Abstract
Objective
The accuracy of the Omron HEM-1040 automated oscillometric upper-arm blood pressure (BP) monitoring device, designed for home self-measurements in adult populations, was tested according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060–2:2013 protocol.
Methods
The requirements of this protocol were precisely followed with the recruitment of 85 individuals in whom sequential systolic BP (SBP) and diastolic BP (DBP) were measured in the same (left) arm and who fulfilled the inclusion criteria involving the ranges of arm circumference, SBP, and DBP.
Results
The mean device-observer difference in the 255 separate pairs of BP data was −2.7 ± 7.14 mmHg for SBP and −3.3 ± 5.70 mmHg for DBP. The data were in accordance with criterion 1 of the ANSI/AAMI/ISO 81060–2:2013 standard requirements (≤ 5 ± ≤ 8 mmHg). In addition, the mean device-observer difference in the 85 participants was −2.7 ± 5.89 mmHg for SBP and −3.3 ± 4.99 mmHg for DBP, fulfilling criterion 2 with a standard deviation of ≤ 6.39 mmHg for SBP and ≤ 6.09 mmHg for DBP.
Conclusion
The OMRON HEM-1040 BP monitor fulfilled the requirements of the ANSI/AAMI/ISO validation standard.
Introduction
As is well known from the golden saying that “a man is as old as his arteries,” by William Osler, atherosclerotic diseases such as cardiovascular accidents pose a threat of deteriorating the life prognosis in aging societies, particularly in developed countries. Hypertension is one of the most influential causes of atherosclerosis and can be relatively easily controlled with antihypertensive agents.Citation1 Blood pressure (BP) is the most important parameter for diagnosing hypertension. BP continuously fluctuates with time, and repeated BP measurements are required for the proper assessment and diagnosis of hypertension. BP monitoring devices are rapidly evolving into not only accurate but also easy-to-use measurement tools. Therefore, individuals who are not health-care professionals can perform accurate self-measurements of BP. The BP levels measured at home are believed to be more valuable than the office BP measured in the clinic for predicting the prognosis of hypertensive patients.Citation2
Another issue is the accuracy of BP measurements of a device. Scientific data are definitely required for device validation.Citation3 Therefore, some international protocols for the validation of BP measuring devices have been proposed. Accordingly, the clinical guidelines for the diagnosis and treatment of hypertension in many countries recommend the use of devices that have been validated according to international protocols.
Therefore, we validated the accuracy of HEM-1040, which can easily measure BP by inserting the arm into a cylinder that encapsulates the cuff of the device, according to the international protocols of the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2013 standard guidelines.Citation4
Materials and Methods
Device
Omron HEM-1040 (Omron Healthcare Co., Ltd. Kyoto, Japan) is an automatic oscillometric device for BP measurements in the upper arm (), and has a pressure range of 20–299 mmHg and a heart rate range of 40–180 beats/min. Systolic BP (SBP), diastolic BP (DBP), and pulse rate are displayed on a liquid crystal digital monitor. The device has an overall weight of approximately 2600 g (without accessories and options), with dimensions of approximately 294 mm × 271 mm × 286 mm (width × height × depth). The cuff can be used for arm circumferences ranging from 22.0 to 42.0 cm. Omron HEM-1040 detects and displays position marks and body movements during BP measurement.
BP Measurements
For each experiment, the manufacturer provided standard production device models. The validation team for each device consisted of three observers who were experienced in measuring BP and trained under the online program of the British and Irish Hypertension Society (https://bihsoc.org/resources/bp-measurement/bp-measurement-auscultatory-tutorials/). BP data were collected by alternating between measurements using the Korotkoff method with a standard mercury sphygmomanometer (ACOMA Medical Industry Co., Ltd, Tokyo, Japan) and measurements using the tested device. Simultaneous auscultations were performed by two observers using the double stethoscope (Y tube) when measuring BP with the Korotkoff method. The two observers were blinded to each other’s readings, and a third observer who served as a supervisor checked the BP readings by the other two observers.
Participant Selection
All participants were recruited as volunteers. This study was approved by the institutional review board of the Biwako Central Hospital (BCH-143) and Omron Healthcare Co., Ltd. (14-shishin-30), with written informed consent obtained from each participant. The measurements were performed in the measurement room of Omron Healthcare Co., Ltd. (Kyoto, Japan), by staff employed by the manufacturer in accordance with the Declaration of Helsinki. The devices were tested on 97 participants according to the ANSI/AAMI/ISO protocol. The participants were screened to ensure that sex, age, arm circumference, and BP readings fulfilled the participation requirements described in the protocol. Participants with arrhythmias, those who moved their arms or bodies during the BP measurements, and those with unclear DBPs during Phase V of the Korotkoff sounds and with great variations in BP of >12/8 mmHg, were excluded from this study.
Procedure
The participants were seated in a quiet room with a comfortable temperature and were instructed to avoid talking during the procedure. The BP measurements started after the participants had rested for 10 min. The participants sat on a chair with their legs uncrossed and their feet flat on the floor. The chair had a supportive back and elbow and forearm rests. The arm circumference was measured in each participant, and the cuff size was adapted for auscultation. All BP measurements were performed in the participants’ left arm at the heart level. The devices were validated according to the same-arm sequential method of the ANSI/AAMI/ISO.
Analysis
Data analysis was performed according to the ANSI/AAMI/ISO requirements. Data are expressed as mean ± standard deviation (SD), and the minimum and maximum values with ranges were calculated. The mean of each pair of observer measurements was calculated as a reference value. The differences between the mean observer values and the test values were calculated according the protocol, and displayed in Bland-Altman plots against the means between the two values.
Results
According to the ANSI/AAMI/ISO guidelines, 97 participants were screened for this experiment. After excluding 12 participants according to the specified participant selection criteria (), a total of 85 participants, 48 men (56%) and 37 women (44%), were finally included. The mean age was 46 ± 13.2 years (range, 24–71 years). The mean arm circumference was 30.0 ± 5.3 cm (range, 22.3–40.8 cm). Of the participants, 60.0% (criterion ≥ 40%), 40.0% (≥ 40%), 40.0% (≥ 20%), and 21.2% (≥ 20%) had an arm circumference of 22.0–32.0, 32.1–42.0, 22.0–27.0, and 37.1–42.0 cm, respectively ().
Table 1 Screening and Recruitment Details
Table 2 Baseline Characteristics and Blood Pressure Distribution
The mean value of the 255 measurements obtained using the mercury sphygmomanometer was 123 ± 24.2 mmHg (range, 84–208 mmHg) for SBP and 78 ± 15.9 mmHg (range, 51–126 mmHg) for DBP ().
The percentages of high SBP (≥ 160 mmHg), medium SBP (≥ 140 mmHg), and low SBP (≤ 100 mmHg) were 8.2% (criterion ≥ 5%), 21.2% (≥ 20%), and 16.5% (≥ 5%), respectively. The percentages of high DBP (≥ 100 mmHg), medium DBP (≥ 85 mmHg), and low DBP (≤ 60 mmHg) were 12.5% (criterion ≥ 5%), 29.4% (≥ 20%), and 15.7% (≥ 5%), respectively ().
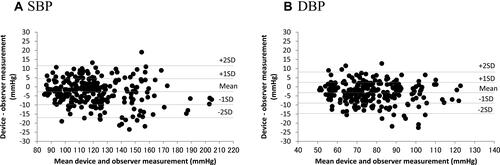
The differences between the two observers were 0 ± 1.4 and 0 ± 1.5 mmHg for SBP and DBP, respectively. The mean differences between the two observers and the Omron HEM-1040 device were −2.7 ± 7.14 mmHg (range, −23.5 to 19.0 mmHg) for SBP and −3.3 ± 5.70 mmHg (range, −22.5 to 12.8 mmHg) for DBP according to criterion 1 (). These data fulfilled the ANSI/AAMI/
Table 3 Validation Results
ISO 81060–2:2013 requirements of ≤ 5 ± ≤ 8 mmHg. The mean differences between the two observers and Omron HEM-1040 were −2.7 ± 5.89 mmHg (range, −19.6 to 9.8 mmHg) for SBP and −3.3 ± 4.99 mmHg (range, −19.6 to 7.8 mmHg) for DBP according to criterion 2 (). Therefore, the SD for SBP was calculated to be < 6.39 mmHg and that for DBP was 6.09 mmHg according to criterion 2. These results were in accordance with the –2:2013 requirements for criteria 1 and 2.
and shows the differences in the SBP and DBP readings, respectively, in relation to the mean differences between the HEM-1040 readings and the mercury sphygmomanometer measurements.
Discussion
The present study found that Omron HEM-1040, an oscillometric BP monitor with a cylindrical-type cuff device, fulfilled validation criteria 1 and 2 of the ANSI/AAMI/ISO 81060-2:2013 guidelines. Therefore, the accuracy of this device for BP measurement is reliable among adult populations without arrhythmias.
However, the range of difference in BP readings between the two methods was widely distributed (−23.5 to 19.0 mmHg in systole, −22.5 to 12.8 mmHg in diastole). The most powerful factor causing the difference was considered to be the same-arm sequential method of the ANSI/AAMI/ISO. BP measurement with auscultation and measurement with the device were not simultaneously, but rather alternately, performed in the left upper arm. Because BP always fluctuates corresponding to each heartbeat, it is natural to find a difference when alternate measurements are performed. The criteria of the ANSI/AAMI/ISO were used to set the significant range of allowance to ensure that our findings are reasonable.Citation4
BP, which is an important indicator of future life prognosis,Citation5 can be accurately monitored using these automated devices. A simple device that can measure BP as easily and accurately as possible is ideal. In this regard, Omron HEM-1040 is a useful tool not only for health-care professionals but also for ordinary persons.
Device Strengths and Limitations
The distinguishing feature of the Omron HEM-1040 is the cuff; as the universal cuff is enclosed in a plastic cylinder, it does not need to be wrapped around the arm when measuring BP. This is advantageous since it is difficult to properly wrap the cuff around the upper arm using the other hand when performing self-BP measurements at home and its design avoids hazardous situations when measuring BP. We validated the Omron HBP-9031C,Citation6 which is a desktop-type device with a similar cuff, and found it to be accurate according to the ANSI/AAMI/ISO protocol. However, one limitation of this device is the universal cuff, which is intended for an arm circumference of 22.0 to 42.0 cm, and is therefore unsuitable for users with an arm circumference outside of this range. The selection of an appropriate cuff size according to the user’s arm circumference is required to assess accurate BP.Citation7 Another limitation is that the study was performed only in adults without arrhythmias. As BP in selected patient groups, such as pregnant women, patients with diabetes and/or obesity, and children, may be different, another study including special patient groups and children is needed to confirm the accuracy of this device in these groups. Lastly, the study was performed by the staff employed by the manufacturer of this device, which may pose potential conflicts of interest. To avoid bias, observers were blinded to each other’s readings, and a hypertension specialist (H. Takahashi) supervised the study. Despite this, conflicts of interest may remain.
Conclusion
The results of this study showed that the Omron HEM-1040 device met the requirements of the ANSI/AAMI/ISO international protocol in adult populations without arrhythmias.
Disclosure
Kanako Saito is employed by and receives a salary from Omron Healthcare Co., Ltd., the manufacturer of Omron HEM-1040. The authors report no other conflicts of interest in this work.
References
- Lüscher TF. Arterial and pulmonary hypertension: risk assessment and current pharmacological and interventional management. Eur Heart J. 2018;39(47):4127–4131. doi:10.1093/eurheartj/ehy824
- Stergiou GS, Kario K, Kollias A, et al. Home blood pressure monitoring in the 21st century. J Clin Hypertens (Greenwich). 2018;20(7):1116–1121. doi:10.1111/jch.13284
- Palatini P, Frick GN. Techniques for self‐measurement of blood pressure: limitations and needs for future research. J Clin Hypertens (Greenwich). 2012;14(3):139–143. doi:10.1111/j.1751-7176.2011.00586.x
- Association for the Advancement of Medical Instrumentation American National Standard. ANSI/AAMI/ISO 81060-2:2013 non-invasive sphygmomanometers – part 2: clinical investigation of automated measurement type. Available from: http://my.aami.org/aamiresources/previewfiles/8106002_1306_preview.pdf#search=%27American+National+Standard.+ANSI%2FAAMI%2FISO+810602%3A2013+Noninvasive%27. Accessed September 15, 2020.
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Saito K, Hishiki Y, Takahashi H. Validation of the Omron HBP-9031C professional office blood pressure monitor in the general population according to the ANSI/AAMI/ISO 81060-2:2013 protocol. J Hum Hypertens. 2020;34(10):735–738. doi:10.1038/s41371-020-0301-0
- Ringrose J, Millay J, Babwick SA, et al. Effect of overcuffing on the accuracy of oscillometric blood pressure measurements. J Am Soc Hypertens. 2015;9(7):563–568. doi:10.1016/j.jash.2015.04.007