface markers CD31+, CD34 bright, and CD45, AC133, CD14, CD14a, CD235a, Live/Dead Violet negative. Of importance for identification of the EPC is the ability to become endothelial cells (ECs) in culture. While CD34+ and/or CD133+ cells in culture may become ECs, the CD34+ and/or CD133+ cells could be a mixture of subpopulations. However, the cells identified as CD34+ and/or CD133+ may be more effective in providing paracrine factors and stimulating neovascularization than the commonly used BM MNCs. Tongers et alCitation27 recently described the results of a clinical trial for patients with refractory angina treated with intramyocardial autologous CD34+ cells, finding significant improvements in angina frequency and exercise tolerance. There is one clinical trial currently underway for treatment with CD34+ in patients with dilated cardiomyopathy, and five clinical trials underway for the treatment of MI, CAD, and heart failure with CD133+ cells. One study, NCT01187654, will compare the treatment of CD133+ cells and BM MNC in MI patients. This comparison could be informative as to whether the CD133+ cells have an advantage over the more frequently used BM MNC. Bissels et alCitation28 found that microRNAs were expressed differentially in CD133+, CD34+, and CD133- cells involved in differentiation, prevention of apoptosis, and cytoskeletal remodeling.
Mesenchymal SCs (MSCs)
The MSCs are found in the BM and other tissues. MSCs are positive for CD44, CD73, CD90 (Thy1), and CD105, and negative for the hematopoietic markers, CD45, lineage markers, EC (CD31), and macrophage (CD11b/MAC-1).Citation29 The MSCs have advantages over HSCs.Citation27,Citation30 Compared with HSCs, MSCs are more abundant, readily proliferate in culture, and are easily differentiated into different cell types, such as adipocytes, fibroblasts, osteocytes, and myoblasts. Further, studies suggest that MSCs may be more potent for cardiac repair than HPSCs.Citation31 Although the MSCs can be differentiated into cardiomyocytes, immortalization was important and could increase the potential of tumor formation. Citation15 In addition to BM, adipose tissue can also be used as an abundant source of MSCs.Citation32,Citation33 The MSCs from UCB, adipose tissue, and BM expressed the same cell surface markers; however, there are some differences in the percentage of certain markers and colony heterogeneity. Gaebel et alCitation34 compared treatment of MI in mice with MSCs from UCB, adipose tissue, and BM. Cells from BM, adipose tissue, and UCB CD105+ showed improvements in heart functions, decreased infarct size, and capillary density. UCB CD105 treated mice had reduced collagen deposition compared with BM and adipose tissue cells, and BM and UCB CD105 cells additionally had reduced apoptosis when compared with mice treated with adipose tissue cells. This study suggests that the function of the MSCs may be dependent on the source. Clinical trials with MSCsCitation35–Citation37 are promising, and currently there are 19 clinical trials underway.Citation7,Citation38 In a recent randomized, double blind, placebo-controlled studyCitation37 with MSC therapy after acute MI; there was improvement in the global assessment of cardiac function at 6 months in 45% of the patients.
Cardiac progenitor cells (CPCs)
Although it had been believed for a long time that cardiac myocytes were terminally differentiated, dividing myocytes found in the heart implied that there are resident or noncardiac cardiomyocyte progenitor cells.Citation39 There have been intensive efforts to identify the cardiomyocyte stem and progenitor cells in the last 10 years.Citation39 Purified cardiomyocytes isolated from rodent hearts dedifferentiate and divide, expressing SC markers such as c-kit, Sca-1, Isl1, and Abcg2.Citation40–Citation45 CPCs have been isolated from human myocardial biopsies.Citation46,Citation47 These same cells can organize into spheres and re-differentiate into myocytes and ECs.Citation48 Yamada et alCitation49,Citation50 suggested that CD133+ cells from brown adipose tissue were highly effective in differentiation into cardiomyocytes compared with HPSCs, and that mouse BAT CD133+ cells efficiently induced BM SCs into cardiomyocytes (CD45- CD31- CD105+) differentiation. There are four ongoing clinical studies to test autologous CPCs (); one study (ALCADIA) will use cardiac-derived SCs to treat ischemic cardiomyopathy, and two studies will take advantage of the cardiosphere-derived stem/progenitor cells (derived from cell outgrowth of autologous cardiac biopsy) for patients with a recent MI (CADUCEUS) or heart failure (TICAP). In the SCIPIO trial, patients with ischemic cardiomyopathy are treated with c-kit+lin− CPCs derived from the right atrial appendage, and initial results from 16 patients report that LVEF increased and infarct size decreased.Citation51
Adipose tissue-derived SCs (ASCs)
Cells isolated from adipose tissue can be separated by centrifugation into adipocytes and stromal vascular cells. The stromal vascular fraction may contain preadipocytes, pericytes and EPCs, adult multipotent MSCs, circulating blood cells, fibroblasts, ECs, smooth-muscle cells, and immune cells. This stromal vascular fraction may differentiate into a number of cell lineages, including the adipocytes, cartilage, bone skeletal muscle, neuronal cells, ECs, cardiomyocytes, and smooth-muscle cells.Citation52,Citation53 ASCs are defined as CD44 and CD105 positive, and Cd11b, CD34, and CD45 negative cells. Although there is disagreement regarding the capacity of ASCs to differentiate into ECs, freshly isolated human ASCs also consist of EPCs (CD11b, CD34, and CD45 positive cells) and when cultured they have a cobblestone appearance and take up acetylated low-density lipoprotein. Bai et alCitation54 found that human freshly isolated adipocytes or cultured adipose tissue-derived cells underwent cardiomyogenesis through a fusion-independent pathway. Takahashi et alCitation55 reported that in rat femoral artery injury, ASCs did not differentiate into ECs, but were able to inhibit neointimal formation by the secretion of paracrine factors. There is one ongoing clinical trial (NCT01216995) testing adipose tissue-derived cells in patients after an acute MI.
Induced pluripotent stem (iPS) cells
Another potential source of SCs is iPS cells.Citation56 This source relies on in vitro de-differentiation of adult cells to embryonic-like SCs and then reprogramming using specific culture conditions to induce cardiac lineage cells including cardiomyocytes, smooth-muscle cells, and ECs. Adult cells most commonly used for iPS cells are fibroblasts and may be derived from a variety of tissues such as dermal, liver, stomach, pancreas, and neural and hematopoietic cells. Endogenous non-BM SC and iPS cells have been characterized in animal models and some have been identified in adult humans. Defining these cells and their requirements for proliferation and mobilization will provide additional options for enhanced efficacy of SC therapy.
Embryonic SCs (ESCs)
The ESCs are the ideal SCs, due to the fact that cultures of embryonic cells when stimulated can develop into >200 adult cell types.Citation38,Citation57,Citation58 Current efforts focus on establishing the conditions for directed differentiation of cells by altering the chemical composition of the culture medium, altering the culture surface, or inserting genes.Citation58 A major challenge is the potential of uncontrolled differentiation when injected directly into an animal, and the potential for tumor formation. The promise of ESCs is to genetically modify lethal debilitating chronic disease. There are currently four clinical trials in progress of human ESCs for spinal cord injury and macular degeneration, but unfortunately none for cardiac disease.Citation38
Expansion of SCs
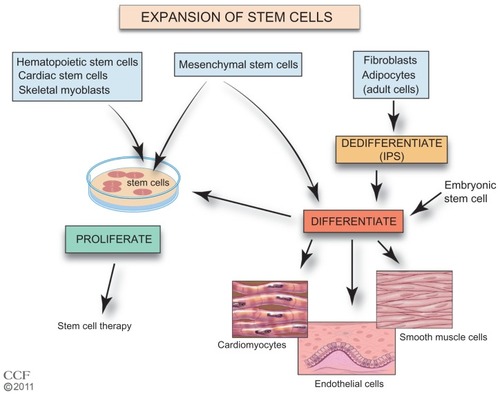
A critical step for improved SC therapy is the expansion of accessible SCs (). The homing of cells to injured tissues is very inefficient, and increasing the number of cells that are available for treatment would be beneficial. Autologous BM cells, adipose tissue, myocardial, and UCB are cultured ex vivo to increase the number of cells. Culturing the tissue also allows selection of specific cells. The ESCs and iPS cells require additional steps prior to expansion of a preparation. The iPS cells require de-differentiation as an initial step and then both iPS cells and ESCs are induced to differentiate prior to expansion. SCs in culture form colonies, and proliferation without differentiation requires a specific sequence and timing of the availability of growth factors and cytokines.Citation59–Citation66 In addition, these cells must maintain their pluripotency. Cells need to be free of feeder-cells, serum proteins, and microbial agents. Large-scale expansion with maintenance of pluripotency and transplant safety is required.Citation58,Citation67 Currently, effective cell culture proliferation is limited,Citation61 and further studies are needed to understand the requirements for expansion. New approaches are being investigated including the use of nanofibers with growth factors, mesenchymal stromal cells in cultures of HSCs, and genetic manipulation of UCB HSCs.Citation68–Citation72 To improve SC therapy, improved methods of SC ex vivo expansion are required.
Figure 2 Expansion of stem cells.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
Abbreviations: ESC, embryonic stem cell; iPS, induced pluripotent stem; MSC, mesenchymal stem cell.
Identify mobilizing agents with improved effectiveness
SC niches
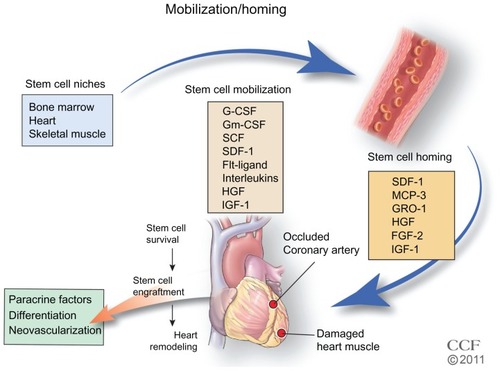
Intensive studies are underway to identify new sources of stem and progenitor cells for therapy. In addition to BM, SC niches have been identified () in heart. The SC niches are defined as a microenvironment with one or more SC that regulates self-renewal and progeny in vivo.Citation73,Citation74 Self-renewal occurs in all tissues and in addition to BM, niches of SCs have been identified in heart, arteries, veins, gonads, intestine, epidermal tissue, and neural tissue.Citation73,Citation75–Citation77 The non-BM SCs were initially defined by immunofluorescence in tissue, but given the number of markers needed, this became untenable, and isolation and identification of SCs by flow cytometry using multiple markers simultaneously has made it possible to isolate and investigate the function of these cells. Recently, lineage mapping has been utilized to locate niches in animal models by genetically labeling SC markers and identifying their location in adult tissue.Citation78,Citation79 An example of lineage mapping is the recent study of Tamura et alCitation78 of neural crest-derived SCs found in the heart that migrate and differentiate into cardiomyocytes after MI. The lineage mapping has been utilized for locating SC niches in a variety of developing organisms.Citation79 The number of quiescent SCs is small, and better detection methods are necessary. Further, identifying the regulation and recruitment of these endogenous SCs in adults is critical.
Figure 3 Stem cell mobilization and homing. Growth factors and cytokines stimulate the mobilization of the stem cells from their niche to injured tissue.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
Abbreviations: G-CSF, granulocyte colony-stimulating factor; Gm-CSF, granulocyte-macrophage colony-stimulating factor; SCF, stem cell factor/c-kit ligand; SDF-1, stromal cell-derived factor 1; MCP-3, monocyte chemotactic protein-3; GRO-1, growth regulated oncogene 1; HGF, hepatic growth factor; FGF-2, fibroblast growth factor; IGF-1, insulin-like growth factor.
Mobilization of BM SCs
In the BM, SCs reside in an endosteal niche along with stromal cells, mesenchymal cells, and ECs. The SCs are retained in the BM with high concentrations of stromal-derived factor (SDF)-1, the major chemoattractant for SCs. The SDF-1 SC receptor, CXCR4, is found in low concentrations. Stimulation with cytokines or growth factors may interrupt ligand/receptor balance. With a decrease in SDF-1 and an increase in CXCR4 expression, a signaling gradient with the PB allows the egress of the SCs from the BM (). Granulocyte colony-stimulating factor (G-CSF) is widely used clinically for SC mobilization and sometimes in conjunction with other factorsCitation57,Citation80 including granulocyte-macrophage colony-stimulating factor, stem cell factor, fms-like tyrosine kinase (Flt)-3 ligand, and interleukin-1, -3, -6, -7, -8, -11, and -12 (). AMD3100, an inhibitor that blocks SDF-1 binding to CXCR4; CTCE-0021, a CXCR4 agonist; recombinant human growth hormone, a pleiotrophic cytokine; parathyroid hormone; pegfilgrastim, pegylated G-CSF with a prolonged half-life, and thrombopoietin, a cytokine that regulates mega-karyocytopoiesis, are also being investigated.Citation80
Figure 4 Bone marrow-derived stem cell mobilization. Bone marrow stem cells may be mobilized by reducing the ligand SDF-1 and increasing the stem cell receptor CXCR4 to create a chemotatic gradient with the peripheral blood. G-CSF treatment increases MMP-9 to regulate changes in SDF-1/CXCR4 pathway, which is dependent on plasmin activation of MMP-9.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
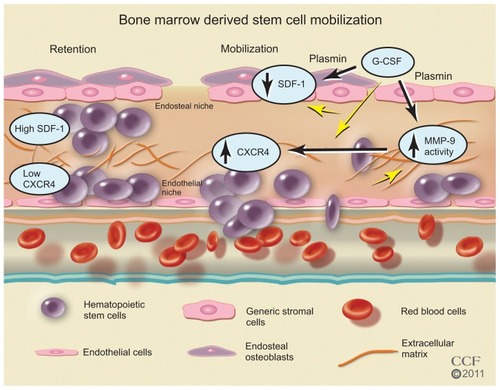
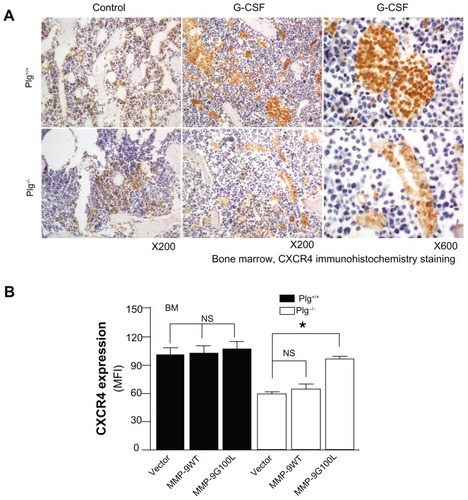
In addition to cytokines and growth factors, proteases such as neutrophil elastase, cathespin G, plasmin, and matrix metalloproteinase (MMP)-9 have been implicated in BM SC mobilization.Citation81–Citation86 After G-CSF treatment, these proteases increase in BM as well as in plasma; however, studiesCitation83 in mice deficient in neutrophil elastase or cathespin G suggest these two proteases are not required for HPSC mobilization. The results of studiesCitation81,Citation83,Citation87,Citation88 in MMP-9 deficient mice are not consistent. While some studiesCitation83,Citation88 report MMP-9 is not required, other studiesCitation81,Citation86,Citation87 suggest MMP-9 plays an important role. These differences may be due to the differences in genetic background of the mice and to differences in the dose of the mobilizing agent. In a recent study,Citation86 the authors of this present paper report that plasmin/MMP-9 is a major proteolytic pathway required for SC mobilization from BM (). Plasmin activation of MMP-9 regulates the SDF-1/CXCR4 signaling. In addition, plasmin also promotes direct degradation of the ECM during SC mobilization.Citation85 G-CSF induced HSC MMP-9 degrades BM SDF-1.Citation83,Citation89,Citation90 The increase in the number of SC mobilized with G-CSF treatment may not be sufficient for the cardiac remodeling after MI, and some patients are resistant to G-CSF.Citation91–Citation93 AMD3100, an inhibitor of CXCR4, is a promising HSC mobilizer under clinical investigation. Studies report mild and reversible side effectsCitation94–Citation96 and that it works synergistically with G-CSF to increase CD34+ cells and total white blood count.Citation94,Citation96–Citation98 However, Dai et al recently reported that chronic AMD100 exacerbates cardiac dysfunction after MI in mice.Citation99
Figure 5 Plasminogen regulates CXCR4 after G-CSF stimulation. (A) CXCR4 immunostaining of bone marrow from Plg+/+ and Plg−/− mice treated with saline (control) or G-CSF. CXCR4 expressing cells (brown color) increased two fold after G-CSF treatment in Plg+/+ mice, but CXCR4 did not change in Plg−/− mice. (B) Lentivirus expression of act MMP-9 in Plg−/− restored CXCR4 expression. Plasminogen activation of MMP-9 is required for CXCR4 expression after G-CSF treatment.
Abbreviations: CXCR4, C-X-C receptor 4; G-CSF, granulocyte colony-stimulating factor; MFI, mean fluorescence intensity; MMP-9, matrix metalloproteinase-9; Plg, plasminogen.
Mobilization of CPCs
A number of cardiomyocyte progenitor pools have been identifiedCitation76 that have common and unique markers, including: side population (SP) CPCs; c-kit+ CPCs; Sca-1+ CPCs; cardiospheres and cardiosphere-derived cells; stage specific-embryonic antigen-1+ (SSEA-1+) CPCs; LIM-homeodomain transcription factor+ (Islet-1+) CPCs; and epicardium-derived cells. The CPCs demonstrate greater proliferation potential in the infarct border compared with the necrotic core. These cells have the potential to differentiate into cardiomyocytes, smooth-muscle cells, and ECs, but the stimulatory factors for differentiation vary. The SP CPCsCitation100,Citation101 can be stimulated by SDF-1 and are both c-kit and Sca-1 positive, but are also positive for the ATP-binding cassette transporter (ABCG2). The cardiac SP cells are a mixture of subpopulations, and proof that these cells are SC is not definitive. The c-kit marker was used to identify and isolate HSCs, but their ability to differentiate into cardiomyocytes is controversial.Citation102,Citation103 Cells positive for c-kit isolated from human and rodent tissue express specific cardiac transcription factors, GATA4, GATA5, MEF2C, and Kkx2, and when cultured express mature cardiomyocyte markers, cardiac actinin, cardiac myosin, desmin, and connexin 43.Citation45,Citation104 The CPCs may be stimulated by insulin-like growth factor-1 (IGF-1); hepatic growth factor (HGF) high-mobility group box protein-1 (HMGB1), a chromatin-binding protein secreted by necrotic cells, and SDF-1.Citation105 The CPCs possess growth factor receptors and when activated increase proliferation, migration, and differentiation. Tamoxifen-treated double-transgenic miceCitation48 expressed dedifferentiated cardiomyocytes that expressed CPC markers and ~2/3 expressed c-kit. Studies in zebrafish and mammalian development suggest the potential of the epicardium-derived cells, the epithelial cells in the outermost layer of the heart, to develop into cardiomyocytes in vivo.Citation106 Smart et alCitation107 reported that in mouse heart, thymosin β4 can release the quiescent EPDCs. Development of small molecules to release the cells is underway.Citation106 Isl1+ CPCs are prominent during development, and in the postnatal rat, mouse, and human myocardium, Isl1+, c-kit-, Sca-l-, and CD31- cells have been defined as cardioblasts. Both iPS cells and ESCs give rise to this lineage in vivo. The Isl-1+ cells are rare in the myocardium and the possibility of endogenously recruiting or in vitro expansion appears to be limited. The SSEA-1+ CPCsCitation108 give rise to myocardial and endocardial cells during development in the neonatal and adult rat heart, but can progress to more committed c-kit+, Sca-1, and abcg2+ cells. When transplanted into rat heart, improved regeneration of infarcted myocardium results. Sca-1+ CD31+ cells are found in the heart as small interstitial cells that lack the HSC lineage markers of c-kit, Flt- 1, Flk-1, CD45, and CD34.Citation41 Using transgenic mice, cardiac Sca-1+ cells were found to play a role in the regulation the signaling required for efficient myocardial regeneration.Citation42,Citation109 Studies with ESCs and their requirements for cardiomyocyte differentiation may shed light on the factors that induce differentiation and proliferation of the endogenous CPCs.Citation110 A better understanding of SC mobilization from cardiogenic niches may lead to more effective agents for not only recruiting cells for ex vivo expansion, but to mobilize endogenous sources.
Strategies for improving SC homing, survival, and engraftment in the injured heart
SC delivery
Available routes of SC delivery include intravenous, intracoronary, epicardial, endocardial, and coronary sinus injection.Citation2,Citation111 While the intravenous injection of SCs is the least invasive method of delivery, retention of cells in the lungs is problematic. After an MI, intracoronary injection through a catheter is the preferred method of delivery. The epicardial and transendocardial are more invasive, but the most reliable. The transendocardial administration uses a percutaneous catheter-based approach. The coronary sinus delivery provides access to the infarcted and ischemic tissue, but may not be available to all patients. In the clinical trials, SCs were delivered by either bolus or multiple intracoronary injections, but only a small percentage reached the heart.Citation1,Citation112 At least 90% of injected cells die by apoptosis. Alternative methods of delivery are being investigated, such as use of biodegradable scaffold-based engineered tissue.Citation113,Citation114 An advantage is the variable size, but problematic issues are thickness of the patch and toxicity of the degraded material. Only limited improvement in cardiac function has been noted. A recent studyCitation115 tested sheets of cardiomyocytes progenitor cell and reported an increase in cardiogenesis and improved function. The development of safe and more effective materials for use in SC delivery is necessary.
Homing
Homing is the migration of SCs from endogenous and exogenous sources through the blood or tissue to a destination where they differentiate and replace or repair injured tissue. After an MI, expression of several factors has been observed, including transient increases in cardiac cytokines, SDF-1, MCP-3, GRO-1, that are chemo-attractants for SCs.Citation116–Citation123 After acute MI, the expression of these factors leads to SC homing to the infarcted tissue. However, many of the homing factors are expressed for only a short period of time after MI. SDF-1, the most studied homing factor, is expressed by the injured cardiac tissue for less than 1 weekCitation123 and MCP-3 for less than 10 days after MI.Citation124 In preclinical studies, genetic engineering of these factors into delivered SCs is effective in increasing SC homing.Citation123,Citation125 For example, the delivery of SDF-1 to the myocardium, either through cell-based gene therapy,Citation123,Citation126 gene transfer,Citation127 or protein-enhancedCitation128 homing of SCs, results in revascularization and improvement in cardiac function. Furthermore, overexpressing SDF-1 receptor CXCR4 in SCs leads to greater homing of SCs and improved left ventricular function when the cells were delivered within 24 hours of MI.Citation129–Citation131 Studies in animals show that engineering cells to induce the expression of SC homing factors or their receptors in myocardial tissue can promote SC homing from BM to the injured myocardium; however, these have not to date been tested in humans.Citation132
Survival/engraftment
Survival and engraftment of SCs is perhaps the most important challenge for SC therapy, and the factors necessary for effective survival and engraftment are not necessarily the same as those required for homing. After an MI, there is an enormous loss of cardiomyocytes and supporting cells that need to be replaced. The environmental signals that may guide SCs to the cardiomyocyte lineage or to the secretion of paracrine factors may be absent in the infarcted tissue, and SCs may provide these signals. Many studies have focused on strategies to optimize SC migration through injured myocardial tissue. Proteases, adhesion molecules, and integrins are important in regulating SC migration through injured myocardial tissue and modulation of the connective tissue microenvironment to improve SC engraftment.Citation133–Citation136
Several proteases have been identified to have significant effects on SC mobilization or SC migration and engraftment in cardiac tissue. SDF-1 and other factors induce the secretion of matrix metalloproteinase MMP-2 and MMP-9.Citation137–Citation139 Of significant interest, proteolytic enzymes, including neutrophil elastase, cathepsin G, and MMP-2/9, also negatively regulate cell migration by cleaving the N-terminal region of SDF-1 or cleaving CXCR4.Citation90,Citation139–Citation142 Those proteolytic enzymes are involved in spatial temporal changes in the locomotion machinery of SCs, thus mediating SC recruitment and engraftment.
Integrins are also key factors for adhesion, rolling and transmigration of SCs across the endothelium. The HSCs express several adhesion molecules including multiple integrins. In particular, a dominant role for the α4β1 integrin very-late antigen [VLA]-4 interaction with vascular cell adhesion molecule (VCAM)-1 has been suggested by studies in which exposure to blocking antibodies to VLA-4 or VCAM-1 significantly reduced the engraftment of transplanted HSCs.Citation143–Citation145 CD18 expression by the EPCs is necessary for its interaction with EC surface ICAM-1, and a CD18 neutralizing antibody significantly inhibits SC engraftment after acute MI.Citation146 These studies suggest the potential targets for the genetic enhancement of SC recruitment and engraftment.
Several other strategies have been proposed: identifying natural mediators; pre-translational directed differentiation of SCs to cardiomyocytes; activation of growth factors (FGF-2, IGF-1a)Citation132 and antiapoptotic factors (p-Akt, SDF-1, BCl-1, and PDGF); and genetically engineered SCs.Citation125,Citation132 The challenge to improve survival in SC therapy is to identify effective ways to increase the number of cells that reach and survive in the injured heart area.
Assessment of SC therapy
The goals of SC therapy are to: replace lost cardiomyocytes; increase ECs to improve blood flow; provide paracrine cytokines and growth factors; and improve measurable cardiac function, including an increase in LVEF; decrease left ventricular end-diastolic diameter; increase myocardial perfusion; and importantly increase exercise capacity. In clinical trials, methods to measure cardiac function include echocardiography, single photon emission computed tomography, and magnetic resonance imaging (MRI).Citation1,Citation3,Citation37,Citation147–Citation149 These methods are well established, but more sensitive methods are necessary to evaluate SC homing and engraftment. Techniques to evaluate the timing and specific role of narrow populations of cells, such as MRICitation150–Citation152 and SC labeling with geneticCitation153,Citation154 and immunofluorescence detectable tagsCitation155 are being investigated in animal models. The lineage/fate mappingCitation110,Citation156–Citation158 has proved to be an informative tool, and further studies in animal models and ex vivo SC labeling of cells for therapy will continue to be valuable.
Conclusion
SC therapy is an exciting and dynamic area of research with the potential to improve recovery of CVD, the leading cause of death. While animal models clearly show benefits of SC therapy to improve cardiac function after MI and ischemic heart failure, clinical trials have been disappointing. However, the results of clinical trials are promising. Better methods are needed to improve the isolation and identification of SCs, increase ex vivo expansion of SCs, and increase delivery effectiveness. A clearer understanding of mobilization and homing of SCs is needed to identify new and more effective agents. Delineating the function of specific SCs in remodeling injured tissue and how resident cardiac SCs may be enhanced is needed to improve SC engraftment and survival.
Acknowledgments
This study was funded by grants from American Heart Association (AHA0625331B and 09BGIA2050157) and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL078701). The authors thank Beth Halasz, CMI, for the artwork for the figures.
Disclosure
The authors report no conflicts of interest in this work.
References
- GeorgeJCStem cell therapy in acute myocardial infarction: a review of clinical trialsTrans Res201015511019
- MozidAMArnousSSammutECMathurAStem cell therapy for heart diseasesBr Med Bull20119814315921596713
- Sanz-RuizRGutierrez IbanesEArranzAVFernandez SantosMEFernandezPLFernandez-AvilesFPhases I–III clinical trials using adult stem cellsStem Cells Int2010201057914221076533
- WenYMengLXieJOuyangJDirect autologous bone marrow-derived stem cell transplantation for ischemic heart disease: a meta-analysisExpert Opin Biol Ther201111555956721388335
- HirschANijveldtRvan der VleutenPAIntracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trialEur Heart J201132141736174721148540
- HuikuriHVKervinenKNiemelaMEffects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarctionEur Heart J200829222723273218845667
- ClinicalTrials.gov [homepage on the Internet] Available from: http://www.ClinicalTrials.govAccessed January 2, 2012
- TillJEMcCullochEASiminovitchLA stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cellsProc Natl Acad Sci U S A196451293614104600
- MenaschePTowards the second generation of skeletal myoblasts?Cardiovasc Res200879335535618508855
- AgbulutOVanderveldeSAl AttarNComparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardiumJ Am Coll Cardiol200444245846315261948
- TaylorDAAtkinsBZHungspreugsPRegenerating functional myocardium: improved performance after skeletal myoblast transplantationNat Med1998489299339701245
- GershBJSimariRDBehfarATerzicCMTerzicACardiac cell repair therapy: a clinical perspectiveMayo Clin Proc2009841087689219797777
- HaiderHLeiYAshrafMMyoCell, a cell-based, autologous skeletal myoblast therapy for the treatment of cardiovascular diseasesCurr Opin Mol Ther200810661162119051139
- FinneyMRGrecoNJHaynesworthSEDirect comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemiaBiol Blood Marrow Transplant200612558559316635794
- AgarwalUGhalayiniWDongFRole of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarctionCirc Res2010107566767620634485
- FeldmanEJGergisUManagement of refractory acute myeloid leukemia: re-induction therapy or straight to transplantation?Curr Hematol Malig Rep2011 [Epub ahead of print]
- StoneRMO’DonnellMRSekeresMAAcute myeloid leukemiaHematology Am Soc Hematol Educ Program20049811715561679
- LodiDIannittiTPalmieriBStem cells in clinical practice: applications and warningsJ Exp Clin Cancer Res201130921241480
- SieburgHBChoRHDykstraBUchidaNEavesCJMuller-SieburgCEThe hematopoietic stem compartment consists of a limited number of discrete stem cell subsetsBlood200610762311231616291588
- AlevCIiMAsaharaTEndothelial progenitor cells: a novel tool for the therapy of ischemic diseasesAntioxid Redox Signal201115494996521254837
- PraterDNCaseJIngramDAYoderMCWorking hypothesis to redefine endothelial progenitor cellsLeukemia20072161141114917392816
- PeichevMNaiyerAJPereiraDExpression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursorsBlood200095395295810648408
- TimmermansFVan HauwermeirenFDe SmedtMEndothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursorsArterioscler Thromb Vasc Biol20072771572157917495235
- CaseJMeadLEBesslerWKHuman CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitorsExp Hematol20073571109111817588480
- MundJACaseJThe role of circulating endothelial progenitor cells in tumor angiogenesisCurr Stem Cell Res Ther20116211512121190536
- EstesMLMundJAIngramDACaseJIdentification of endothelial cells and progenitor cell subsets in human peripheral bloodCurr Protoc Cytom2010Chapter 9Unit 933.11120373498
- TongersJLosordoDWLandmesserUStem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challengesEur Heart J201132101197120621362705
- BisselsUWildSTomiukSCombined characterization of microRNA and mRNA profiles delineates early differentiation pathways of CD133+ and CD34+ hematopoietic stem and progenitor cellsStem Cells201129584785721394831
- CoplandIBMesenchymal stromal cells for cardiovascular diseaseJ Cardiovasc Dis Res20112131321716750
- WagnerJKeanTYoungRDennisJECaplanAIOptimizing mesenchymal stem cell-based therapeuticsCurr Opin Biotechnol200920553153619783424
- RipaRSHaack-SorensenMWangYBone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trialCirculation200711611 SupplI243017846310
- KernSEichlerHStoeveJKluterHBiebackKComparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissueStem Cells20062451294130116410387
- WagnerWWeinFSeckingerAComparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord bloodExp Hematol200533111402141616263424
- GaebelRFurlaniDSorgHCell origin of human mesenchymal stem cells determines a different healing performance in cardiac regenerationPLoS One201162e1565221347366
- LeeJSHongJMMoonGJLeePHAhnYHBangOYA long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic strokeStem Cells20102861099110620506226
- ChenSLFangWWYeFEffect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarctionAm J Cardiol2004941929515219514
- HareJMTraverseJHHenryTDA randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarctionJ Am Coll Cardiol200954242277228619958962
- TrounsonAThakarRGLomaxGGibbonsDClinical trials for stem cell therapiesBMC Med201195221569277
- CarvalhoABde CarvalhoACHeart regeneration: past, present and futureWorld J Cardiol20102510711121160711
- MartinCMMeesonAPRobertsonSMPersistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heartDev Biol2004265126227514697368
- OhHBradfuteSBGallardoTDCardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarctionProc Natl Acad Sci U S A200310021123131231814530411
- MatsuuraKNagaiTNishigakiNAdult cardiac Sca-1- positive cells differentiate into beating cardiomyocytesJ Biol Chem200427912113841139114702342
- MessinaEDe AngelisLFratiGIsolation and expansion of adult cardiac stem cells from human and murine heartCirc Res200495991192115472116
- LaugwitzKLMorettiALamJPostnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineagesNature2005433702664765315703750
- BeltramiAPBarlucchiLTorellaDAdult cardiac stem cells are multipotent and support myocardial regenerationCell2003114676377614505575
- DavisDRKizanaETerrovitisJIsolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsiesJ Mol Cell Cardiol201049231232120211627
- DavisDRRuckdeschel SmithRMarbanEHuman cardiospheres are a source of stem cells with cardiomyogenic potentialStem Cells201028590390420309960
- ZhangYLiTSLeeSTDedifferentiation and proliferation of mammalian cardiomyocytesPLoS One201059e1255920838637
- YamadaYYokoyamaSWangXDFukudaNTakakuraNCardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytesStem Cells20072551326133317289932
- YamadaYWangXDYokoyamaSFukudaNTakakuraNCardiac progenitor cells in brown adipose tissue repaired damaged myocardiumBiochem Biophys Res Commun2006342266267016488397
- BolliRChughARD’AmarioDCardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised Phase 1 trialLancet201137898061847185722088800
- MadonnaRGengYJDe CaterinaRAdipose tissue-derived stem cells: characterization and potential for cardiovascular repairArterioscler Thromb Vasc Biol200929111723172919628786
- MadonnaRDe CaterinaRAdipose tissue: a new source for cardiovascular repairJ Cardiovasc Med20101127180
- BaiXYanYSongYHBoth cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarctionEur Heart J201031448950120037143
- TakahashiMSuzukiEObaSAdipose tissue-derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral arteryAm J Physiol Heart Circ Physiol20102982H41542319940081
- Martinez-FernandezANelsonTJTerzicANuclear reprogramming strategy modulates differentiation potential of induced pluripotent stem cellsJ Cardiovasc Transl Res20114213113721207217
- ThomsonJAItskovitz-EldorJShapiroSSEmbryonic stem cell lines derived from human blastocystsScience19982825391114511479804556
- BraamSRDenningCMummeryCLGenetic manipulation of human embryonic stem cells in serum and feeder-free mediaMethods Mol Biol201058441342319907990
- BhatiaMBonnetDKappUWangJCMurdochBDickJEQuantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo cultureJ Exp Med199718646196249254660
- Varnum-FinneyBXuLBrashem-SteinCPluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signalingNat Med20006111278128111062542
- KellySSSolaCBde LimaMShpallEEx vivo expansion of cord bloodBone Marrow Transplant2009441067368119802023
- BoitanoAEWangJRomeoRAryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cellsScience201032959971345134820688981
- PeledTMandelJGoudsmidRNPre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamineCytotherapy20046434435516146887
- ZhangCCKabaMIizukaSHuynhHLodishHFAngiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantationBlood200811173415342318202223
- ReyaTDuncanAWAillesLA role for Wnt signalling in self-renewal of haematopoietic stem cellsNature2003423693840941412717450
- MurdochBChadwickKMartinMWnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivoProc Natl Acad Sci U S A200310063422342712626754
- Perez-SimonJALopez-VillarOAndreuEJMesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a Phase I/II clinical trialHaematologica20119671072107621393326
- LuJAggarwalRPompiliVJDasHA novel technology for hematopoietic stem cell expansion using combination of nanofiber and growth factorsRecent Pat Nanotechnol20104212513520420564
- WalendaTBokermannGVentura FerreiraMSSynergistic effects of growth factors and mesenchymal stromal cells for expansion of hematopoietic stem and progenitor cellsExp Hematol201139661762821356269
- DahlbergADelaneyCBernsteinIDEx vivo expansion of human hematopoietic stem and progenitor cellsBlood2011117236083609021436068
- DelaneyCRatajczakMZLaughlinMJStrategies to enhance umbilical cord blood stem cell engraftment in adult patientsExpert Rev Hematol20103327328320835351
- MadonnaRDe CaterinaRStem cells and growth factor delivery systems for cardiovascular diseaseJ Biotechnology20111544291297
- KrankelNSpinettiGAmadesiSMadedduPTargeting stem cell niches and trafficking for cardiovascular therapyPharmacol Ther20111291628120965213
- VoogJJonesDLStem cells and the niche: a dynamic duoCell Stem Cell20106210311520144784
- MartinezECKofidisTAdult stem cells for cardiac tissue engineeringJ Mol Cell Cardiol201150231231920709074
- BolliniSSmartNRileyPRResident cardiac progenitor cells: at the heart of regenerationJ Mol Cell Cardiol201150229630320643135
- MorrisonSJSpradlingACStem cells and niches: mechanisms that promote stem cell maintenance throughout lifeCell2008132459861118295578
- TamuraYMatsumuraKSanoMNeural crest-derived stem cells migrate and differentiate into cardiomyocytes after myocardial infarctionArterioscler Thromb Vasc Biol201131358258921212399
- BarkerNCleversHTracking down the stem cells of the intestine: strategies to identify adult stem cellsGastroenterology200713361755176018054544
- NerviBLinkDCDiPersioJFCytokines and hematopoietic stem cell mobilizationJ Cell Biochem200699369070516888804
- HeissigBHattoriKDiasSRecruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligandCell2002109562563712062105
- HeissigBLundLRAkiyamaHThe plasminogen fibrinolytic pathway is required for hematopoietic regenerationCell Stem Cell20071665867018371407
- LevesqueJPLiuFSimmonsPJCharacterization of hematopoietic progenitor mobilization in protease-deficient miceBlood20041041657215010367
- PelusLMBianHKingAGFukudaSNeutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4Blood2004103111011912958067
- TjwaMMouraRMoonsLFibrinolysis-independent role of plasmin and its activators in the haematopoietic recovery after myeloablationJ Cell Mol Med20091311–124587459519210287
- GongYFanYHoover-PlowJPlasminogen regulates stromal cell-derived factor-1/CXCR4-mediated hematopoietic stem cell mobilization by activation of matrix metalloproteinase-9Arterioscler Thromb Vasc Biol20113192035204321719761
- CramerDEWagnerSLiBMobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9Stem Cells20082651231124018339771
- RobinsonSNPisarevVMChavezJMSinghRKTalmadgeJEUse of matrix metalloproteinase (MMP)-9 knockout mice demonstrates that MMP-9 activity is not absolutely required for G-CSF or Flt-3 ligand-induced hematopoietic progenitor cell mobilization or engraftmentStem Cells200321441742712832695
- JinFZhaiQQiuLDegradation of BM SDF-1 by MMP-9: the role in G-CSF-induced hematopoietic stem/progenitor cell mobilizationBone Marrow Transplant200842958158818679363
- McQuibbanGAButlerGSGongJHMatrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1J Biol Chem200127647435034350811571304
- StiffPGingrichRLugerSA randomized Phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin’s disease or non-Hodgkin’s lymphomaBone Marrow Transplant200026547148111019835
- HolmMNot all healthy donors mobilize hematopoietic progenitor cells sufficiently after G-CSF administration to allow for subsequent CD34 purification of the leukapheresis productJ Hematother1998721111139597568
- AnderliniPPrzepiorkaDSeongCFactors affecting mobilization of CD34+ cells in normal donors treated with filgrastimTransfusion19973755075129149776
- DevineSMFlomenbergNVesoleDHRapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphomaJ Clin Oncol20042261095110215020611
- HendrixCWFlexnerCMacFarlandRTPharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteersAntimicrob Agents Chemother20004461667167310817726
- LilesWCBroxmeyerHERodgerEMobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonistBlood200310282728273012855591
- FlomenbergNDevineSMDipersioJFThe use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF aloneBlood200510651867187415890685
- LackNAGreenBDaleDCA pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100Clin Pharmacol Ther200577542743615900288
- DaiSYuanFMuJChronic AMD3100 antagonism of SDF- 1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarctionJ Mol Cell Cardiol201049458759720655922
- LiangSXTanTYGaudryLChongBDifferentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic modelInt J Cardiol20101381404919254813
- YamaharaKFukushimaSCoppenSRHeterogeneic nature of adult cardiac side population cellsBiochem Biophys Res Commun2008371461562018413147
- ScherschelJASoonpaaMHSrourEFFieldLJRubartMAdult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardiumMol Ther20081661129113718431364
- OrlicDKajsturaJChimentiSBone marrow cells regenerate infarcted myocardiumNature2001410682970170511287958
- BearziCRotaMHosodaTHuman cardiac stem cellsProc Natl Acad Sci U S A200710435140681407317709737
- BocchiLSaviMGraianiGGrowth factor-induced mobilization of cardiac progenitor cells reduces the risk of arrhythmias, in a rat model of chronic myocardial infarctionPLoS One201163e1775021445273
- VieiraJMRileyPREpicardium-derived cells: a new source of regenerative capacityHeart2011971151920884787
- SmartNBolliniSDubeKNDe novo cardiomyocytes from within the activated adult heart after injuryNature2011474735364064421654746
- OttHCMatthiesenTSBrechtkenJThe adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cellsNat Clin Pract Cardiovasc Med20074Suppl 1S273917230213
- TateishiKAshiharaETakeharaNClonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regenerationJ Cell Sci2007120Pt 101791180017502484
- ChiriacANelsonTJFaustinoRSBehfarATerzicACardiogenic induction of pluripotent stem cells streamlined through a conserved SDF-1/VEGF/BMP2 integrated networkPLoS One201054e994320376342
- DibNKhawajaHVarnerSMcCarthyMCampbellACell therapy for cardiovascular disease: a comparison of methods of deliveryJ Cardiovasc Transl Res20114217718121181320
- MusialekPTekieliLKostkiewiczMRandomized transcoronary delivery of CD34(+) cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of (m)Tc-labeled cells activityJ Nucl Cardiol201118110411621161463
- GuoHDCuiGHWangHJTanYZTransplantation of marrow-derived cardiac stem cells carried in designer self-assembling peptide nanofibers improves cardiac function after myocardial infarctionBiochem Biophys Res Commun20103991424820637726
- KaiDPrabhakaranMPJinGRamakrishnaSGuided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineeringJ Biomed Mater Res B Appl Biomater201198B237938621681953
- ZakharovaLMastroeniDMutluNTransplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves functionCardiovasc Res2010871404920118202
- FukudaSBianHKingAGPelusLMThe chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftmentBlood2007110386086917416737
- HristovMZerneckeABidzhekovKImportance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injuryCirc Res2007100459059717272812
- PennMSKhalilMKExploitation of stem cell homing for gene deliveryExpert Opin Biol Ther200881173018081534
- LapidotTDarAKolletOHow do stem cells find their way home?Blood200510661901191015890683
- KurdiMBoozGWG-CSF-based stem cell therapy for the heart-unresolved issues part A: paracrine actions, mobilization, and deliveryCongest Heart Fail200713422122717673875
- QianHTryggvasonKJacobsenSEEkblomMContribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrinsBlood200610793503351016439681
- MayorgaMFinanAPennMPre-transplantation specification of stem cells to cardiac lineage for regeneration of cardiac tissueStem Cell Rev200951516019184567
- AskariATUnzekSPopovicZBEffect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathyLancet2003362938569770312957092
- SchenkSMalNFinanAMonocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factorStem Cells200725124525117053210
- KempfTZarbockAWideraCGDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in miceNat Med201117558158821516086
- ZhangMMalNKiedrowskiMSDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarctionFASEB J200721123197320717496162
- HiasaKEgashiraKKitamotoSBone marrow mononuclear cell therapy limits myocardial infarct size through vascular endothelial growth factorBasic Res Cardiol200499316517215088101
- SegersVFTokunouTHigginsLJMacGillivrayCGannonJLeeRTLocal delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarctionCirculation2007116151683169217875967
- KahnJBykTJansson-SjostrandLOverexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulationBlood200410382942294915070669
- ChengZOuLZhouXTargeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performanceMol Ther200816357157918253156
- ZhangDFanGCZhouXOver-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardiumJ Mol Cell Cardiol200844228129218201717
- TakeharaNTsutsumiYTateishiKControlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarctionJ Am Coll Cardiol200852231858186519038683
- IpJEWuYHuangJZhangLPrattREDzauVJMesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftmentMol Biol Cell20071882873288217507648
- BorgTKMarkwaldRPeriostin: more than just an adhesion moleculeCirc Res2007101323023117673682
- XiangGSchusterMDSekiTWitkowskiPEshghiSItescuSDownregulated expression of plasminogen activator inhibitor-1 augments myocardial neovascularization and reduces cardiomyocyte apoptosis after acute myocardial infarctionJ Am Coll Cardiol200546353654116053971
- ShimazakiMNakamuraKKiiIPeriostin is essential for cardiac healing after acute myocardial infarctionJ Exp Med2008205229530318208976
- BykTKahnJKolletOCycling G1 CD34+/CD38+ cells potentiate the motility and engraftment of quiescent G0 CD34+/CD38−/low severe combined immunodeficiency repopulating cellsStem Cells200523456157415790777
- Janowska-WieczorekAMarquezLADobrowskyARatajczakMZCabuhatMLDifferential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokinesExp Hematol200028111274128511063876
- ZhengYSunAHanZCStem cell factor improves SCID-repopulating activity of human umbilical cord blood-derived hematopoietic stem/progenitor cells in xenotransplanted NOD/SCID mouse modelBone Marrow Transplant200535213714215543197
- PetitISzyper-KravitzMNaglerAG-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4Nature Immunology20023768769412068293
- LevesqueJPHendyJTakamatsuYSimmonsPJBendallLJDisruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamideJ Clin Invest2003111218719612531874
- DelgadoMBClark-LewisILoetscherPRapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytesEur J Immunol200131369970711241273
- PapayannopoulouTCraddockCNakamotoBPriestleyGVWolfNSThe VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleenProc Natl Acad Sci U S A19959221964796517568190
- PapayannopoulouTPriestleyGVNakamotoBZafiropoulosVScottLMHarlanJMSynergistic mobilization of hemopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient miceBlood20019751282128811222371
- BonigHPriestleyGVPapayannopoulouTHierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokinesBlood20061071798616141352
- WuYIpJEHuangJEssential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardiumCirc Res200699331532216825578
- GuXXieYGuJRepeated intracoronary infusion of peripheral blood stem cells with G-CSF in patients with refractory ischemic heart failure – a pilot studyCirc J201175495596321325723
- LasalaGPSilvaJAKusnickBAMinguellJJCombination stem cell therapy for the treatment of medically refractory coronary ischemia: a Phase I studyCardiovasc Revasc Med2011121293421241969
- SurderDSchwitterJMoccettiTCell-based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI)Am Heart J20101601586420598973
- YaoYLiYMaGIn vivo magnetic resonance imaging of injected endothelial progenitor cells after myocardial infarction in ratsMol Imaging Biol201113230331320552286
- EbertSNTaylorDGNguyenHLNoninvasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniquesStem Cells200725112936294417690182
- ZhouRIdiyatullinDMoellerSSWIFT detection of SPIO-labeled stem cells grafted in the myocardiumMagn Reson Med20106351154116120432286
- TangJWangJKongXVascular endothelial growth factor promotes cardiac stem cell migration via the PI3 K/Akt pathwayExp Cell Res2009315203521353119800880
- HiguchiTAntonMSarasteAReporter gene PET for monitoring survival of transplanted endothelial progenitor cells in the rat heart after pretreatment with VEGF and atorvastatinJ Nucl Med200950111881188619837770
- AdlerEDBystrupABriley-SaeboKCIn vivo detection of embryonic stem cell-derived cardiovascular progenitor cells using Cy3-labeled Gadofluorine M in murine myocardiumJACC Cardiovasc Imaging2009291114112219761992
- HsiehPCSegersVFDavisMEEvidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injuryNat Med200713897097417660827
- SynnergrenJAkessonKDahlenborgKMolecular signature of cardiomyocyte clusters derived from human embryonic stem cellsStem Cells20082671831184018436862
- MaQZhouBPuWTReassessment of Isl1 and Nkx2–5 cardiac fate maps using a Gata4-based reporter of Cre activityDev Biol200832319810418775691