Abstract
Transforming growth factor β (TGF-β1) is a pleiotropic cytokine with many and complex effects in cell and tissue physiology. This is made possible by a very complex and interwoven signaling system, whose regulation continues to be the focus of a growing line of research. This complex regulation translates to a key role in cardiovascular physiology, hemostasis, and the blood–vessel interface. In accordance with this, the TGF-β1 pathway appears to be deregulated in related disorders, such as atherosclerotic vascular disease and myeloproliferative syndromes. It is expected that the growing amount of experimental and clinical research will yield medical advances in the applications of knowledge of the TGF-β1 pathway to diagnosis and therapeutics.
Introduction
Transforming growth factor β (TGF-β1) is a pleiotropic cytokine, which has been demonstrated to regulate a wide array of biological processes. It plays a major role in the regulation of vascular function and hemostasis. Therefore, it can be considered as a putative therapeutic target in disorders of the blood–vessel interface, such as atherosclerosis and myeloproliferative syndromes. The present narrative review highlights the most important advances in the knowledge of TGF-β regulation in cardiovascular disease. This review has been prepared after a comprehensive search through MEDLINE. Search terms were “TGF beta”, “cardiovascular”, “atherosclerosis”, “myeloproliferative syndromes”, “pathway”, and “regulation”. A broader coverage of research strategy can be found in Gasparyan et al.Citation1
Regulation of TGF-β1 physiology
The canonical TGF-β1 pathway
The regulation of TGF-β1 is shown in . Briefly, active TGF-β1 is released from its latency-associated peptide by activating proteases. Then, it binds to the TGF-β-RII, which acts as a Ser/Thre kinase.Citation2 This Ser/Thre kinase activity phosphorylates TGF-β-RI which may be present in several isoforms termed activinlike kinases (ALKs).Citation3 In general, TGF-β1 stimulates ALK-5 and phosphorylates second messenger proteins termed Smads.Citation4 Smad2 or Smad3 form a heterodimer with Smad4, and internalize into the nucleus to decrease the proliferation/apoptosis ratio,Citation5 increase differentiation,Citation6 and inhibit the expression of inflammatory molecules.Citation7 In endothelial cells, TGF-β1 can stimulate ALK-4/5/7 and Smad2/3, or stimulate ALK-1 and Smad1/5/8 and increase cell proliferation.Citation8 Smad6 and 7 are inhibitory Smads, since they bind Smad4 and inhibit its internalization into the nucleus.Citation9 Endoglin is an accessory to the TGF-β receptor that seems to modulate receptor binding and ALK stimulation.Citation10 It is mutated in hereditary hemorrhagic telangiectasia type 1,Citation11 whereas ALK-1 is mutated in hereditary hemorrhagic telangiectasia type 2.Citation12
Figure 1 Summary of the main regulators of the TGF-β pathway.
Abbreviatons: TGF-β, transforming growth factor beta; Smad, second messenger protein; ALKs, activin-like kinases.
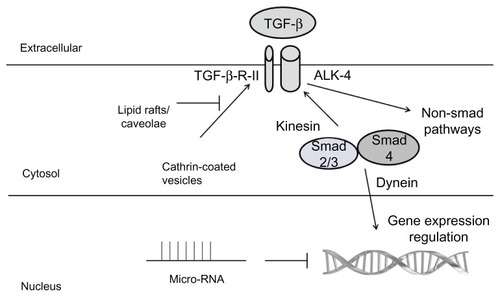
In addition to this classical Smad-dependent pathway, other crosstalks have been described among TGF-β1 and several signaling pathways,Citation13 including mitogen-activated protein (MAP) kinasesCitation14 and small GTPases, such as RhoA.Citation15 Non-Smad pathways seem to be especially important to regulate the TGF-β-mediated fibrotic effect. In particular, focal adhesion kinase has been shown to regulate TGF-β-mediated fibrosis. This is mediated by a TGF-β-mediated recruitment of the p85 subunit of PI3K to focal adhesion kinase to regulate signal transduction, which is independent of tyrosine kinase activation.Citation16 Among MAP kinases, JNK plays a necessary role in mediating TGF-β-mediated epithelial-to-mesenchymal transition in rat peritoneal fibroblasts, in cooperation with Smad3.Citation17 In addition, the ERK MAP kinase regulates epithelial-to-mesenchymal transition in mesothelial cells by involving nuclear factor-κB (NFκB);Citation18 Conversely, p38 MAP kinase seems to inhibit this effect in the same cell model.Citation19 This antifibrotic effect of TGF-β is also mediated by peroxisome proliferator-activated receptor-γ by preventing p300 recruitment, subsequent histone H4 hyperacetilation, and eventual collagen synthesis.Citation20
New insights in TGF-β1 regulation
In recent years, a growing body of experimental medicine suggests an important role of several factors which may act (in a real-time manner) as rheostats for the fine tuning of the TGF-β1 pathway, and thus adapt cell response of TGF-β1 to a given cellular circumstance.
The first important factor is receptor endocytosis. Recent reports indicate that receptor endocytosis is a key event in proper signaling and receptor recycling.Citation21 Moreover, it has been clarified that clathrin-coated pits-mediated endocytosis enhances TGF-β function,Citation22 and that early endosomes behave as signaling organelles to promote TGF-β signaling. Conversely, however, the lipid rafts-caveolae endocytic system inhibits TGF-β signaling.Citation23 Interestingly, it has been described that, given that caveolae localize in cholesterol-rich membrane domains, cholesterol itself inhibits TGB-β signaling in vitro,Citation24 and this might mediate, at least in part, atherogenic effects of cholesterol in vivo. In fact, caveolin, a protein that is a key component of caveolae, physically interacts with TGF-β-R-I to block Smad signaling.Citation25
The second key event is micro-RNA (miRNA) regulation.Citation26 This is achieved by noncoding RNA fragments which are able to silence gene expression. In particular, the miRNA 200 family (miR-200a, miR-200b, miR-200c, miR-141, and miR-429) cooperate with TGF-β signaling to regulate epithelial-to-mesenchymal transition.Citation27 Interestingly, other miRNAs are able to inhibit the antitumor effects of TGF-β and thus are accumulated in human tumors, as seen with the miRNA 25 cluster (miRNA 106b, miRNA 93, and miRNA 25) in gastric cancer,Citation28 neuroblastoma, and multiple myeloma; and the miRNA 17–92 cluster (miRNA 17, miRNA 18a, miRNA 19a, miRNA 20a, miRNA 19b-1, miRNA 92a) in diffuse large B cell lymphoma and small-cell lung cancer.Citation26 MiRNAs miRNA 106b-25 and miR-92 regulate TGF-β-mediated antiproliferative and apoptotic effects.Citation29 In particular, miR-106b and miR-93 inhibit TGF-β-mediated cell cycle arrest, whereas miR-25 inhibits TGF-β-mediated apoptosis.Citation26,Citation29 Of note, this crosstalk between the TGF-β pathway and miRNA seems to be a bidirectional process. Thus, miRNA not only affects the TGF-β pathway, but also, miR-21 is directly upregulated by TGF-β1, and plays a key role in vascular smooth muscle cell differentiation.Citation30 It is expected that this TGF-β-mediated effect also takes place in many other miRNAs. These effects seem to take place postranscriptionally by affecting the Drosha miRNA-stabilizing machinery.Citation26 Therefore, miRNAs are plausible key regulators of the cell response of TGF-β, in a real-time and context-dependent manner.
A third emerging mechanism to control the complex regulation of TGF-β1 signaling is the cytoskeleton. Smads are tightly anchored to the cytoskeleton and constantly shuttle the cytoplasm and the nucleus in basal cell conditions.Citation31 In fact, all Smads are associated to cell microtubules and their trafficking is controlled, at least in part, by microtubules-related proteins, such as kinesin for going to cell membrane receptors,Citation32 and dynein when they direct to the nucleus.Citation33 In cell culture models, nuclear accumulation of Smads has been considered to be a direct marker of TGF-β1 signaling,Citation34,Citation35 and this could be mediated by nuclear phosphatases, which dephosphorylate the C-terminal di-serine motifs Smads.Citation36
Yet again, this link between TGF-β and cytoskeleton acts in a bidirectional manner, given that TGF-β regulates actin polymerization by the non-Smad-signaling pathways RhoA and p38,Citation37 and by affecting epithelial-to-mesenchymal transition.
In recent years, several mathematical models have been developed that will predict the cell response of TGF-β1 in a given circumstance in silico, and thus help to design target-designed novel molecules, in order to modulate the important roles exerted by TGF-β1 signaling in health and disease.Citation38 These models are based on a network of molecular components. In order to describe how a given parameter changes with time, systems of ordinary differential equations were incorporated to build a kinetic model, since these could express molecular changes (concentrations and biochemical modifications) over time, and relate these data to empirical ones.Citation38
TGF-β1 in cardiovascular disease
TGF-β1 in vascular and hemostasis physiology
In general, TGF-β1 is considered as an anti-inflammatory cytokine in the vessel wall.Citation39 In normal vessels, TGF-β1 inhibits endothelialCitation40 and vascular smooth muscleCitation41 cell proliferation. It also increases apoptosis to avoid excessive cellular accumulation,Citation42 and stimulates vascular cell differentiation,Citation43 with a parallel decrease of the expression of inflammatory molecules.Citation44 In the blood–vessel interface, TGF-β1 decreases expression of cell adhesion molecules in vascular cells.Citation45 In addition, in leukocytes, it decreases the activation of integrinsCitation46 and stimulates the function of endothelial progenitor cells, which may help to restore the denuded vessel wall.Citation47 In hemostasis, TGF-β1 seems to behave as an antifibrinolytic factorCitation48 and stimulates platelet-induced vascular repair.Citation49 TGF-β1 is a normal component of platelet alpha granules.Citation50 In fact, the vast majority of serum TGF-β1 comes from platelet degranulation.Citation51
TGF-β1 in atherosclerosis
In atherosclerosis, TGF-β1 is considered to be an antiatherogenic factor, especially in the early stages of the disease, according to what has been termed the protective cytokine hypothesis.Citation52 Thus, TGF-β1 inhibits excessive vascular smooth muscle cell accumulation in the neointima,Citation53 and avoids plaque rupture by means of its stimulation of extracellular matrix synthesis and tissue repair.Citation54 In addition, it controls local inflammation by stimulating Th3 function and regulatory T cells (CD3+/CD25+ cells).Citation55 Therefore, it avoids the excessive immune attack (from both innate and acquired immunity mechanisms) that characterizes atherosclerotic vascular disease. Citation56 In the clinical arena, decreased serum levels of TGF-β1 have been correlated to clinical atherosclerosis.Citation51 However, this parameter has remained an elusive atherosclerosis marker, given that these levels may vary according to the time course of the disease and age.Citation57 The majority of serum TGF-β levels originate from platelets and thus are markers of platelet activation in atherosclerosisCitation2 and rheumatoid arthritis.Citation58 TGF-β levels can be calculated as an active or total (active and acid-activatable) form. In systemic lupus erythematous patients, a lower serum activation index has been associated with increased lymphocyte apoptosis, irreversible organ damage, disease duration, low-density lipoprotein, and increased carotid intima-media thickness.Citation59 Endoglin is an accessory TGF-β receptor and soluble endoglin may interfere with TGF-β interaction with membrane-bound receptors and thus decrease TGF-β signaling.Citation60 Increased levels of soluble endoglin have been related to atherosclerosis,Citation61 as well as preeclampsia. Citation62
However, in late stages of the disease, TGF-β1 seems to behave as a proatherogenic factor by increasing excessive extracellular matrix,Citation63 promotion of in-stent restenosis,Citation64 and induction of pathologic vascular remodeling.Citation65 In fact, it has been demonstrated that end-organ damage in hypertension has been related to increased levels of TGF-β1 in serum and urine.Citation66 Moreover, in atherosclerotic vascular disease, it has been demonstrated that cells become insensitive to TGF-β1 signaling by means of decreased TGF-β1 activation,Citation67 decreased receptorCitation2,Citation68 and Smad downregulation,Citation2,Citation69 altered endocytosis and intracellular trafficking pattern,Citation21 or alteration of any of the other multiple cellular pathways that crosstalk with the TGF-β1 signaling pathway.Citation2 Interestingly, many of these factors may be altered at the genetic level by means of congenital atherosclerotic-related plymorphismsCitation70–Citation72 and genetic determinants of aortic aneurysms.Citation73 Moreover, even acquired mutations have been postulated to modulate these facts.Citation74
TGF-β1 in myeloproliferative syndromes
Philadelphia-negative myeloproliferative syndromes (polycythemia vera, essential thrombocytosis, essential myelofibrosis) are clonal hematological neoplasms in which an increased risk of arterial thrombosis occurs. A growing body of clinical and experimental evidence suggests that these variable phenotypes can follow a graded natural history, from initial essential thrombocytosis to subsequent polycythemia vera, and eventual spent-phase secondary myelofibrosis.Citation75 These three disorders are associated with increased risk of arterial thrombosis, and in polycythemia vera, this risk is 20% at 10 years.Citation75
In essential thrombocytosis, the abnormal clone seems to lose sensitivity to the proliferation-control effects of TGF-β1.Citation76 In polycythemia vera, the abnormal clone equally loses response to the cytokine.Citation77
However, essential or secondary myelofibrosis is the chronic myeloproliferative syndrome which is associated with the shortest survival rate.Citation78 The risk of thrombosis is similar to that found in essential thrombocythemia (1%–3% per patient per year).Citation79 This is the variant of myeloproliferative syndrome which has the strongest link to dysregulation of the TGF-β1 pathway.Citation78 In fact, TGF-β increases myelofibrosis in murine models.Citation80 In cell culture models, TGF-β secretion seems to be regulated by NFκB.Citation81 In myeloproliferative syndromes, there seems to be a progressive grading in TGF-β1 in the prefibrotic state, although established myelofibrosis has lower TGF-β1 levels.Citation82 Higher TGF-β levels seem to be correlated with increased allelic charge of JAK2, and decreased EPC levels.Citation82,Citation83
Therapy of myelofibrosis is a clinical challenge and supportive care has been the only treatment to date.Citation77 Current JAK-2 inhibitors have shown only a limited benefit in regard to spleen size.Citation84 Advances in the knowledge of TGF-β signaling in myeloproliferative syndromes may guide the choice of synergistic novel therapies such as small-molecule TGF-β pathway blockers including, SB-431542/ALK-4 inhibitor of the ALK-4 kinase activityCitation2, heat-shock protein inhibitors, NFκB inhibitors,Citation85 or epigenetic drugs.Citation86
Thus, progressive TGF-β dysfunction can be considered as a shared pathogenic event in atherosclerosis and Philadelphia-negative myeloproliferative syndromes, and a putative diagnostic and therapeutic tool.
Conclusion
TGF-β1 is a key factor in diseases affected by cardiovascular disorders, such as atherosclerosis and myeloproliferative syndromes. The current knowledge of the complex TGF-β1 regulation of physiological and pathological processes may help to design novel diagnostic techniques and target-designed innovative therapies.
Authors’ contributions
SR conceived the idea of the manuscript and wrote the first schematic draft. SR and TT designed the search strategy. SR, JN-D, MR, UM, and TT performed the review of the literature. All authors collaborated in the definitive version, and approved the final draft of the manuscript.
Acknowledgment/disclosure
Our lab received support by FISS (Health Research Fund, PI080920) and Red Temática de Investigación Cardiovascular RECAVA (RD06/0014/1007), both from the Instituto de Salud Carlos III, Spanish Ministry of Health (ISCIII). The authors report no conflicts of interest in this work.
References
- GasparyanAYAyvazyanLBlackmoreHKitasGDWriting a narrative biomedical review: considerations for authors, peer reviewers, and editorsRheumatol Int201131111409141721800117
- RedondoSSantos-GallegoCGTejerinaTTGF-beta1: a novel target for cardiovascular pharmacologyCytokine Growth Factor Rev2007183–427928617485238
- LebrinFGoumansMJJonkerLEndoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transductionEMBO J200423204018402815385967
- LoureiroJSchilteMAguileraABMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposureNephrol Dial Transplant20102541098110820067910
- RedondoSRuizESantos-GallegoCGPadillaETejerinaTPioglitazone induces vascular smooth muscle cell apoptosis through a peroxisome proliferator-activated receptor-gamma, transforming growth factor-beta1, and a Smad2-dependent mechanismDiabetes200554381181715734860
- TangYYangXFrieselREVaryCPLiawLMechanisms of TGF-β-induced differentiation in human vascular smooth muscle cellsJ Vasc Res201148648549421832838
- AzumaMMotegiKAotaKYamashitaTYoshidaHSatoMTGF-beta1 inhibits NF-kappaB activity through induction of IkappaB-alpha expression in human salivary gland cells: a possible mechanism of growth suppression by TGF-beta1Exp Cell Res1999250121322210388535
- GoumansMJValdimarsdottirGItohSActivin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signalingMol Cell200312481782814580334
- TojoMTakebeATakahashiSSmad7-deficient mice show growth retardation with reduced viabilityJ Biochem2012151662163122383537
- LebrinFDeckersMBertolinoPTen DijkePTGF-beta receptor function in the endotheliumCardiovasc Res200565359960815664386
- McAllisterKAGroggKMJohnsonDWEndoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1Nat Genet1994843453517894484
- BergJNGallioneCJStenzelTTThe activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2Am J Hum Genet199761160679245985
- MoustakasAHeldinCHNon-Smad TGF-beta signalsJ Cell Sci2005118Pt 163573358416105881
- YangFChungACHuangXRLanHYAngiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3Hypertension200954487788419667256
- TsaparaALuthertPGreenwoodJHillCSMatterKBaldaMSThe RhoA activator GEF-H1/Lfc is a transforming growth factor-beta target gene and effector that regulates alpha-smooth muscle actin expression and cell migrationMol Biol Cell201021686087020089843
- HongMWilkesMCPenheiterSGGuptaSKEdensMLeofEBNon-Smad transforming growth factor-β signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinaseJ Biol Chem201128620178411785021454615
- LiuQZhangYMaoHA crosstalk between the Smad and JNK signaling in the TGF-β-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cellsPLoS One201272e3200922384127
- StrippoliRBenedictoIPérez LozanoMLCerezoALópez-CabreraMdel PozoMAEpithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathwayDis Model Mech200814–526427419093035
- StrippoliRBenedictoIForondaMp38 maintains E-cadherin expression by modulating TAK1-NF-kappa B during epithelial-to-mesenchymal transitionJ Cell Sci2010123Pt 244321433121098640
- GhoshAKBhattacharyyaSWeiJPeroxisome proliferator-activated receptor-gamma abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivatorFASEB J20092392968297719395477
- ChenYGEndocytic regulation of TGF-beta signalingCell Res2009191587019050695
- HayesSChawlaACorveraSTGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2J Cell Biol200215871239124912356868
- RazaniBZhangXLBitzerMvon GersdorffGBöttingerEPLisantiMPCaveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptorJ Biol Chem200127696727673811102446
- ChenCLLiuIHFlieslerSJHanXHuangSSHuangJSCholesterol suppresses cellular TGF-beta responsiveness: implications in atherogenesisJ Cell Sci2007120Pt 203509352117878231
- ZhaoBWangQDuJLuoSXiaJChenYGPICK1 promotes caveolin-dependent degradation of TGF-β type I receptorCell Res2012
- IkushimaHMiyazonoKCellular context-dependent “colors” of transforming growth factor-beta signalingCancer Sci2010101230631220067465
- GregoryPABertAGPatersonELThe miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1Nat Cell Biol200810559360118376396
- PetroccaFVisoneROnelliMRE2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancerCancer Cell200813327228618328430
- VenturaAYoungAGWinslowMMTargeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clustersCell2008132587588618329372
- KumarswamyRVolkmannIJazbutyteVDangwalSParkDHThumTTransforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21Arterioscler Thromb Vasc Biol201232236136922095988
- HataADavisBNControl of microRNA biogenesis by TGFbeta signaling pathway-A novel role of Smads in the nucleusCytokine Growth Factor Rev2009205–651752119892582
- BatutJHowellMHillCSKinesin-mediated transport of Smad2 is required for signaling in response to TGFbeta ligandsDev Cell200712226127417276343
- JinQDingWMulderKMRequirement for the dynein light chain km23-21 in a Smad2-dependent transforming growth factor-beta signaling pathwayJ Bio Chem200728226191221913217420258
- KurisakiAKoseSYonedaYHeldinCHMoustakasATransforming growth factor-beta induces nuclear import of Smad3 in an importin-beta1 and Ran-dependent mannerMol Biol Cell20011241079109111294908
- ChenHBRudJGLinkXuHNuclear targeting of transforming growth factor-beta-activated Smad complexesJ Biol Chem200528022213292133615799969
- KnockaertMSapkotaGAlarcónCMassaquéJBrivanlouAHUnique players in the BMP pathway: small C-terminal domain phosphatases dephosphorylate Smad1 to attenuate BMP signalingProc Natl Acad Sci U S A200610332119401194516882717
- VardouliLMoustakasAStournarasCLIM kinase and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-betaJ Biol Chem200528012114481145715647284
- ClarkeDCLiuXDecoding the quantitative nature of TGF-beta/Smad signalingTrends Cell Biol200818943044218706811
- LebastchiAHKhanSFQinLTransforming growth factor beta expression by human vascular cells inhibits interferon gamma production and arterial media injury by alloreactive memory T cellsAm J Transplant201111112332234121812925
- CastanaresCRedondo-HorcajoMMagán-MarchalNten DijkePLamasSRodríguez-PascualFSignaling by ALK5 mediates TGF-beta-induced ET-1 expression in endothelial cells: a role for migration and proliferationJ Cell Sci2007120Pt 71256126617376964
- RedondoSSantos-GallegoCGGanadoPAcetylsalicylic acid inhibits cell proliferation by involving transforming growth factor-betaCirculation2003107462662912566377
- RuizERedondoSGordillo-MoscosoATejerinaTPioglitazone induces apoptosis in human vascular smooth muscle cells from diabetic patients involving the transforming growth factor-beta/activin receptor-like kinase-4/5/7/Smad2 signaling pathwayJ Pharmacol Exp Ther2007321243143817267584
- JaffeMSestiCWashingtonIMTransforming growth factor-β signaling in myogenic cells regulates vascular morphogenesis, differentiation, and matrix synthesisArterioscler Thromb Vasc Biol2012321e1e1121979435
- FeinbergMWWatanabeMLebedevaMATransforming growth factor-beta1 inhibition of vascular smooth muscle cell activation is mediated via Smad3J Biol Chem200427916163881639314754879
- WalsheTEDoleVSMaharajASPatternISWagnerDDD’AmorePAInhibition of VEGF or TGF-{beta} signaling activates endothelium and increases leukocyte rollingArterioscler Thromb Vasc Biol20092981185119219461051
- BasoniCNoblesMGrimshawAInhibitory control of TGF-beta1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytesFASEB J200519782282415746186
- RedondoSHristovMGümbelDTejerinaTWeberCBiphasic effect of pioglitazone on isolated human endothelial progenitor cells: involvement of peroxisome proliferator-activated receptor-gamma and transforming growth factor-beta1Thromb Haemost200797697998717549301
- VayalilPKOlmanMMurphy-UllrichJEPostlethwaitEMLiuRMGlutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogenAm J Physiol Lung Cell Mol Physiol20052896L937L94516258002
- AnituaEAguirreJJAlgortaJEffectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcersJ Biomed Mater Res B Appl Biomater200884241542117595032
- MeyerAWangWQuJPlatelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overloadBlood201211941064107422134166
- GraingerDJMosedaleDEMetcalfeJCWeissbergPLKempPRActive and acid-activatable TGF-beta in human sera, platelets and plasmaClin Chim Acta1995235111317634487
- GraingerDJTransforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesisArterioscler Thromb Vasc Biol200424339940414699019
- LutgensEGijbelsMSmookMTransforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progressionArterioscler Thromb Vasc Biol200222697598212067907
- DaiJMichineauSFranckGLong term stabilization of expanding aortic aneurysms by a short course of cyclosporine A through transforming growth factor-beta inductionPLoS One2011612e2890322194945
- JiQWGuoMZhengJSDownregulation of T helper cell type 3 in patients with acute coronary syndromeArch Med Res200940428529319608018
- KoenenRRWeberCChemokines: established and novel targets in atherosclerosisEMBO Mol Med201131271372522038924
- OkamotoYGotohYUemuraOTanakaSAndoTNishidaMAge-dependent decrease in serum transforming growth factor (TGF)-beta 1 in healthy Japanese individuals; population study of serum TGF-beta 1 level in JapaneseDis Markers2005212717415920293
- GasparyanAYStavropoulos-KalinoglouAMikhailidisDPDouglasKMKitasGDPlatelet function in rheumatoid arthritis: arthritic and cardiovascular implicationsRheumatol Int201131215316420390282
- JacksonMAhmadYBruceINCoupesBBrenchleyPEActivation of transforming growth factor-beta1 and early atherosclerosis in systemic lupus erythematosusArthritis Res Ther200683R8116646981
- López-NovoaJMBernabeuCThe physiological role of endoglin in the cardiovascular systemAm J Physiol Heart Circ Physiol20102994H959H97420656886
- Blázquez-MedelaAMGarcía-OrtizLGómez-MarcosMAIncreased plasma soluble endoglin levels as an indicator of cardiovascular alterations in hypertensive and diabetic patientsBMC Med201088621171985
- López-NovoaJMSoluble endoglin is an accurate predictor and a pathogenic molecule in pre-eclampsiaNephrol Dial Transplant200722371271417210583
- DeesCAkhmetshinaAZerrPPlatelet-derived serotonin links vascular disease and tissue fibrosisJ Exp Med2011208596197221518801
- PalleroMATalbert RodenMChenYFStainless steel ions stimulate increased thrombospondin-1-dependent TGF-beta activation by vascular smooth muscle cells: implications for in-stent restenosisJ Vasc Res201047430932220016205
- Kieć-WilkBStolarz-SkrzypekKSliwaAZdienickaAKawecka-JaszczKPeripheral blood concentrations of TGFβ1, IGF-1 and bFGF and remodelling of the left ventricle and blood vessels in hypertensive patientsKardiol Pol2010689996100220859888
- LaviadesCVaroNDíezJTransforming growth factor beta in hypertensives with cardiorenal damageHypertension200036451752211040229
- SakamotoYMiyazakiATamagawaHWangGPHoriuchiSSpecific interaction of oxidized low-density lipoprotein with thrombospondin-1 inhibits transforming growth factor-beta from its activationAtherosclerosis20051831859315907858
- McCaffreyTADuBFuCThe expression of TGF-beta receptors in human atherosclerosis: evidence for acquired resistance to apoptosis due to receptor imbalanceJ Mol Cell Cardiol19993191627164210471347
- KalininaNAgrotisAAntropovaYSmad expression in human atherosclerotic lesions: evidence for impaired TGF-beta/Smad signaling in smooth muscle cells of fibrofatty lesionsArterioscler Thromb Vasc Biol20042481391139615166010
- RaoMGuoDJaberBLTighiouartHPereiraBJBalakrishnanVSfor HEMO Study GroupTransforming growth factor-beta 1 gene polymorphisms and cardiovascular disease in hemodialysis patientsKidney Int200466141942715200451
- SieMPUitterlindenAGBosMJTGF-beta 1 polymorphisms and risk of myocardial infarction and stroke: the Rotterdam StudyStroke200637112667267117023672
- PengZZhanLChenSXuEAssociation of transforming growth factor-β1 gene C-509T and T869C polymorphisms with atherosclerotic cerebral infarction in the Chinese: a case-control studyLipids Health Dis20111010021679448
- BirosENormanPEJonesGTMeta-analysis of the association between single nucleotide polymorphisms in TGF-β receptor genes and abdominal aortic aneurysmAtherosclerosis2011219121822321855067
- ClarkKJCaryNRGraceAAMetcalfeJCMicrosatellite mutation of type II transforming growth factor-beta receptor is rare in atherosclerotic plaquesArterioscler Thromb Vasc Biol200121455555911304472
- Alvarez-LarránAPereiraACervantesFAssessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia veraBlood201211961363136922160617
- KurodaHMatsunagaTTeruiTDecrease of Smad4 gene expression in patients with essential thrombocythaemia may cause an escape from suppression of megakaryopoiesis by transforming growth factor-beta1Br J Haematol2004124221122014687032
- LiJBenchAJHuntlyBJGreenARMutation and methylation analysis of the transforming growth factor beta receptor II gene in polycythaemia veraBr J Haematol2001115487288011843821
- VannucchiAMManagement of myelofibrosisHematology Am Soc Hematol Educ Program2011201122223022160038
- PonceCCde LourdesFChauffailleMIharaSSSilvaMRThe relationship of the active and latent forms of TGF-β1 with marrow fibrosis in essential thrombocythemia and primary myelofibrosisMed Oncol12272011 [Epub ahead of print.]
- GastinneTVigantFLavenu-BombledCAdenoviral-mediated TGF-beta1 inhibition in a mouse model of myelofibrosis inhibit bone marrow fibrosis developmentExp Hematol2007351647417198875
- RameshwarPNarayananRQianJDennyTNColonCGasconPNF-kappa B as a central mediator in the induction of TGF-beta in monocytes from patients with idiopathic myelofibrosis: an inflammatory response beyond the realm of homeostasisJ Immunol200016542271227710925316
- CampanelliRRostiVVillaniLEvaluation of the bioactive and total transforming growth factor β1 levels in primary myelofibrosisCytokine201153110010620801055
- SozerSWangXZhangWCirculating angiogenic monocyte progenitor cells are reduced in JAK2V617F high allele burden myeloproliferative disordersBlood Cells Mol Dis200841328429118715806
- VerstovsekSKantarjianHMEstrovZLong-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controlsBlood2012120612011209
- Wagner-BallonOPisaniDFGastinneTProteasome inhibitor bortezomib impairs both myelofibrosis and osteosclerosis induced by high thrombopoietin levels in miceBlood2007110134535317374740
- ThepotSItzyksonRSeegersVfor Groupe Francophone des Myelodysplasies (GFM)Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM)Blood2010116193735374220664061