Abstract
Background
Stage 2 hypertension often requires combination antihypertensive therapy. Ambulatory blood pressure monitoring (ABPM) is a useful tool for assessing antihypertensive drugs and their combinations.
Objective
To compare the effect of a moderate dose of angiotensin receptor blocker/calcium channel blocker (ARB/CCB) combined with a diuretic versus a maximal dose of ARB with a diuretic on 24-hour ambulatory blood pressure monitoring (ABPM) and other derived ambulatory blood pressure (ABP) parameters.
Methods
The EXforge As compared to Losartan Treatment ABPM substudy was a randomized, double-blind, parallel-group, active-control, forced-titration study of patients with Stage 2 hypertension that compared the efficacy of initial treatment with valsartan/amlodipine 160/5 mg (n = 48) or losartan 100 mg (n = 36). At week 3, hydrochlorothiazide (HCTZ) 25 mg was added in both treatment groups. ABP was measured at baseline and at week 6. Additionaly, 24-hour ABP, nighttime (10 pm to 6 am) and daytime (6 am to 10 pm) ABP, and ABP load (percentage of readings above 140/90 mmHg) were determined.
Results
Eighty-four patients (48 ARB/CCB/HCTZ, 36 ARB/HCTZ) had ABPM at baseline and at week 6. Reductions of systolic/diastolic ABP were greater in the ARB/CCB/ HCTZ group than in the ARB/HCTZ group for 24-hour mean ABP (−22.0/−13.3 versus −17.4/−8.1 mmHg), as well as nighttime ABP (−22.2/−13.3 versus −16.2/−7.4 mmHg), daytime ABP (−21.9/−13.0 versus −18.1/−8.6 mmHg), ABP in the last 4 hours of the dosing period (−21.5/−13.5 versus −17.0/−7.7 mmHg), and ABP load (21.7%/12.8% versus 30.8%/20.0%).
Conclusion
Initiating antihypertensive treatment with moderate doses of ARB/CCB with a diuretic is more effective in lowering nighttime and daytime ABP and reducing ABP load than a maximal dose of an ARB with a diuretic.
Background
There is overwhelming evidence that ambulatory blood pressure (ABP) monitoring (ABPM) is better at predicting future risk for cardiovascular morbidity and mortality compared with usual office blood pressure (BP) measurements in both untreated and treated hypertensive patients.Citation1–Citation4 The BP load, defined as the percentage of readings in 24-hour ABP in which BP is above 140/90 mmHg, has also been shown to predict target organ damage in patients with arterial hypertension.Citation5,Citation6 ABPM is a useful tool for studying antihypertensive drugs and their combinations and provides greater insight into circadian BP variation than does clinic BP monitoring. Other clinically important features of ABPM are its ability to assess the capacity of a given antihypertensive treatment to adequately control BP throughout the 24-hour dosing period, and to provide information on the possible need to differentiate dosing times of different drugs.Citation7,Citation8
Among patients with Stage 2 hypertension, the combination of two or more agents is recommended as initial treatment to achieve BP control.Citation9–Citation11 Many renin-angiotensin II-aldosterone system (RAAS)-based single-pill combinations include either an angiotensin-converting-enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) with a diuretic (eg, hydrochlorothiazide [HCTZ]), or an ACE inhibitor or ARB with a calcium-channel blocker (CCB). RAAS/ Diuretic and RAAS/CCB combinations have fully additive BP-lowering effects.Citation11 However, based on findings from the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension study, a RAAS/CCB combination may control BP more effectively and reduce cardiovascular morbidity and mortality, as compared with a RAAS/diuretic combination.Citation12,Citation13
The additional BP reduction that results from combining antihypertensive agents from two different classes is approximately five times greater than would result from doubling the dose of a single antihypertensive agent.Citation14 Thus, it was hypothesized that a triple-therapy combination of moderate doses of an ARB and a CCB with a diuretic would be more effective than a dual-therapy combination of the maximal dose of an ARB with a diuretic. Using clinic BP measures, the authors reported in the EXforge As compared to Losartan Treatment (EXALT) study that early initiation of a tripletherapy ARB/CCB/HCTZ combination lowered BP more effectively than a maximal-dose dual-therapy ARB/HCTZ combination in 488 patients with Stage 2 hypertension.Citation15 ABP outcomes are reported here for the subgroup of patients from the EXALT study who had ABPM at baseline and after 6 weeks of study treatment.
Methods
The design of the EXALT study has been reported in full previously.Citation15 It was a randomized, double-blind, parallel-group, active-control, forced-titration trial that was conducted between July 2009 and January 2010 at 80 centers in the USA. Twenty centers with capabilities to use the ABP device participated in the ABPM substudy. Ethics committee and/or institutional review board approval was granted at all participating centers, and all patients provided written informed consent before enrollment. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Eligibility criteria
Male and female outpatients aged ≥18 years were enrolled in the EXALT study if they had Stage 2 systolic hypertension, defined as a mean in-office sitting systolic BP (MSSBP) of ≥160 mmHg and <200 mmHg at randomization. Patients were excluded if they were taking more than three antihypertensive medications at study enrollment or if BP was ≥140/90 mmHg while on triple therapy (one therapy of which was a diuretic) at the optimal dose of each drug. Other key exclusion criteria were: MSSBP ≥200 mmHg or mean sitting diastolic BP (MSDBP) ≥110 mmHg at the time of enrollment (ie, prior to washout); use of four or more antihypertensive medications in the past 30 days; secondary hypertension; significant heart disease (eg, recent stroke/transient ischemic attack, heart failure, coronary artery bypass graft surgery, coronary intervention, unstable angina, myocardial infarction, heart block, atrial fibrillation/ flutter, or valve disease); significant renal or hepatic impairment; or baseline serum sodium <135 mEq/L, serum potassium <3.5 mEq/L or ≥5.5 mEq/L, or glycosylated hemoglobin >9%. Exclusion criteria specific to the ABPM substudy were: arm circumference >42 cm, employment requiring night-shift work, or a history of sleep apnea.
Study design
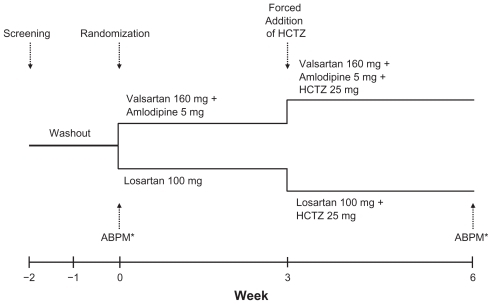
Study treatment schedules for the EXALT study have been presented previously.Citation15 Study treatment in the ABPM substudy was force titrated as summarized in . After screening and a 1- to 2-week washout, eligible patients were randomized and initiated treatment with valsartan/ amlodipine 160/5 mg or losartan 100 mg. At week 3, HCTZ 25 mg was added in both treatment groups. ABPM was performed for 24 hours before study visits at baseline and at week 6.
Figure 1 ABPM substudy design. Study medication was force titrated at week 3. ABP was measured for 24 hours before the visits at week 0 and week 6.
Abbreviations: ABP, ambulatory blood pressure; ABPM, ambulatory blood pressure monitoring; HCTZ, hydrochlorothiazide.
Patients were discontinued from the study if they had MSSBP ≥200 mmHg or MSDBP ≥110 mmHg, or if they could not be force titrated to the next dose due to adverse events or hypotension (MSSBP <100 mmHg or MSDBP <60 mmHg). Patients took double-blinded study medication once daily, in the morning. On study visit days, clinic BP measures were obtained before the daily dose of study medication. Patients were not permitted to take other antihypertensive or diuretic treatments during the study.
BP assessments
Patients enrolled in the EXALT study had clinic BP measurements at each study visit with an automated digital brachial artery BP device; the full methods for clinic BP measurements have been reported previously.Citation15 At the 20 centers that participated in the ABPM substudy, patients who expressed interest in participating in the substudy and satisfied the entry criteria were enrolled.
After baseline clinic BP measurement, the ABP device (SpaceLabs 90207, Redmond, WA) was placed on the patient’s nondominant arm. Readings were correlated with clinic BP measurements at the time of device placement. Following the correlation procedure, BP and heart rate were recorded every 20 minutes during the 24-hour monitoring period. Qualitycontrol criteria for ABPM were defined as starting between 7 and 10 am, with at least 24 hours of ABP data after the beginning of the test time, at least 80% of expected readings captured during the 24-hour period, and no more than two nonconsecutive hours with no valid BP reading. If baseline ABPM was successful, the patient was randomized into the ABPM substudy. If baseline ABPM was unsuccessful, their randomization was postponed and the ABP device was reapplied within 48 hours. If the second ABP measurement was successful, the patient was randomized into the study, and ABP was measured again at the week-6 study visit. Patients who had two unsuccessful attempts to measure mean 24-hour ABP at baseline were discontinued from the ABPM substudy but were allowed to continue in the main study.
Study endpoints
The primary study efficacy variable was the change from baseline in MSSBP at week 6 in the overall population, as has been reported previously.Citation15 The following efficacy endpoints were evaluated for both systolic and diastolic ABP in this ABPM substudy: 24-hour ABP, nighttime ABP (10 pm to 6 am), daytime ABP (6 am to 10 pm), ABP in the last 4 hours of the dosing period, and hourly ABP values. ABP load was defined as the percentage of systolic ABP readings that were >140 mmHg and the percentage of diastolic ABP readings that were >90 mmHg. Adverse events were recorded at each study visit.
Statistical analysis
The ABPM substudy population for efficacy and safety analyses in this report included all patients with valid ABPM at baseline and at week 6. Baseline demographics and clinical characteristics were compared between treatment groups with a two-sample t-test for continuous variables and chi-square or Fisher’s exact test for categorical variables (excluding missing values). Patients were analyzed for efficacy endpoints according to the treatment they were assigned at randomization. The least-squares mean and standard error of the mean (SEM) for changes in systolic and diastolic ABP from baseline to week 6 were analyzed for 24-hour mean ABP, nighttime ABP, daytime ABP, and ABP in the last 4 hours of the dosing period. Least-squares mean and SEM for changes in clinic BP measures (MSSBP and MSDBP) from baseline to week 6 were also analyzed for patients participating in the ABPM substudy. Analysis of covariance (ANCOVA) with baseline MSSBP and treatment regimen as explanatory variables was used to test each efficacy endpoint for superiority of the valsartan/ amlodipine group over the losartan group. For ABP load, both ANCOVA and a nonparametric Wilcoxon rank-sum test were used to determine between-treatment differences. Adverse events were coded using Medical Dictionary for Regulatory Activities terminology to give a system organ class and preferred term for each event.Citation16
Results
Patients
Of the 488 patients enrolled in the EXALT study (241 in the valsartan/amlodipine/HCTZ group and 247 in the losartan/ HCTZ group), 416 (85%) completed the study.Citation15 Of the 114 patients who were enrolled in the ABPM substudy, 84 patients (36 in the valsartan/amlodipine/HCTZ group and 48 in the losartan/HCTZ group), satisfied the eligibility criteria and provided valid ABPM data at the baseline visit. All 84 of these patients also completed the follow-up ABP assessment successfully at week 6. Baseline characteristics among the patients in the ABPM substudy were balanced between treatment groups () and were similar to those of the whole study population.Citation15
Table 1 Baseline demographics and characteristics by treatment (ABPM substudy population)
Efficacy
ABP
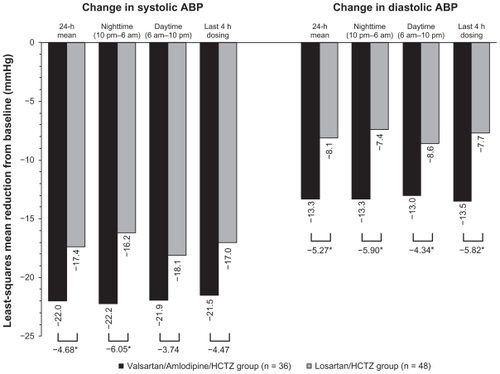
Baseline values for 24-hour mean ABP ± standard deviation (SD) were 150.1 ± 15.0/87.5 ± 12.0 mmHg and 148.1 ± 13.8/86.1 ± 11.4 mmHg in the valsartan/ amlodipine/ HCTZ and losar tan/HCTZ groups, respectively. Least-squares mean ± SEM reductions in 24-hour mean ABP at week 6 were significantly greater in the valsartan/amlodipine/HCTZ group than in the losartan/ HCTZ group (−22.0 ± 1.7/−13.3 ± 1.0 mmHg and −17.4 ± 1.5/−8.1 ± 0.8 mmHg, respectively; P = 0.043/P < 0.001; ). Reductions in nighttime ABP (10 pm to 6 am), daytime ABP (6 am to 10 pm), and ABP in the last 4 hours of the dosing period (the early morning hours prior to the next dose) are shown in . At week 6, significantly greater reduction in systolic ABP was seen for nighttime ABP (10 pm to 6 am) in the valsartan/amlodipine/ HCTZ group compared with the losartan/HCTZ group.
Figure 2 LSM change from baseline to week 6 in ABP. Changes were evaluated for the 24-hour mean values, the average of nighttime values (from 10 pm to 6 am), the average of daytime values (from 6 am to 10 pm), and the average values during the last 4 hours before the next dose of study medication.
Abbreviations: ABP, ambulatory blood pressure; HCTZ, hydrochlorothiazide; ANCOVA, analysis of covariance; LSM, least squares mean.
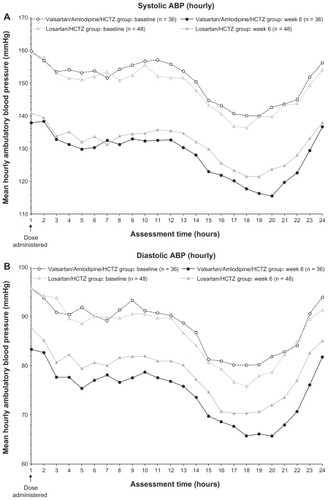
After 6 weeks of treatment, valsartan/amlodipine/HCTZ provided greater and more consistent reduction of ABP throughout the 24-hour measuring period than losartan/ HCTZ ().
Figure 3 Mean hourly ambulatory blood pressure (ABP) at baseline and at week 6; (A) systolic ABP; (B) diastolic ABP.
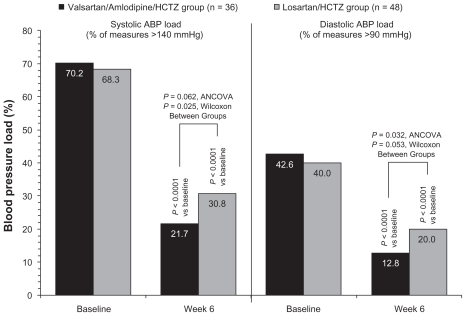
Mean values for systolic ABP load (the percentage of readings that were >140 mmHg) and diastolic ABP load (the percentage of readings that were >90 mmHg) are shown in . Reduction of systolic ABP load in the valsartan/ amlodipine/HCTZ group (from 70.2% to 21.7%) was greater than in the losartan/HCTZ group (from 68.3% to 30.8%; ANCOVA, P = 0.062; Wilcoxon, P = 0.025). Reduction of diastolic ABP load in the valsartan/amlodipine/HCTZ group (from 42.6% to 12.8%) was also greater than in the losartan/ HCTZ group (from 40.0% to 20.0%; ANCOVA, P = 0.032; Wilcoxon, P = 0.053).
Figure 4 Mean ambulatory blood pressure (ABP) load at baseline and at week 6. Systolic ABP load and diastolic ABP load were defined as the percentage of readings (during the 24-hour ABP monitoring) that were >140 mmHg and >90 mmHg, respectively.
Clinic BP
Among the patients in the ABPM substudy population, mean ± SD baseline values for clinic MSSBP/MSDBP were 168.8 ± 6.9/98.4 ± 8.6 mmHg and 170.5 ± 10.2/98.3 ± 9.0 mmHg in the valsartan/amlodipine/ HCTZ group and losartan/HCTZ group, respectively. Least-squares mean ± SEM reductions of MSSBP/MSDBP at week 6 were −31.2 ± 2.8/−14.3 ± 1.9 mmHg and −30.8 ± 2.4/−11.0 ± 1.7 mmHg in the valsartan/amlodipine/ HCTZ group and losartan/HCTZ group, respectively (P = 0.93/P = 0.194).
Safety
Full safety results for the overall population have been reported previously.Citation15 In the ABPM substudy population, 12/36 (33%) patients in the valsartan/amlodipine/HCTZ group and 18/48 (38%) patients in the losartan/HCTZ group had at least one adverse event by week 6. The most commonly reported adverse events (valsartan/amlodipine/HCTZ, losartan/HCTZ) were: dizziness (1 [3%], 3 [6%]), back pain (2 [6%], 1 [2%]), and pain in extremity (2 [6%], 0 [0%]). One patient in each group had an adverse event of hypotension.
Discussion
In the EXALT ABPM sub-study, ABP reductions were significantly greater with the valsartan/amlodipine/HCTZ combination than with the losartan/HCTZ combination. These results were consistent with the clinic BP findings of the main study, which was designed to investigate whether triple-combination therapy with moderate doses of an ARB and a CCB with a diuretic was comparable or superior to dual-combination therapy with the maximal available fixed combination dose of an ARB with a diuretic.Citation15 Thus, the observed differences in ABPM lowering were attributable to the combination of moderate doses of a CCB with an ARB + diuretic versus the maximal dose of an ARB + diuretic.
In contrast to the clinic BP findings of the main study,Citation15 among the patients in the ABPM substudy, there was no significant difference in clinic BP results between the two treatment groups. This observation could have been due to the small number of patients in the substudy. For the main study, a sample size of at least 460 patients was projected to provide adequate power to detect a 5 mmHg difference in clinic BP between treatment groups. Only 84 patients were evaluable in the ABPM substudy; thus, treatment differences using clinic BP measures were not observed. These findings provide additional support for previous evidence that ABPM is a more sensitive measure than clinic BP to evaluate the BP-lowering efficacy of treatment.Citation3
In addition to its increased sensitivity for changes in BP control, ABPM can be used to assess the BP-lowering efficacy of a treatment over the entire dosing interval and circadian patterns of BP control.Citation17 In this analysis, significant treatment differences were seen not only for 24-hour mean ABP, but also for nighttime ABP (10 pm to 6 am), and ABP load (percentage of readings >140 mmHg [systolic] or >90 mmHg [diastolic]). The differences between treatment groups in nighttime ABP were more pronounced than the differences in daytime ABP, which shows that triple-therapy combination provides even greater control at nighttime. Diminished nighttime decline in ABP has been shown to predict cardiovascular eventsCitation18–Citation20 and cardiovascular mortality.Citation4,Citation21 Similarly, ABP load has been reported to predict end-organ damage in patients with systolic hypertension.Citation5,Citation6
Two other recent reports described the effects of triple-therapy or dual-therapy combinations of an ARB, CCB, and diuretic on ABP endpoints in 283 patientsCitation22 and 380 patients,Citation23 respectively. In those studies, the fulldose triple-therapy combinations (valsartan/amlodipine/ HCTZ 320/10/25 mg or olmesartan/amlodipine/HCTZ 40/10/25 mg) lowered 24-hour, nighttime, and daytime ABP significantly more effectively than any dual-therapy combination at the same doses. The authors of the present paper compared lower doses of the ARB and CCB in the triple-therapy combination (valsartan/amlodipine/HCTZ 160/5/25 mg) to dual therapy with full-dose losartan with HCTZ (100/25 mg). Although comparing the findings of the present study to the other studies is limited by differences in study populations and study designs, the treatment difference of 5.4 mmHg in favor of the triple-therapy combination for the reduction of 24-hour mean systolic ABP in this study was similar to the 6.4 mmHg difference between the full-dose triple-therapy combinations and the ARB/HCTZ dual-therapy combinations in each of the previous studies.Citation22,Citation23
The main limitation of this analysis is the small number of patients in the ABPM substudy. Because of the small sample size, it was not possible to perform subgroup analyses of triple versus dual therapy within different subgroups (eg, gender, age, race, hypertension severity): future studies in these subgroups would be helpful. Another potential limitation is that postbaseline ABP evaluation was done only at week 6, which provided a single point estimate of the effects of study treatment on ABP.
Despite these limitations, the findings show that using ABPM, initiating treatment with a combination of moderate doses of an ARB, and a CCB with HCTZ lowers BP more effectively than a maximal dose of an ARB with HCTZ. These results support both the efficacy of combining lower doses of multiple antihypertensive agents and the use of ABP instead of clinic BP as a better measure of treatment efficacy in hypertension.
Acknowledgments
This work was supported by Novartis Pharmaceuticals Corporation. Dr Duprez provided the outline of the contents and detailed verbal discussion of the manuscript during a meeting held in May 2011. All authors edited the manuscript for scientific intellectual content and approved the final version of the manuscript submitted for publication. The authors thank Jonathan Latham of PharmaScribe, LLC, for editorial assistance with support from Novartis Pharmaceuticals Corporation. The authors also thank Katrina Zapotulko of Novartis Pharmaceuticals Corporation for project management support of the clinical trial, the participating investigators for their contributions to the conduct of the study, and study coordinators at the investigative sites for their diligent efforts.
Disclosure
Dr Duprez has received grant support from Novartis Pharmaceuticals Corporation and Roche, and has served as a consultant to Novartis Pharmaceuticals Corporation and Pfizer Inc. Dr Ferdinand has received research support and honoraria from AstraZeneca Pharmaceuticals LP, Merck and Co, Inc, Novartis Pharmaceuticals Corporation, Pfizer Inc, Forest Labs, and Daiichi Sankyo. Drs Purkayastha and Samuel are employees of and hold stock in Novartis Pharmaceuticals Corporation. Dr Wright has received research support from Novartis Pharmaceuticals Corporation, Atri-tech, and St Jude Medical; honoraria from Novartis Pharmaceuticals Corporation, Boehringer-Ingelheim, Forest Pharmaceutical, and GlaxoSmithKline; and served as a consultant to Novartis Pharmaceuticals Corporation.
References
- ConenDBambergFNoninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysisJ Hypertens2008261290129918550999
- ClementDLDe BuyzereMLDe BacquerDAPrognostic value of ambulatory blood-pressure recordings in patients with treated hypertensionN Engl J Med2003348242407241512802026
- PickeringTGWhiteWBGilesTDWhen and how to use self (home) and ambulatory blood pressure monitoringJ Am Soc Hypertens201042566120400049
- DolanEStantonAThijsLSuperiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome studyHypertension200546115616115939805
- FalquiVViazziFLeonciniGBlood pressure load, vascular permeability and target organ damage in primary hypertensionJ Nephrol200720Suppl 12S636718050146
- BauwensFDuprezDDe BuyzereMClementDLBlood pressure load determines left ventricular mass in essential hypertensionInt J Cardiol19923433353381532954
- WhiteWBHow well does ambulatory blood pressure predict targetorgan disease and clinical outcome in patients with hypertension?Blood Press Monit19994Suppl 2S172110822418
- HermidaRCAyalaDESmolenskyMHPortaluppiFChronotherapy in hypertensive patients: administration-time dependent effects of treatment on blood pressure regulationExpert Rev Cardiovasc Ther20075346347517489671
- WhiteWBImproving blood pressure control and clinical outcomes through initial use of combination therapy in stage 2 hypertensionBlood Press Monit200813212312918347448
- ChobanianAVBakrisGLBlackHRSeventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressureHypertension20034261206125214656957
- GradmanAHBasileJNCarterBLBakrisGLCombination therapy in hypertensionJ Am Soc Hypertens201041425020374950
- JamersonKBakrisGLDahlofBExceptional early blood pressure control rates: the ACCOMPLISH trialBlood Press2007162808617612905
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med2008359232417242819052124
- WaldDSLawMMorrisJKBestwickJPWaldNJCombination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trialsAm J Med2009122329030019272490
- WrightRFDuprezDPurkayasthaDSamuelRFerdinandKCCombination angiotensin-receptor blocker (ARB)/calcium channel blocker with HCTZ vs the maximal recommended dose of an ARB with HCTZ in patients with stage 2 hypertension: the EXforge as compared to Losartan Treatment in Stage 2 systolic hypertension (EXALT) studyJ Clin Hypertens (Greenwich)201113858859721806769
- Maintenance and Support Services Organization (MSSO)Medical Dictionary for Regulatory ActivitiesChantilly, VAMSSO2011 [updated October 27]. Available from: http://www.meddramsso.com/Accessed October 31, 2011
- LefebvreJPoirierLLacourciereYMethodology to determine duration of action for antihypertensive drugsAnn Pharmacother200236587488111978167
- StaessenJAThijsLFagardRPredicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic hypertension in Europe trial investigatorsJAMA1999282653954610450715
- VerdecchiaPPorcellatiCSchillaciGAmbulatory blood pressure. An independent predictor of prognosis in essential hypertensionHypertension19942467938017995639
- KarioKPickeringTGMatsuoTHoshideSSchwartzJEShimadaKStroke prognosis and abnormal nocturnal blood pressure falls in older hypertensivesHypertension200138485285711641298
- OhkuboTHozawaAYamaguchiJPrognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama studyJ Hypertens200220112183218912409956
- LacourcièreYCrikelairNGlazerRDYenJCalhounDA24-hour ambulatory blood pressure control with triple-therapy amlodipine, valsartan and hydrochlorothiazide in patients with moderate to severe hypertensionJ Hum Hypertens2011251061562221248785
- IzzoJMelinoMFernandezVLeeJHeyrmanRTwenty-four hour efficacy and safety of full-dose, triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazideJ Clin Hypertens (Greenwich)201012Suppl 1A3435