Abstract
Aim
Chronic heart failure is associated with endothelial dysfunction and insulin resistance. The aim of this investigation was to study insulin-stimulated endothelial function and glucose uptake in skeletal muscles in patients with heart failure in comparison to patients with type 2 diabetes.
Methods
Twenty-three patients with systolic heart failure and no history of diabetes, seven patients with both systolic heart failure and type 2 diabetes, 19 patients with type 2 diabetes, and ten healthy controls were included in the study. Endothelial function was studied by venous occlusion plethysmography. Insulin-stimulated endothelial function was assessed after intra-arterial infusion of insulin followed by co-infusion with serotonin in three different dosages. Forearm glucose uptake was measured during the insulin infusion.
Results
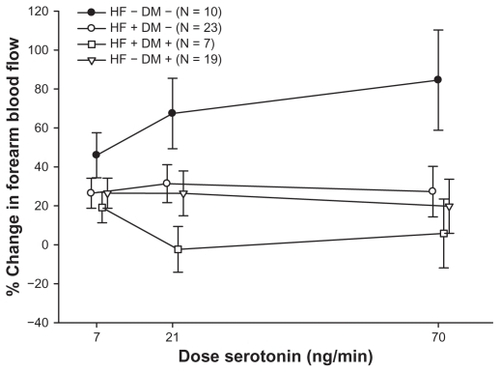
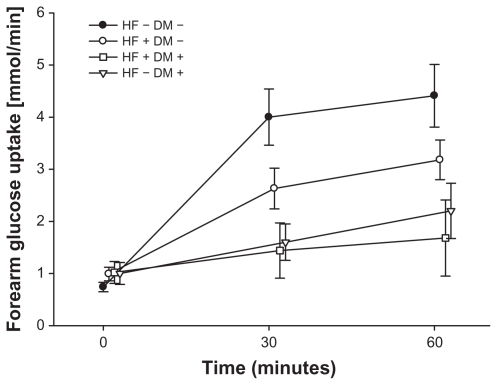
Patients with systolic heart failure had impaired insulin-stimulated endothelial function. The percentage increase in blood flow during co-infusion with insulin and serotonin dose response study was 24.74% ± 6.16%, 23.50% ± 8.32%, and 22.29% ± 10.77% at the three doses respectively, compared to the healthy control group 45.96% ± 11.56%, 67.40% ± 18.11% and 84.57% ± 25.73% (P = 0.01). Insulin-stimulated endothelial function was similar in heart failure patients and patients with type 2 diabetes, while it was further deteriorated in patients suffering from both heart failure and diabetes with a percentage increase in blood flow of 19.15% ± 7.81%, −2.35% ± 11.76%, and 5.82% ± 17.70% at the three doses of serotonin, respectively. Forearm glucose uptake was impaired in patients with heart failure compared to healthy controls (P = 0.03) and tended to be further impaired by co-existence of diabetes (P = 0.08).
Conclusion
Systolic heart failure and type 2 diabetes result in similar vascular insulin resistance and reduced muscular insulin-stimulated glucose uptake. The effects of systolic heart failure and type 2 diabetes appear to be additive.
Introduction
The prognosis in heart failure (HF) has improved, but remains poor with a 5-year mortality rate of nearly 50%.Citation1 Insulin resistance predisposes to the development of HF and aggravates the prognosis in HF patients.Citation2 HF is also associated with insulin resistance irrespective of the etiology.Citation3,Citation4 The severity of HF has also been shown to be proportional to the degree of insulin resistance.Citation5,Citation6
Insulin resistance is associated with reduced muscle glucose uptake as well as endothelial dysfunctionCitation7 and HF patients have impairment of insulin-stimulated peripheral glucose deposition during a hyperinsulinemic euglycemic clamp.Citation8 Previous studies have shown that patients with HF have endothelial dysfunction.Citation9 Also, in patients with HF, endothelial dysfunction is an independent prognostic marker of death, hospitalization, and worsening of HF.Citation10
In healthy subjects, insulin induces endothelial-dependent vasodilatation by increasing endothelial nitric oxide production.Citation11 Nitric oxide has vascular protective functions besides vasodilatation; it prevents platelet adhesion and inhibits smooth muscle cell proliferation, all components of the physiological development of atherosclerosis. Impairment of insulin-stimulated vasodilatation has been shown to exist in patients with type 2 diabetes and patients with insulin resistance,Citation12–Citation14 and may contribute to deterioration of vascular function leading to atherosclerosis.
As seen in type 2 diabetes, patients with HF may have co-existing insulin resistance of both skeletal muscle and endothelium which constitutes a new pathophysiological approach.
We therefore studied insulin-stimulated endothelial function and insulin-stimulated forearm glucose uptake in patients with HF; patients with co-existing HF and type 2 diabetes; patients with type 2 diabetes and no co-existing HF, and in a healthy control group.
Patients and methods
Patients
Thirty patients with stable systolic HF were included in the study. Patients were eligible for the study if they met the inclusion criteria of documented systolic HF with a Left Ventricular Ejection Fraction (LVEF) of ≤35% at the time of entering the study. Before entering the study, all patients had a transthoracic echocardiogram (TTE) performed to ensure the existence of systolic HF. Ischemic heart disease was the cause of chronic HF in 24 patients and the remaining six patients had idiopathic cardiomyopathy. All patients were kept on their usual medication for at least 2 months before entering the study. Both men and women were included in the study and were between 46 and 80 years old. Exclusion criteria were decompensated HF, uncontrolled hypertension, hypotension, and bradycardia. A subgroup of seven patients had both HF and documented type 2 diabetes (HF+DM+) and the remaining 23 patients had HF but no type 2 diabetes (HF+DM−).
Nineteen patients with type 2 diabetes (HF−DM+) were included for the study of endothelial function. These patients met the diagnostic criteria for type 2 diabetes, as defined by the American Diabetes Association.Citation15 Patients with a history of peripheral atherosclerotic disease or known diabetic retinopathy, nephropathy, or neuropathy were excluded and none of the patients had been diagnosed with HF or ischemic heart disease. None of the patients with diabetes were treated with insulin. Additional exclusion criteria were also uncontrolled hypertension, hypotension, or bradycardia. Ten individuals without a medical history of cardiovascular disease, hypertension, dyslipidemia, or diabetes served as a healthy control group (HF−DM−). None of the individuals in the healthy control group received any kind of medication.
All participants were Caucasian. All patients in the HF−DM− as well as the HF+DM− group had an oral glucose tolerance test (OGTT) done before entering the study, to ensure no coexisting diabetes or impaired glucose tolerance (IGT).Citation15
Participants were recruited by advertisement in newspapers or from the local out-patient clinic. All gave informed consent before entering the study. The study was approved by the ethics committee of the city of Copenhagen (ref KF 02-071/03), as well as the Danish Medicines Agency (ref 2612-2423) and complies with the Declaration of Helsinki. The study has been registered at clinicaltrials.gov (identifier: NCT00497003).
Methods
Venous occlusion plethysmography
In all four groups, patients with HF (HF+DM−); patients with HF and type 2 diabetes (HF+DM+); patients with type 2 diabetes (HF−DM+) as well as the healthy control group (HF−DM−), endothelium-dependent and endothelium-independent vasodilatation were studied by using venous occlusion plethysmography. The studies of endothelial function were done after an overnight fast with abstinence from smoking and none in the patient groups took their usual medication on the day of examination. The patients lay supine with the forearm at a horizontal level with the right atrium. All examinations were performed in a quiet room with the temperature kept constant during the day of examination.
All studies began with examinations of endothelium dependent vasodilatation by infusing increasing doses of serotonin (7, 21, 70 ng/minute) (Serotonin; Clinalfa, Läufelfingen, Switzerland) intra-arterially for 4 minutes at each dose level before measurements of forearm blood flow. Then studies of insulin-stimulated endothelial function were assessed after co-infusion of serotonin and insulin (Actrapid [Novo Nordisk Scandinavia, Malmö, Sweden] in a 1% human albumin solution [vehicle]). The infusion rate of insulin was 0.05 mU/kg body weight/minute for 60 minutes before vaso-reactivity studies with serotonin were done.
The insulin stimulated endothelial function was calculated as the percentage increase in actual flow (mL/minute) after co-infusion with insulin and serotonin compared to serotonin.
Endothelium-independent vasodilatation in the forearm was examined by exchanging serotonin infusion with increasing doses of sodium nitroprusside (Nitropress; Abbott Laboratories, North Chicago, IL). To determine the NO-dependent fraction of insulin-stimulated serotonin response, an intra-arterial co-infusion of NG-monomethyl-L-arginine (L-NMMA; Clinalfa) was infused for 10 minutes, with a dose of 3.3 mg/minute, followed by a dose-response study with serotonin. To allow wash-out between measurements, all infusions were stopped for at least 30 minutes while saline was infused at a rate of 60 mL/hour to maintain the cannula patent. The infusion protocol is shown in .
Forearm glucose uptake
During the examination day, blood samples were drawn simultaneously from a venous catheter in both the infused and the non-infused arm as well as from the arterial cannula. The venous catheter in the infused arm was placed retrograde to flow to collect blood, representing a product of muscle metabolism in the forearm. Forearm glucose uptake was calculated as the arterio-venous difference (AV-difference) in glucose concentration multiplied by the actual forearm blood flow.Citation16 The catheter in the non-infused arm served as a control for systemic changes in the concentration of glucose and insulin during insulin infusion.
Plasma glucose concentrations were determined by the glucose oxidase method (Vitros Chemistry; Johnson and Johnson, Rochester, NY) and serum insulin concentrations by a chemiluminescent immunometric assay (Immulite 2500; DPC, Los Angeles, CA).
Statistical analysis
Unless otherwise specified, results are expressed as means ± standard error of the mean (SEM).
Comparisons of differences between the groups were performed using unpaired Student’s t-test. Differences between groups for single parameters were compared with two-way ANOVA. Changes in forearm blood flow as well as changes in forearm glucose uptake were subject to analysis of variance for repeated measurements using the proc mixed procedure in the Statistical Analysis Software (v 8.0; SAS Institute, Cary, NC). Forearm glucose uptake measurements were log transformed to satisfy assumptions of normal distribution and homogeneity of variance of residuals. Subjects and interaction between subject and their response to serotonin entered the model as random variables whereas infusion sequence and serotonin doses entered the model as fixed values.
Power calculations showed, that with a sample size of ten patients in each group, a difference of 20% in forearm blood flow and forearm glucose uptake can be found, with a statistical significance of 5%.
Results
Baseline data for all four groups have been presented in . In general, more patients in the HF group received treatment with aspirin, statins, and either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor antagonist (ATII antagonist) than patients with type 2 diabetes. All patients with HF – both with and without diabetes – were treated with the beta blocker carvedilol. The patients with diabetes and no HF had the least beneficial metabolic profile with higher body mass index (BMI) and blood glucose. They also tended to have higher blood pressure, but more patients with HF also received blood pressure-lowering agents. The lipid profile in the group of patients with diabetes was similar to the patients with HF.
Table 1 Baseline data for all participants
Endothelial function
In the group of patients with HF and no diabetes (HF+DM−), the percentage increase in endothelium-dependent vasodilatation at the three dose levels of serotonin after co-infusion of insulin was 26.44% ± 7.73%, 31.37% ± 9.77%, and 27.31% ± 12.98%, which is significantly lower compared to the healthy control group (HF−DM−) 45.96% ± 11.56%, 67.40% ± 18.11%, and 84.57% ± 25.73% (P = 0.03). Impairment of insulin-stimulated endothelial function in heart failure (HF+DM−) was comparable to that observed in the group of patients with type 2 diabetes (HF−DM+): 26.48% ± 7.74%, 26.40% ± 11.52%, and 19.75% ± 13.87%. Remarkably, the combination of HF and type 2 diabetes (HF+DM+) had an additive effect on limiting the insulin-stimulated endothelial function: to 19.15% ± 7.81%, −2.35% ± 11.76%, and 5.82% ± 17.7% ().
Insulin-stimulated forearm glucose uptake
Forearm glucose uptake during insulin infusion in patients with HF (both patients with and those patients without diabetes) was significantly deteriorated compared to the healthy control group (HF−DM−) (P = 0.03). Glucose uptake during the 60 minutes of intra-arterial infusion of insulin in the HF group without diabetes (HF+DM−) was 0.99 ± 0.13 mmol/minute before the insulin infusion to 2.63 ± 0.39 mmol/minute after 30 minutes of infusion and 3.18 ± 0.38 mmol/minute after 60 minutes. In the healthy control group (HF−DM−) the glucose uptake during insulin stimulation was 0.74 ± 0.09 mmol/minute, 4.00 ± 0.54 mmol/minute, and 4.41 ± 0.60 mmol/minute at 0, 30, and 60 minutes, respectively (P = 0.04) (). For the group with both diabetes and HF (HF+DM+) the forearm glucose uptake was even further impaired with glucose uptake of 1.02 ± 0.21 mmol/minute before insulin infusion, 1.44 ± 0.53 mmol/minute after 30 minutes insulin infusion, and 1.68 ± 0.73 mmol/minute (P = 0.02 in comparison to healthy controls) (P = 0.08 in comparison to HF+DM−) and is comparable to forearm glucose uptake in patients with known diabetes and no heart failure (HF−DM+). During the intra-arterial insulin infusion in patients with HF, serum-insulin increased in the infused arm from 13 ± 4 μU/mL to 133 ± 13 μU/mL but did not result in systemic changes of serum insulin levels.
Discussion
In this study we are the first to show that patients with HF have an impairment of insulin-stimulated endothelial function to a similar extent to what is found among patients with known type 2 diabetes (). Furthermore, the combination of HF and type 2 diabetes has an additive effect on limiting vascular insulin stimulation. Concerning forearm glucose uptake, we found, as it was expected, a decreased response in patients with type 2 diabetes compared to healthy controls (). Interestingly, this response also tended to be impaired in the studied group of patients with HF to a similar extent as observed in the group of patients with known type 2 diabetes. There was also a trend towards lower forearm glucose uptake in the group of patients with both HF and type 2 diabetes, even though this difference was not significant ().
Altogether, these findings indicate that both vascular and metabolic insulin resistance, at least concerning skeletal muscle glucose uptake, are independently and additionally impaired when patients suffer from the combination of type 2 diabetes and HF.
Several studies have found patients with HF to be systemic insulin resistant when examined by use of the golden standard method hyperinsulinemic euglycemic clamp technique.Citation6,Citation17,Citation18 But to the best of our knowledge, HF has not been found to be associated with vascular insulin resistance. Even in one study on patients with HF it was not possible to find impaired insulin-stimulated baseline blood flow during a hyperinsulinemic glucose clamp.Citation8
The novel finding of our study, that the endothelium in patients with HF had a reduced ability of insulin-enhanced vasodilatation during serotonin stimulation, may have several explanations. The endothelium has a pivotal role in the vascular homeostasis with a number of factors balanced to protect against atherosclerosis. Many of the effects of the endothelium depend on its ability to release nitric oxide (NO) after activation of nitric oxide synthase (e-NOS). NO inhibits vascular smooth cell proliferation, reduces platelet aggregation, platelet and leukocyte adhesion, and prevents oxidative stress. Endothelial dysfunction has been found in groups of patients with risk factors of cardiovascular disease such as hypertension, type 2 diabetes, hyperinsulinemia, dyslipidemia, obesity, and smoking among others.Citation7,Citation19,Citation20 Impaired endothelial function has shown to be associated with an increased risk of developing cardiovascular disease.Citation21,Citation22
Patients with heart failure have a decreased skeletal muscle blood flow, thought to be responsible for the reduced exercise tolerance seen in these patients.Citation23 The vasodilatory actions of insulin, have been thought to play an important role in patients with HF. With increasing severity of HF, an increased insulin resistance has been found but also an inverse association between severity of HF and the vasodilatory capacity of insulin.Citation18 Insulin-stimulated vasodilatation has previously been proven to be NO-dependent.Citation11 By inducing local hyperinsulinemia in the brachial artery, but keeping the systemic insulin level stable, we aimed to avoid the confounding effects of systemic neurohumoral activation by insulin. The serum concentrations reached at the end of intra-arterial insulin infusion in the infused arm were within a physiological range and to be compared with the concentrations reached at a postprandial situation. We were therefore able to test the local actions of insulin artificially increased to postprandial levels, on the endothelium, with a co-infusion of serotonin, an endothelial dependent agonist of e-NOS activation, and thereby NO production. The impaired insulin-stimulated serotonin response in patients with HF may lead to a reduced glucose delivery to skeletal muscle and thereby enhancing insulin resistance, which could explain our results ().
The reduced vasodilatory response of insulin could lead to reduced glucose uptake in skeletal muscle and could be a possible factor for the reduced exercise capacity seen in patients with HF.Citation24
Examinations of endothelial-independent vasodilatation showed that the findings in this study are not a result of vascular smooth muscle dysfunction (data not shown). Studies of co-infusion of L-NMMA showed that the insulin-stimulated vasodilatation was NO-dependent (data also not shown).
Limitations to the study
The heart failure group was slightly older than the type 2 diabetes (T2DM) group and there were more smokers in the heart failure group; both age and smoking are factors that impair endothelium function.
The small number of patients in the group of patients with both diabetes and heart failure is a limitation to the study and a possible explanation for the fact that we did not reach statistical significance. As patients were allocated to the study and some of the patients withdrew consent, the numbers in different groups were less well-balanced. Also, due to the limited sample size, subgroup analysis with respect to ischemic heart disease as a cause of heart failure was not performed.
It would have been an advantage to the study to do sub-group analysis between patients with ischemic heart disease and idiopathic HF, to see if there were any differences. The size of the study was too small to do subgroup analysis.
The results of this study indicate that insulin resistance and reduced peripheral blood flow in patients with HF could be explained by vascular insulin resistance. We also found that the peripheral glucose uptake was reduced during insulin stimulation. HF can therefore be considered as an insulin- resistant state with vascular consequences comparable to patients with type 2 diabetes and the combination of heart failure and diabetes further aggravates the vascular metabolic reactions. When treating patients with HF, it is of great importance to keep in mind the possible metabolic disadvantages of the drug and to monitor potential glucose metabolic changes.
Acknowledgments
Grants were provided for Britt Falskov from The Danish Heart Foundation and Bispebjerg Hospital Research Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- FromAMLeibsonCLBursiFDiabetes in heart failure: prevalence and impact on outcome in the populationAm J Med2006119759159916828631
- KamaleshMSubramanianUSawadaSEckertGTemkitMTierneyWDecreased survival in diabetic patients with heart failure due to systolic dysfunctionEur J Heart Fail20068440440816309953
- PaternostroGPaganoDGnecchi-RusconeTBonserRSCamiciPGInsulin resistance in patients with cardiac hypertrophyCardiovasc Res199942124625310435017
- SabelisLWSendenPJZonderlandMLDeterminants of insulin sensitivity in chronic heart failureEur J Heart Fail20035675976514675854
- SwanJWAnkerSDWaltonCInsulin resistance in chronic heart failure: relation to severity and etiology of heart failureJ Am Coll Cardiol19973025275329247528
- AdachiHOhnoTOguriMOshimaSTaniguchiKEffect of insulin sensitivity on severity of heart failureDiabetes Res Clin Pract200777Suppl 1S25826217467109
- SteinbergHOChakerHLeamingRJohnsonABrechtelGBaronADObesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistanceJ Clin Invest19969711260126108647954
- ParsonageWHetmanskiDCowleyADifferentiation of the metabolic and vascular effects of insulin in insulin resistance in patients with chronic heart failureAm J Cardiol200289669670311897212
- KatzSDHryniewiczKHriljacIVascular endothelial dysfunction and mortality risk in patients with chronic heart failureCirculation2005111331031415655134
- FischerDRossaSLandmesserUEndothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or deathEur Heart J2005261656915615801
- SteinbergHOBrechtelGJohnsonAFinebergNBaronADInsulin- mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide releaseJ Clin Invest1994943117211798083357
- Rask-MadsenCIhlemannNKrarupTInsulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart diseaseDiabetes200150112611261811679442
- LaaksoMEdelmanSVBrechtelGBaronADImpaired insulin- mediated skeletal muscle blood flow in patients with NIDDMDiabetes1992419107610831499861
- LaaksoMEdelmanSVBrechtelGBaronADDecreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistanceJ Clin Invest1990856184418522189893
- American Diabetes AssociationDiagnosis and classification of diabetes mellitusDiabetes Care200629Suppl 1S434816373932
- ZierlerKLTheory of the use of arteriovenous concentration differences for measuring metabolism in steady and non-steady statesJ Clin Invest196140122111212516695873
- PaolissoGDe RiuSMarrazzoGVerzaMVarricchioMD’OnofrioFInsulin resistance and hyperinsulinemia in patients with chronic congestive heart failureMetabolism19914099729771895963
- ParsonageWAHetmanskiDCowleyAJBeneficial haemodynamic effects of insulin in chronic heart failureHeart200185550851311302998
- CreagerMACookeJPMendelsohnMEImpaired vasodilation of forearm resistance vessels in hypercholesterolemic humansJ Clin Invest19908612282342195060
- CelermajerDSSorensenKEGeorgakopoulosDCigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adultsCirculation1993885 Pt 1214921558222109
- PerticoneFCeravoloRPujiaAPrognostic significance of endothelial dysfunction in hypertensive patientsCirculation2001104219119611447085
- MeyerBMortlDStreckerKFlow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptideJ Am Coll Cardiol20054661011101816168284
- MullerAFBatinPEvansSHawkinsMCowleyAJRegional blood flow in chronic heart failure: the reason for the lack of correlation between patients’ exercise tolerance and cardiac output?Br Heart J19926764784811622698
- WilsonJRMartinJLSchwartzDFerraroNExercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscleCirculation1984696107910876713612