Abstract
Cardiovascular disease (CVD) is responsible for significant morbidity and mortality within the United States and worldwide. Although targeting low-density lipoprotein cholesterol (LDL-C) in the prevention of CVD has been shown to be effective, evidence exists to indicate that significant cardiovascular (CV) risk remains in patients receiving 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) – a risk that may be correlated with low levels of high-density lipoprotein cholesterol (HDL-C). Among the various tactics under investigation to increase HDL-C, inhibition of cholesteryl ester transfer protein (CETP) appears the most adept to raise these levels. Although torcetrapib, a CETP inhibitor, demonstrated significant beneficial changes in HDL-C and LDL-C after 12 months of therapy when coadministered with atorvastatin, patients in the torcetrapib arm experienced a rise in mortality, including increased risk of death from CV and non-CV causes as well as a significant rise in major CV events. Later studies established that the adverse effects of torcetrapib were produced from molecule-specific off-target effects and not to the mechanism of CETP inhibition. These untoward outcomes have not been detected with anacetrapib, the third of the CETP inhibitors to enter Phase III trials. Furthermore, treatment with anacetrapib revealed both a statistically significant decrease in LDL-C and increase in HDL-C over placebo. While the place in therapy of niacin and fibrates to reduce CV events is currently in question secondary to the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes and the Action to Control CV Risk in Diabetes trials, the ongoing large-scale, randomized–placebo, controlled-outcomes study with anacetrapib coadministered with statin treatment will not only test the hypothesis if CETP inhibition lowers residual CV risk but will also provide insight as to which patient subgroups might benefit the most from anacetrapib despite aggressive therapy with statins.
Cardiovascular disease (CVD) is responsible for significant morbidity and mortality within the United States (US) and worldwide. The prevalence of CVD in the US is projected to be 37.8% by 2015 – an estimate which will only increase in subsequent years.Citation1
Current guidelines for the prevention of coronary heart disease (CHD) identify low-density lipoprotein cholesterol (LDL-C) as the primary target for lipid-lowering therapy.Citation2,Citation3 Numerous randomized controlled clinical trials have solidified 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) as the cornerstone of LDL-C-lowering therapy. More importantly, in addition to lowering surrogate levels of LDL-C, the use of statins in the primary and secondary prevention of CVD have been linked to significant reductions in cardiovascular (CV) outcomes and all-cause mortality.Citation4–Citation6 These reductions are believed to stem from the pleiotropic effects of statins in addition to their LDL-C-lowering ability.Citation7
Although targeting LDL-C in the prevention of CVD has been shown to be effective, evidence exists to indicate that significant CV risk remains in patients receiving statin-based therapy – a risk that may be correlated with subsequently low levels of high-density lipoprotein cholesterol (HDL-C). In a post hoc analysis of the Treating to New Targets study in which randomized patients received atorvastatin 10 mg daily or atorvastatin 80 mg daily, the frequency of major CV events increased with decreasing levels of HDL-C in both treatment arms.Citation8 This relationship was seen even among patients obtaining LDL-C levels less than 70 mg/dL. This inverse relationship between HDL-C and the risk of CVD has long been established through several epidemiological studies.Citation9–Citation12 The landmark Framingham Study concluded that HDL-C was the most significant lipid risk factor for CHD.Citation9 One evaluation of four epidemiologic studies put forward that for each 1 mg/dL increase in HDL-C, there was a decrease in CHD of 1.9%–2.9%.Citation13 As a result of this epidemiological evidence, targeting HDL-C to further reduce CV risk provides an appealing alternative to LDL-C-lowering therapy.
Cholesteryl ester transfer protein inhibitors
Among the various tactics under investigation to increase HDL-C, inhibition of cholesteryl ester transfer protein (CETP) appears the most adept to raise such levels.Citation14,Citation15 Initially, torcetrapib (CP-529414; Pfizer, La Jolla, CA), a CETP inhibitor, demonstrated promising results. In a Phase I trial conducted in healthy volunteers, torcetrapib at doses of 120 mg once daily and twice daily for 14 days increased HDL-C by 73% and 91%, respectively, and decreased LDL-C by 21% and 42%, respectively, with no evident adverse effects.Citation16 When torcetrapib 120 mg daily was administered with atorvastatin 20 mg daily, an LDL-C reduction of 17% (P = 0.02) beyond that achieved with atorvastatin alone and an HDL-C increase of 61% (P < 0.001) occurred after 4 weeks.Citation17 Eventually, early trials brought torcetrapib under scrutiny when results demonstrated an elevation in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of 1.3 to 2.2 and 0.9 to 1.1 mmHg at doses of 60 or 90 mg daily, respectively. Consequently, future trials with torcetrapib were restricted to utilize a dose of 60 mg daily.Citation18,Citation19
In the fourth quarter of 2006, all the torcetrapib trials were suspended due to the results of the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE) trial, which enrolled 15,067 high-risk CV patients. The participants were randomized to receive either atorvastatin 10 to 80 mg daily and placebo or atorvastatin and torcetrapib 60 mg daily. Despite a 72.1% increase in HDL-C and a 24.9% decrease in LDL-C after 12 months of therapy with the combination regimen, patients in the torcetrapib arm experienced a rise in mortality, including increased risk of death from both CV and non-CV causes as well as a significant rise in major CV events of 25% (95% confidence interval [CI]: 1.09–1.44; P = 0.001).Citation20 These results were confirmed by simultaneous trials: Investigation of Lipid Level Management Using Coronary Atherosclerosis by CETP Inhibition and HDL Elevation (ILLUSTRATE), Rating Atherosclerosis Disease Change with a New CETP Inhibitor (RADIANCE)-1 and RADIANCE-2.Citation21–Citation23
Later studies established that the adverse effects of torcetrapib were produced from molecule-specific off-target effects and not to the mechanism of CETP inhibition.Citation24–Citation26 Regardless of the 60-mg dose cap per day in ILLUMINATE, ILLUSTRATE, RADIANCE-1, and RADIANCE-2, the mean SBP elevations were 5.4, 4.6, 2.8, and 5.4 mmHg, respectively.Citation20–Citation23 Further analyses of ILLUSTRATE, RADIANCE- 1, and RADIANCE-2 pointed to a mineralcorticoid effect accompanied by an elevation in serum sodium and decreased serum potassium in patients who received torcetrapib. Forrest et al demonstrated that torcetrapib increased blood pressure through a CETP-independent pathway in mice (both with and without a CETP transgene), rats, dogs, and rhesus monkeys.Citation26 These untoward outcomes have not been detected with the other two CETP inhibitors, anacetrapib (MK-0859; Merck, Whitehouse Station, NJ) or dalcetrapib (JTT-705; Roche, Nutley, NJ), both of which entered Phase III trials.Citation27
Dalcetrapib was halted in May 2012 due to lack of efficacy in the Phase III dAL-OUTCOMES trial, a study in stable CHD patients with recent acute coronary syndrome.Citation28 In comparison to the other CETP inhibitors, anacetrapib and torcetrapib, dalcetrapib was a significantly less potent inhibitor of CETP.Citation29 Evacetrapib (LY2484595; Eli Lilly, Indianapolis, IN), DRL-17822 (Dr Reddy’s Laboratories, Hyderabad, India), and JTT-302 (Japan Tobacco, Tokyo, Japan) are currently undergoing Phase II investigation, while AT-103 (AFFiRiS AG, Vienna, Austria), a vaccine against CETP, and TA-8995 (Mitsubishi Tanabe, Osaka, Japan) are in early stage development. Anacetrapib, the third of the CETP inhibitors to commence Phase III trials, will be discussed in detail in this manuscript.
The role of CETP in cholesterol metabolism
Cholesterol is maintained by means of two homeostatic processes that lead cholesterol away from and back to the liver. Lipids secreted from hepatocytes in the form of very low-density lipoprotein cholesterol (VLDL-C), intermediate-density lipoprotein cholesterol, and LDL-C particles are taken up by the peripheral tissues via the LDL receptor. Alternatively, excess cholesterol in the periphery is removed and transported back to the liver by HDL-C via scavenger receptors for recycling and excretion from the body. This mechanism is most commonly termed reverse cholesterol transport (RCT) ().Citation30,Citation31 The role HDL-C plays in the removal of cholesterol from the body by means of RCT is essential in maintaining cholesterol equilibrium and is believed to subsequently give HDL-C its atheroprotective properties. Further contributing to the beneficial effects of HDL-C mediated RCT are the anti-inflammatory, antioxidative, antiapoptotic, antithrombotic, vasodilatory, and anti-infectious properties.Citation32
Figure 1 The function of CETP in RCT.
Abbreviations: CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; FC, free cholesterol; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; HL, hepatic lipase; LCAT, lecithin cholesterol acyltransferase; LDL, low-density lipoprotein; LDL-R, low-density lipoprotein receptor; LPL, lipoprotein lipase; RCT, reverse cholesterol transport; SR-B1, scavenger receptor-B1; TG, triglyceride; VLDL, very low-density lipoprotein.
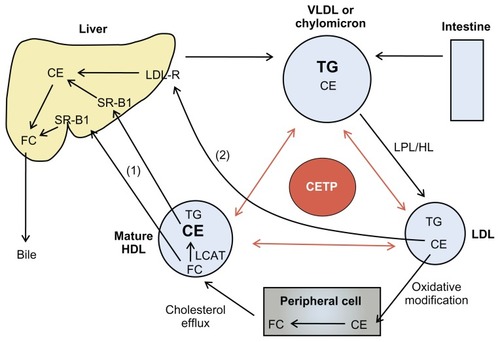
Within the RCT pathway exists CETP, a hepatically derived hydrophobic glycoprotein secreted from the liver that binds to HDL-C. CETP mediates the equimolar transfer of cholesteryl ester (CE) from HDL to apolipoprotein (apo) B lipoproteins (chylomicra, VLDL-C, and LDL-C) and the equimolar transfer of triglycerides (TGs) from VLDL-C and LDL-C to HDL-C.Citation33 The involvement of CETP in the RCT pathway is believed to result in both antiatherogenic and atherogenic activity. CETP-mediated transfer of CE accounts for the return of cholesterol from the peripheral cells to the liver via LDL receptors. Cholesterol can then be secreted into bile and eliminated from the body, leading to potentially antiatherogenic results. Conversely, when LDL receptors are unable to function adequately, CETP leads to the accumulation of LDL-C in the plasma.Citation34 The exchange of CE from HDL-C to apo B lipoproteins may also prevent efflux of cholesterol from peripheral cells and diminish circulating levels of HDL-C, which can reduce return of cholesterol from the arterial walls back to the liver.Citation35 In addition, CETP activity causes HDL-C and LDL-C to become TG-heavy, giving way to small-dense HDL-C and LDL-C. The small-dense LDL-C has an increased susceptibility to oxidative modification by TG lipase, which promotes uptake within the arterial wall by macrophage scavenger receptors.Citation36 Predominance of this smaller less buoyant LDL-C has been associated with up to a threefold increased risk of developing CAD.Citation37 Considering the potentially atherogenic effects of CETP, inhibition of this protein and the ability to increase antiatherogenic HDL-C has generated great interest in the prevention and treatment of CAD and atherosclerosis.
The correlation between CETP deficiencies and CV risk remains unclear at this point. Initial studies indicated that deficiencies in CETP were associated with an increased risk of CAD.Citation38,Citation39 However, the Women’s Genome Health Study revealed opposing results, suggesting cardioprotective benefits of CETP inactivity through an HDL-C-mediated pathway.Citation40 Finally, results of the Honolulu Heart Study revealed that men with CETP mutations had the lowest risk of CHD; however, these results were not statistically significant.Citation41 Therefore, the outcomes of these trials and the potential CV benefit of CETP modulation merit further research in this field.
Anacetrapib is a once daily, orally administered, CETP inhibitor that is currently undergoing Phase III clinical trials. Similar to torcetrapib, anacetrapib exhibits its effect by forming a reversible bond between CETP and HDL-C. Formation of this bond results in the inhibition of CETP-mediated CE and TG transfer between HDL-C and apo B lipoproteins, consequently increasing circulating antiatherogenic HDL-C.Citation27 In comparison to the other CETP inhibitors, torcetrapib and dalcetrapib, anacetrapib shares a similar potency with torcetrapib and is a significantly more potent inhibitor of CETP than dalcetrapib.Citation29
Pharmacokinetics, drug interactions, and pharmacodynamics
The pharmacokinetics of anacetrapib have been evaluated in healthy humans, dyslipidemic subjects, and animal models.Citation25,Citation42–Citation45 Following a 1-hour delay, anacetrapib is promptly absorbed with peak concentrations arising approximately 4 hours after administration.Citation25,Citation43–Citation45 Although anacetrapib is highly plasma protein-bound, the binding is reversible. After a 150-mg dose was administered in dyslipidemic patients, the mean maximum concentration (Cmax) of anacetrapib in plasma was 1861 nM on day 1 and 1960 nM on day 28, depicting a similar concentration after once-daily multiple dosing compared to a single dose upon initiation.Citation25 While the pharmacokinetic profile of anacetrapib is not affected by age, sex, or obesity,Citation43 food significantly enhances the absorption. A low-fat meal increased area under the curve (AUC)0–∞ up to two times and Cmax up to three times, while a high-fat meal increased AUC0–∞ up to six times and Cmax up to nine times versus the fasted state.Citation43 Therefore, anacetrapib is recommended to be administered with meals.Citation25,Citation43,Citation44 Steady state is reached after 7 days.Citation25,Citation44 The three minor radioactive metabolites formed through cytochrome P450 (CYP)3A4-catalyzed oxidation are excreted by the biliary–fecal route.Citation45 Anacetrapib has a biphasic elimination profile. It has a long terminal half-life and it exhibits an effective half-life of approximately 18 hours.Citation25,Citation44 A study by Dansky et al concluded that even after cessation of anacetrapib 8 weeks prior, the continued presence of drug levels coupled with persistent decreases in LDL-C and residual elevations in HDL-C suggested that anacetrapib has a terminal half-life of approximately 3 to 4 weeks.Citation46
When Krishna et al assessed the CYP3A drug interaction potential of anacetrapib in healthy volunteers, the medication did not influence the activity of this enzyme.Citation47 The study did provide evidence that anacetrapib is a moderately sensitive substrate of CYP3A as the plasma samples revealed that anacetrapib activity was elevated by ketoconazole, a potent CYP3A4 inhibitor. Additionally, the pharmacokinetics of drugs that are substrates for CYP pathways are not modified by anacetrapib. When midazolam was used as a probe-sensitive CYP3A substrate, anacetrapib treatment did not affect the activity of this enzyme, as depicted by a lack of variance in the plasma midazolam concentration. Similarly, a study evaluating the pharmacokinetic effects of simvastatin, also a sensitive CYP3A substrate, administered as monotherapy or in combination with anacetrapib, revealed no difference.Citation48 Subsequent to these trials, studies have been performed with digoxin and warfarin.Citation49,Citation50 Digoxin is a substrate of the P-gylcoprotein (Pgp)-mediated transport pathway. Metabolism of digoxin does not rely on nor does it affect CYP metabolism. Multiple dose administration of anacetrapib 100 mg did not affect single-dose pharmacokinetics of digoxin or warfarin in healthy treatment groups. Therefore, no dosage adjustment is required for warfarin or digoxin when used concomitantly with anacetrapib.
Pharmacodynamic studies to analyze the serum CETP inhibitory potential of anacetrapib have been completed.Citation43,Citation44 A study by Krishna et al revealed that while anacetrapib exerted a serum CETP maximum inhibition of nearly 90% at 4 hours on the first day, the inhibitory potential decreased 5% to 10% at the same time point after 14 days. On day 1, the trough inhibitory potential was approximately 80%, and after 14 days of repeated doses, the inhibitory potential was diminished about 20%.Citation44 An elucidation of this occurrence may be due to the threefold increase in CETP concentration observed throughout the study. Although a precise explanation as to the CETP mass increase is not confirmed, Clark et al credited this increase to the strong adherence of CETP for HDL-C with development of a non-productive complex.Citation51 Clark et al further noted that the CETP mass increase should not interfere with HDL-C elevation as the emergence of CETP concentration is not significant compared to the amount of HDL particles. This theory was validated when anacetrapib was dosed for 4 weeks and no associated loss of efficacy was found.Citation25
Clinical trials
The effects of anacetrapib on surrogate markers of CVD when administered alone or in combination with atorvastatin have been examined by Bloomfield et al.Citation52 The study enrolled 589 adult patients with primary hypercholesterolemia or mixed hyperlipidemia who had LDL-C between 100 to 190 mg/dL, 100 to 160 mg/dL if categorized as moderate-risk patients, or 100 to 130 mg/dL if diabetic. The majority (53.8%) of patients had low HDL-C (mean, 50.5 mg/dL) and a median LDL-C of 141.1 mg/dL at baseline. Patients were randomized to one of ten groups: placebo, atorvastatin 20 mg alone, anacetrapib 10, 40, 150, or 300 mg once daily as monotherapy, or atorvastatin 20 mg in combination with anacetrapib at the aforementioned dosage strengths for 8 weeks. Each of the groups included an equal number of patients with TG greater than 150 mg/dL.
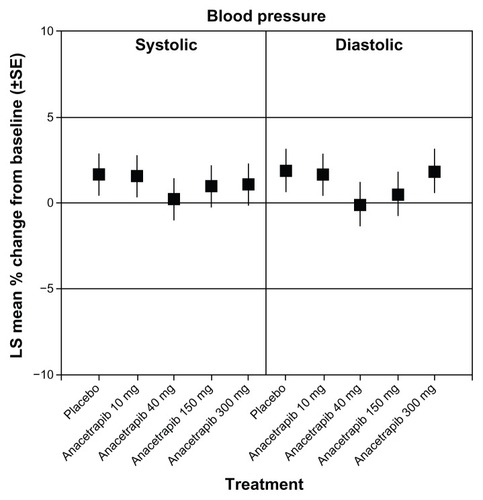
The efficacy endpoints in the study were the percent change from baseline within the different treatment groups in LDL-C (primary), HDL-C, non-HDL-C, total cholesterol, TG, and apo B, apo A-I, and apo E (secondary). Both HDL-C and LDL-C were statistically significantly increased and decreased, respectively, when anacetrapib was administered alone or in combination with atorvastatin ( and ). Additionally, although similar to the percentage change with anacetrapib monotherapy, there was a statistically significant increase in HDL-C with combination treatment versus atorvastatin monotherapy (). There was no further benefit in terms of lipid alteration when the anacetrapib dose was increased from 150 to 300 mg ( and ). Total cholesterol and TG were not affected by anacetrapib monotherapy or with concomitant administration with atorvastatin. Apo E and apo A-I were increased and lipoprotein (a) levels were decreased with elevating doses of anacetrapib. The C-reactive protein reduction of 30.9% with atorvastatin monotherapy was attenuated with combination treatment. All of the treatment arms tolerated anacetrapib monotherapy and combination therapy well, as the incidence of adverse effects were similar between the groups. There were no deaths or serious adverse effects. The most common complaint included constipation, diarrhea, dyspepsia, and myalgia. Most notably, there was no effect on either SBP or DBP (). Although this study revealed significant positive alterations in the lipid profile with anacetrapib alone or when coadministered with atorvastatin, further studies are required to demonstrate how these surrogate markers will translate into positive CV outcomes.
Figure 2 Changes in LDL-C over time. (A) anacetrapib monotherapy versus placebo and (B) anacetrapib + atorvastatin 20 mg versus atorvastatin 20 mg.
Abbreviation: LDL-C, low-density lipoprotein cholesterol.
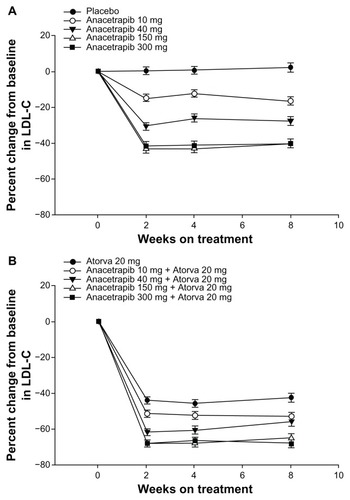
Figure 3 Changes in HDL-C over time. (A) Anacetrapib monotherapy versus placebo and (B) anacetrapib + atorvastatin 20 mg versus atorvastatin 20 mg.
Abbreviation: HDL-C, high-density lipoprotein cholesterol.
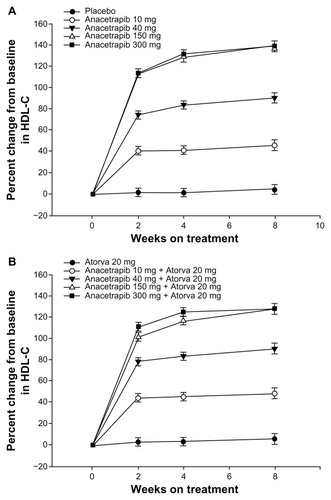
Figure 4 Changes in systolic and diastolic blood pressure at week 8 with anacetrapib monotherapy versus placebo.
Abbreviations: LS, least squares; SE, standard error.
Krauss et al examined the effects of anacetrapib on plasma lipids, lipoprotein subfraction concentrations, and lipoprotein composition in 30 healthy individuals following 14 days of therapy. Patients were randomized to receive anacetrapib 150 mg daily, 20 mg daily, or placebo. In patients receiving 150 mg of anacetrapib, LDL-C was reduced by 26% and HDL-C was increased by 82%. Additionally, there was a 29% decrease in apo B, a 21% increase in apo A-I, and a 43% decrease in lipoprotein (a). Regarding particle protein analysis, anacetrapib 150 mg once daily significantly reduced baseline mean particle concentrations of medium VLDL (22%), small VLDL (31%), large intermediate density lipoproteins (35%), medium LDL2a (35%), LDL2b (39%), and small LDL3a (28%) when compared to placebo. In addition, there was a significant increase in particle concentration of very small LDL4b (75%). Lastly, anacetrapib 150 mg significantly increased large HDL2b by 373%, yet yielded no significant change in smaller HDL2a + 3 particle concentrations.Citation53
A model-based approach was used to identify a suitable dose for future trials.Citation54 Considering the various variables examined (ie, formulation, diet, and study population), a dose of 100 mg for anacetrapib was selected for future study in Phase III trials that was not previously analyzed in the Bloomfield et al Phase IIb trial. The lipid-altering effects demonstrated in a Phase III trial, which was published during the review of the model-based approach, validated the predictions made.Citation55
DEFINE trial
Unlike the trial by Bloomfield et al, patients in The Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib (DEFINE) study were included if they had pre-existing CHD or were at high risk of CHD.Citation55,Citation56 This international, double-blind, Phase III, placebo-controlled trial evaluated the lipid level effects, tolerability, and safety profile of anacetrapib. Of the 2757 patients who were initially screened, 1623 patients were randomized to receive anacetrapib 100 mg or placebo daily in combination with statin therapy with or without other antilipemic agents. Each of the groups included patients aged 18 to 80 years who had LDL-C between 50 to 100 mg/dL, HDL-C less than 60 mg/dL, and TG not more than 400 mg/dL. The majority of patients had CHD, while 45.3% had risk factors for CHD. Only 0.7% of the patients were not on statin treatment.
The efficacy endpoints in the study were the percent change from baseline within the two treatment groups in LDL-C at 24 weeks and the safety and tolerability profile of anacetrapib during the 76 weeks. As in the Bloomfield et al trial, LDL-C was chosen as a primary endpoint because it is a CV risk factor that anacetrapib impacts. The change in LDL-C up to week 76 and change in HDL-C, non-HDL-C, apo B, and apo A-I after 24 weeks and 76 weeks of therapy were secondary efficacy endpoints. In addition to the safety endpoints of CV death, nonfatal myocardial infarction, stroke, and hospitalization due to unstable angina, other variables evaluated included blood pressure and electrolyte levels. The DEFINE trial analyzed the CV endpoints via a Bayesian approach to exclude the 25% CV events that occurred with torcetrapib. After 24 weeks of treatment, DEFINE established both a statistically significant decrease in LDL-C and increase in HDL-C over placebo (). These effects extended to week 76. Although secondary efficacy outcomes were also positively affected versus placebo, confirmation of a lack of benefit with C-reactive protein levels was established. Furthermore, the Bayesian analysis confirmed a 94% probability that anacetrapib would not produce a 25% increase in CV adverse outcomes previously detected with torcetrapib (). Although not all the surrogate markers originally used to test the off-target effects of torcetrapib have been analyzed with anacetrapib, the positive CV results of the DEFINE trial have assisted in reinvestigating the hypothesis that CETP inhibition is cardioprotective. Ideally, a largescale, randomized, placebo-controlled trial representing a variety of ethnic groups while evaluating the effects of long-term reduction of LDL-C to very low levels should be conducted in order to offer definitive outcomes as to the safety and efficacy of anacetrapib.
Figure 5 Changes in cholesterol levels during the study period.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
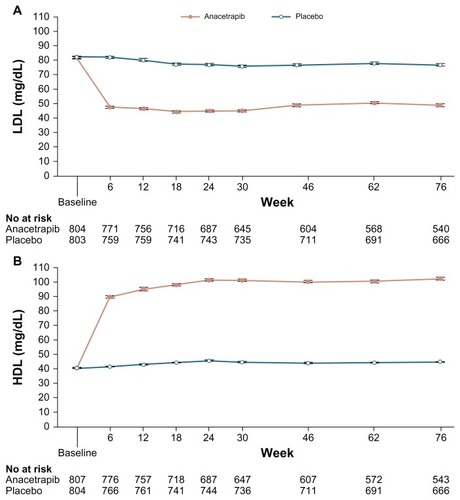
Table 1 Cardiovascular Events during the Treatment Phase of the StudyTable Footnote*
REVEAL trial
Anacetrapib is currently under investigation in the Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification (REVEAL), a large-scale, randomized, placebocontrolled trial of the clinical effects of anacetrapib among people with established vascular disease.Citation57 This double-blind Phase III trial of anacetrapib 100 mg daily will test the hypothesis if anacetrapib will reduce the incidence of major coronary events (ie, coronary death, myocardial infarction, or coronary revascularization procedure) in patients with a history of CVD who are taking statin therapy for lowering LDL-C.
The REVEAL trial is currently enrolling patients 50 years or older who meet at least one of the following inclusion criteria: history of myocardial infarction, cerebrovascular atherosclerotic disease, or peripheral arterial disease; or diabetes mellitus with other evidence of symptomatic CHD (ie, treatment or hospitalization for angina or a history of coronary revascularization or acute coronary syndrome). This international multicenter trial is expected to enroll 30,000 patients and is estimated to be completed by 2017.Citation57
Alternative agents to raise HDL-C
There are three agents in the quiver of currently available pharmacological agents to promote raising HDL-C: nicotinic acid (niacin), fibric acid derivatives (fibrates), and statins. Of these agents, niacin has a more pronounced effect on HDL-C, raising HDL-C by 15% to 35%. In addition to its effects on HDL-C, niacin has also been shown to decrease LDL-C by 5% to 25% and decrease TGs by 20% to 50%.Citation2 Niacin is believed to increase circulating HDL-C by inhibiting the uptake and catabolism of HDL–apo A within the liver without altering HDL–apo A synthesis. By reducing the catabolic rate, niacin prolongs the half-life of HDL-C, allowing for greater accumulation of cholesterol and larger, more antiatherogenic, HDL-C molecules.Citation58,Citation59 Additionally, niacin has also been shown to indirectly increase HDL-C by decreasing the activity of CETP.Citation60
Niacin is available in three oral formulations: immediate release, extended release, and sustained release. Traditional immediate-release niacin is dosed two to three times daily, whereas the newer extended-release formulations can be administered once daily. The most common adverse effects associated with niacin, which may affect tolerability and adherence, include diarrhea, nausea, vomiting, increased cough, pruritus, and most notably flushing of the face and upper body. Niacin-induced flushing is believed to result from rapid elevations in serum nicotinic acid. In an effort to prevent these rapid elevations, extended release formulations of niacin have been created. The incidence and severity of flushing may be reduced by administering aspirin 30 minutes prior to niacin. Additionally, the dosage of niacin should be slowly titrated to minimize this unwanted adverse effect. It should be noted that tolerance to the incidence and severity of niacin develops over several weeks following initiation.Citation61,Citation62
Despite niacin’s ability to substantially increase HDL-C, evidence to support the efficacy of niacin to reduce CV outcomes is inconsistent. A 2010 meta-analysis of niacin alone or in combination with other lipid-lowering agents concluded that the use of niacin significantly reduced major coronary events, stroke, and any CV events.Citation63 Several of the studies included in the analysis took place before statin therapy became the standard of care. In contrast to this meta-analysis, the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes (AIM-HIGH) trial, which evaluated the effects of extended release niacin in addition to intensive statin therapy in the prevention of CV events, was terminated early due to a lack of efficacy.Citation64 Results of AIM-HIGH have called into question the benefits of increasing HDL-C with niacin in addition to intensive statin therapy.
Although fibrates (fenofibrate, fenofibric acid, gemfibrozil) exert a more profound effect on TGs (20% to 50% reduction), these agents have been shown to increase HDL-C levels by 10% to 35% and also lower LDL-C by 5% to 20%.Citation2 The exact mechanism by which fibrates exert their lipid-modifying capabilities has not been fully determined; however, it is understood that these agents activate peroxisome proliferator-activated receptor-α (PPAR-α). Fibrate-mediated activation of PPAR-α results in both upregulation and downregulation of several genes that partake in lipid metabolism and transport within the liver and adipose tissue.Citation65 In addition to the aforementioned atherosclerotic benefits of fibrates, these agents are believed to possess several pleiotropic effects. Fibrates, in particular, have been shown to modulate pro-inflammatory cytokines as well as fibrinogen, plasminogen activator inhibitor-1, and C-reactive protein – each linked to atherosclerosis.Citation66
Within the US, there are two Food and Drug Administration (FDA) approved fibrates: gemfibrozil and fenofibrate. FDA-approved derivates of fenofibrate have also been developed. These include micronized fenofibrate and fenofibric acid. Owing to its preference among patients and physicians, fenofibrate is administered once daily, whereas gemfibrozil is given twice daily with meals. Although fibrates are generally well tolerated, the most commonly reported side effects are gastrointestinal upset, dyspepsia, abdominal pain, cramping, muscle aches, and rash. A major disadvantage of gemfibrozil is the increased risk of rhabdomyolysis when administered with statins. Glucuronidation, a pathway for renal excretion of lipophilic statins, is significantly inhibited by gemfibrozil but not fenofibrate.Citation67 Therefore, fenofibrate is preferred in those who require combined therapy with a statin and fibrate.Citation68–Citation70
Similarly to niacin, the effect of raising HDL-C with fibrates has not consistently resulted in positive CV outcomes. In the Veterans Affairs HDL Intervention Trial study, the use of gemfibrozil to raise HDL-C and lower TG levels in patients with a history of CHD and an LDL-C level of less than 140 mg/dL decreased the rate of nonfatal myocardial infarction and death from CHD by 22%.Citation71 Further analysis of this study revealed that the concentrations of HDL-C achieved in the gemfibrozil treatment group were strongly correlated with significant reductions in coronary events.Citation72 However, there are rather disheartening results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) lipid study. In this landmark trial, the addition of fenofibrate to open-label simvastatin therapy in high-risk diabetic patients failed to reduce the rate of CV events.Citation73 Disappointing results of the AIM-HIGH and ACCORD studies have clouded the previously clear correlation between raising HDL-C and preventing CV endpoints.
Of the agents noted to have an impact on serum HDL-C, statins have been shown to have the smallest effect, raising HDL-C by 5% to 15%.Citation2 Within the statin realm, rosuvastatin and atorvastatin have the greatest potential for raising HDL-C.Citation74 Statins display their effect on cholesterol by competitively inhibiting HMG-CoA reductase, the rate limiting step in cholesterol synthesis. Reductions in hepatocyte cholesterol concentrations result in increased expression of LDL receptors, which promote the removal of circulating LDL-C and LDL precursors. In addition, statins may lower LDL-C independent of LDL receptors by inhibiting the synthesis and secretion of apo B lipoproteins and TG-rich lipoproteins from the liver.Citation75 Although statins do in fact raise HDL-C, the CV benefit seen with this class is believed to be attributed to reductions in LDL-C as well as their aforementioned pleiotropic effects.
There are currently seven available statins approved for use within the US: atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin, each of which are administered once daily. Because the majority of cholesterol synthesis occurs at night, it is recommended that agents with shorter half-lives, such as simvastatin, be administered in the evening or at bedtime. Statins are well tolerated in most patients, and their adverse effects can be seen with each agent throughout the class. The most common adverse effects of statins include myalgia, myopathy, gastrointestinal discomfort, and elevations in liver transaminases. One of the more serious side effects seen with statins is rhabdomyolysis. Therefore, it is of utmost importance that patients newly initiated on statins be monitored for myopathy. Once identified, patients experiencing myopathy should have their statin dose decreased or if not possible, the medication should be discontinued. Additionally, concomitant administration of CYP3A4 inhibitors increase the risk of myopathy and rhabdomyolysis; thus, these patients should be monitored more scrupulously.Citation75
Conclusion
The function of CETP is vital to lipid metabolism, and inhibition of this glycoprotein signifies a potential strategy to manage dyslipidemia. Compared to the current FDA-approved agents, anacetrapib increases HDL-C levels above that observed with niacin or fibrates. The safety and tolerability of anacetrapib was confirmed in multiple studies including DEFINE, and the off-target adverse effects previously reported with torcetrapib were not replicated with anacetrapib. While the place in therapy of niacin and fibrates is currently in question, the ongoing outcomes of the REVEAL study will not only test the hypothesis if CETP inhibition lowers residual CV risk but also provide insight as to which patient subgroups might benefit the most from anacetrapib despite aggressive therapy with statins. Pending the results of the REVEAL trial, anacetrapib may potentially be the first FDA-approved agent in this class to treat patients with dyslipidemia and those with proven atherosclerotic CVD.
Disclosure
The authors report no conflicts of interest in this work.
References
- HeidenreichPATrogdonJGKhavjouOAForecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart AssociationCirculation2011123893394421262990
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)JAMA2001285192486249711368702
- SmithSCJrAllenJBlairSNAHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood InstituteCirculation2006113192363237216702489
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trialLancet2002360932672212114036
- RidkerPMDanielsonEFonsecaFARosuvastatin to prevent vascular events in men and women with elevated C-reactive proteinN Engl J Med2008359212195220718997196
- Scandinavian Simvastatin Survival Study GroupBaseline serum cholesterol and treatment effect in the Scandinavian Simvastatin Survival Study (4S)Lancet19953458960127412757746058
- DavignonJBeneficial cardiovascular pleiotropic effects of statinsCirculation200410923 Suppl 1III394315198965
- BarterPGottoAMLaRosaJCfor Treating to New Targets InvestigatorsHDL cholesterol, very low levels of LDL cholesterol, and cardiovascular eventsN Engl J Med2007357131301131017898099
- GordonTCastelliWPHjortlandMCKannelWBDawberTRHigh density lipoprotein as a protective factor against coronary heart disease. The Framingham StudyAm J Med1977625707714193398
- AssmannGSchulteHvon EckardsteinAHuangYHigh-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transportAtherosclerosis1996124SupplS11208831911
- GoldbourtUMedalieJHHigh density lipoprotein cholesterol and incidence of coronary heart disease – the Israeli Ischemic Heart Disease StudyAm J Epidemiol19791093296308222135
- MillerNEThelleDSFordeOHMjosODThe Tromsø heart-studyHigh-density lipoprotein and coronary heart-disease: a prospective case-control studyLancet19771801996596867464
- GordonDJProbstfieldJLGarrisonRJHigh-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studiesCirculation19897918152642759
- DuffyDRaderDJEmerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transportCirculation20061131140115016505192
- ShahPKEmerging HDL-based therapies for atherothrombotic vascular diseaseCurr Treat Options Cardiovasc Med200791607017378977
- ClarkRWSutfinTARuggeriRBRaising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapibArterioscler Thromb Vasc Biol200424349049714739125
- BrousseauMESchaeferEJWolfeMLEffects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterolN Engl J Med20043501505151515071125
- DavidsonMHMcKenneyJMShearCLRevkinJHEfficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levelsJ Am Coll Cardiol2006481774178117084249
- McKenneyJMDavidsonMHShearCLRevkinJHEfficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels on a background of atorvastatinJ Am Coll Cardiol2006481782179017084250
- BarterPJCaulfieldMErikssonMEffects of torcetrapib in patients at high risk for coronary eventsN Engl J Med20073572109212217984165
- NissenSETardifJCNichollsSJEffect of torcetrapib on the progression of coronary atherosclerosisN Engl J Med20073561304131617387129
- KasteleinJvan LeuvenSIBurgessLEffect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemiaN Engl J Med20073561620163017387131
- BotsMLVisserenFLEvansGWTorcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trialLancet2007370958215316017630038
- KuivenhovenJAde GroothGJKawamuraHEffectiveness of inhibition of cholesteryl ester transfer protein by JTT-705 in combination with pravastatin in type II dyslipidemiaAm J Cardiol2005951085108815842977
- KrishnaRAndersonMSBergmanAJEffect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomized placebo-controlled phase I studiesLancet200737096031907191418068514
- ForrestMJBloomfieldDBriscoeRJTorcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosteroneBr J Pharmacol20081541465147318536749
- VergeerMStroesESThe pharmacology and off-target effects of some cholesterol ester transfer protein inhibitorsAm J Cardiol2009104Suppl32E38E
- RocheHoffmann-LaA randomized, double-blind, placebo-controlled study assessing the effect of ro4607381 on cardiovascular mortality and morbidity in clinically stable patients with a recent acute coronary syndromeClinicalTrials.gov [Internet]Bethesda, MDNational Library of Medicine2000 [cited May 28, 2012]. Available from: http://clinicaltrials.gov/show/NCT00658515. NLM Identifier: NCT00658515
- RanallettaMBieriloKKChenYBiochemical characterization of cholesteryl ester transfer protein inhibitorsJ Lipid Res20105192739275220458119
- GoedekeLFernández-HernandoCRegulation of cholesterol homeostasisCell Mol Life Sci201269691593022009455
- BarterPJKasteleinJJTargeting cholesteryl ester transfer protein for the prevention and management of cardiovascular diseaseJ Am Coll Cardiol200647349249916458126
- ChapmanMJTherapeutic elevation of HDL-cholesterol to prevent atherosclerosis and coronary heart diseasePharmacol Ther2006111389390816574234
- TothPPReverse cholesterol transport: high-density lipoprotein’s magnificent mileCurr Atheroscler Rep2003538639312911849
- OliveiraHCde FariaECCholesteryl ester transfer protein: the controversial relation to atherosclerosis and emerging new biological rolesIUBMB Life201163424825721488146
- ShahPKInhibition of CETP as a novel therapeutic strategy for reducing the risk of atherosclerotic diseaseEur Heart J200728151217121756
- ChaitABrazgRLTribbleDLKraussRMSusceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern BAm J Med19939443503568475928
- KraussRMHeterogeneity of plasma low-density lipoproteins and atherosclerosis riskCurr Opin Lipidol1994553393497858908
- HiranoKYamashitaSKugaYAtherosclerotic disease in marked hyperalphalipoproteinemia. Combined reduction of cholesteryl ester transfer protein and hepatic triglyceride lipaseArterioscler Thromb Vasc Biol199515184918567583564
- ZhongSSharpDSGroveJSIncreased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levelsJ Clin Invest19969712291729238675707
- RidkerPMParéGParkerANZeeRYMiletichJPChasmanDIPolymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: Genomewide analysis among 18 245 initially healthy women from the Women’s Genome Health StudyCirc Cardiovasc Genet200921263320031564
- CurbJDAbbottRDRodriguezBLProspective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderlyJ Lipid Res200445594895314967821
- TanEYHartmannGChenQPharmacokinetics, metabolism, and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in rats and rhesus monkeysDrug Metab Dispos20103845947320016052
- KrishnaRGargAPanebiancoDSingle-dose pharmacokinetics and pharmacodynamics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjectsBr J Clin Pharmacol20096853554519843057
- KrishnaRBergmanAJJinBMultiple-dose pharmacodynamics and pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjectsClin Pharmacol Ther20088467968318580870
- KumarSTanEYHartmannGMetabolism and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in humansDrug Metab Dispos20103847448320016053
- DanskyHMBloomfieldDGibbonsPEfficacy and safety after cessation of treatment with the cholesteryl ester transfer protein inhibitor anacetrapib (MK-0859) in patients with primary hypercholesterolemia or mixed hyperlipidemiaAm Heart J2011162470871621982664
- KrishnaRBergmanAJJinBAssessment of the CYP3A-mediated drug interaction potential of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy volunteersJ Clin Pharmacol2009491808719004846
- KrishnaRGargAJinBAssessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and anacetrapib, a potent cholesteryl ester transfer protein(CETP) inhibitor, in healthy subjectsBr J Clin Pharmacol20096752052619552746
- KrishnaRStypinskiDAliMLack of meaningful effect of anacetrapib on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjectsBr J Clin Pharmacol201274111612422243494
- KrishnaRStypinskiDAliMLack of an effect of anacetrapib on the pharmacokinetics of digoxin in healthy subjectsBiopharm Drug Dispos201132952552922031172
- ClarkRWRuggeriRBCunninghamDBambergerMJDescription of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of actionJ Lipid Res200647353755216326978
- BloomfieldDCarlsonGLSapreAEfficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patientsAm Heart J200915735236019185645
- KraussRMWojnooskiKOrrJChanges in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapibJ Lipid Res201253354054722180633
- KrishnaRBergmanAJGreenMDockendorfMFWagnerJADykstraKModel-based development of anacetrapib, a novel cholesteryl ester transfer protein inhibitorAAPS J201113217919021347617
- CannonCPDanskyHMDavidsonMDesign of the DEFINE trial: determining the efficacy and tolerability of CETP inhibition with anacetrapibAm Heart J2009158451351919781408
- CannonCPShahSDanskyHMDetermining the Efficacy and Tolerability InvestigatorsSafety of anacetrapib in patients with or at high risk for coronary heart diseaseN Engl J Med2010363252406241521082868
- LandrayMBowmanLREVEAL: Randomized EValuation of the Effects of Anacetrapib through Lipid-modification A large-scale, randomized placebo-controlled trial of the clinical effects of anacetrapib among people with established vascular diseaseClinicalTrials.gov [Internet]Bethesda, MDNational Library of Medicine2000 [cited May 28, 2012]. Available from: http://clinicaltrials.gov/show/NCT01252953. NLM Identifier: NCT01252953
- CreiderJCHegeleRAJoyTRNiacin: another look at an underutilized lipid-lowering medicationNat Rev Endocrinol2212012 [Epub ahead of print.]
- ZhangLHKamannaVSGanjiSHXiongXMKashyapMLNiacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein A-I in HepG2 cellsJ Lipid Res201253594195022389325
- van der HoornJWde HaanWBerbéeJFNiacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden. CETP miceArterioscler Thromb Vasc Biol200828112016202218669886
- Abbott LaboratoriesNIASPAN [package insert]North Chicago, ILAbbott Laboratories2010
- GilleABodorETAhmedKOffermannsSNicotinic acid: pharmacological effects and mechanisms of actionAnnu Rev Pharmacol Toxicol2008487910617705685
- BruckertELabreucheJAmarencoPMeta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosisAtherosclerosis2010210235336120079494
- BodenWEProbstfieldJLAndersonTfor AIM-HIGH InvestigatorsNiacin in patients with low HDL cholesterol levels receiving intensive statin therapyN Engl J Med2011365242255226722085343
- ChapmanMJRedfernJSMcGovernMEGiralPNiacin and fibrates in atherogenic dyslipidemia: pharmacotherapy to reduce cardiovascular riskPharmacol Ther2010126331434520153365
- McKeageKKeatingGMFenofibrate: a review of its use in dyslipidaemiaDrugs201171141917194621942979
- BallantyneCMDavidsonMHPossible differences between fibrates in pharmacokinetic interactions with statinsArch Intern Med2003163192394239514581261
- RosensonRSCurrent overview of statin-induced myopathyAm J Med2004116640841615006590
- GoldbergACBaysHEBallantyneCMEfficacy and safety of ABT-335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemiaAm J Cardiol2009103451552219195513
- KhouryNGoldbergACThe use of fibric acid derivatives in cardiovascular preventionCurr Treat Options Cardiovasc Med201113433534221544518
- RubinsHBRobinsSJCollinsDGemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study GroupN Engl J Med1999341641041810438259
- RobinsSJCollinsDWittesJTVA-HIT Study GroupVeterans Affairs High-Density Lipoprotein Intervention Trial. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trialJAMA2001285121585159111268266
- GinsbergHNElamMBLovatoLCfor ACCORD Study GroupEffects of combination lipid therapy in type 2 diabetes mellitusN Engl J Med2010362171563157420228404
- SanteeJLindseyCPaceHRelative efficacy of antilipemic agents in non-high-density lipoprotein cholesterol reductionJ Pharm Pract522012 [Epub ahead of print.]
- MaronDJFazioSLintonMFCurrent perspectives on statinsCirculation2000101220721310637210