Abstract
Venous thromboembolism is a frequent and potentially life-threatening complication of orthopedic surgery. Rivaroxaban is an oral direct factor Xa inhibitor, which was shown to be effective for the prevention of venous thromboembolism after elective hip and knee arthroplasty in the RECORD study program. Rivaroxaban has the potential to overcome the limitations of the current standards of care in the prevention of venous thromboembolism. XAMOS (Xarelto® in the prophylaxis of post-surgical venous thromboembolism after elective major orthopedic surgery of hip or knee) is an international, noninterventional, parallel-group study to gain insight into the safety (major bleeding, side effects) and effectiveness (prevention of symptomatic thromboembolic events) of rivaroxaban in daily clinical practice. XAMOS will follow 15,000 patients after major orthopedic surgery in approximately 200 centers worldwide, with about 7500 patients receiving rivaroxaban and about 7500 standard of care. XAMOS will supplement the clinical data obtained in the Phase III RECORD 1, 2, 3, and 4 trials in which rivaroxaban was shown to be superior for the primary efficacy endpoints, and with a safety profile similar to that of enoxaparin after hip or knee replacement surgery. XAMOS was started in 2009 and will complete recruitment and follow-up in 2011.
Background
Venous thromboembolism is a frequent and potentially fatal complication in patients undergoing major hip or knee surgery. Without prophylaxis, patients have a 40%–60% risk of deep vein thrombosis detected by screening and a 1%–30% risk of pulmonary embolism (0.1%–7.5% fatal), depending on expositional and dispositional risks.Citation1 However, with routine use of thromboprophylaxis, fatal pulmonary embolism is uncommon, and symptomatic venous thromboembolism is reported in 1.3%–10% of patients within 3 months after surgery.Citation1 Most symptomatic venous thromboembolism occurs after discharge from hospital and the risk continues for at least 2 months and is the most common cause for readmission of postsurgical patients to the hospital.Citation2,Citation3
Contemporary prophylaxis
For pharmacologic prophylaxis of venous thromboembolism in patients with total hip replacement, total knee replacement, and hip fracture surgery, three treatments are recommended in the current American College of Chest Physicians guidelines,Citation4 ie, low molecular weight heparins, fondaparinux, and adjusted- dose vitamin K antagonists. Prophylaxis is recommended to continue for at least 10 days but, because of the prolonged risk, it may be extended for up to 35 days.Citation4,Citation5
While the parenteral agents, ie, low molecular weight heparin and fondaparinux, are effective, their use is limited by the requirement for subcutaneous injection. This may be regarded as desirable in the initial phase after surgery, because administration by health professionals ensures complete coverage of the perioperative period without reliance on patient compliance. However, hesitance to self-inject (fearing the needle stick) and the potential negative impact on patient compliance have to be considered when patients are discharged from hospital. Because current hospitalization in many countries averages ≤4 days, this not only becomes important for extended prophylaxis but also for the mandatory initial phase of anticoagulation (1–10 days). A further concern in the use of low molecular weight heparins and likely also with fondaparinux is the potential for heparin-induced thrombocytopenia. With low molecular weight heparin, heparin-induced thrombocytopenia is 10 times less frequent compared with unfractionated heparins, for which an incidence of 3% has been reported in patients following hip replacement surgery.Citation6–Citation8 The potential of fondaparinux to evoke heparin-induced thrombocytopenia is controversial,Citation6,Citation9 and patients with potential heparin-induced thrombocytopenia have been successfully switched from unfractionated heparin or low molecular weight heparin to fondaparinux.Citation10
Vitamin K antagonists are recommended by the guidelines based on a 44% reduction of deep vein thrombosis and 77% reduction of pulmonary embolism versus placebo.Citation11 Vitamin K antagonists are considered to be less effective than low molecular weight heparin (relative risk 1.51 for total and proximal deep vein thrombosis)Citation11 and fondaparinux in preventing asymptomatic and symptomatic inhospital venous thromboembolism, with a slight but nonsignificant increase in surgical bleeding and wound hematoma. However, they are often used during the ambulatory phase of anticoagulation because their oral administration is perceived less of a barrier to use. Accordingly, for total hip replacement, adjusted-dose oral vitamin K antagonists with warfarin is a common form of thromboprophylaxis in North America.Citation12 On the other hand, vitamin K antagonists are limited by a delayed onset of action, which in some cases makes bridging for 2–3 days into full anticoagulation with low molecular weight heparin necessary, and are also limited by the fact that vitamin K antagonists have a high interindividual variability, which is in part influenced by genetic polymorphism.Citation13 In addition, they exhibit a high propensity for drug–drug and drug–food interactions, making close supervision (International Normalized Ratio [INR] monitoring) with frequent dose adjustments necessary. While these prerequisites are generally met in clinical trials, where a considerable portion of patients are reported to be in the desirable INR range,Citation11,Citation14 clinical practice data have shown that the actual average proportion of patients in the desired INR range may be as low as 19%, despite using dosing nomograms.Citation15
Rivaroxaban
Rivaroxaban (Xarelto®) is an oral direct factor Xa inhibitor. The oral bioavailability of rivaroxaban 10 mg is >90%, and peak plasma concentrations are achieved within 2.5 to 4 hours.Citation16,Citation17 The clinical efficacy and safety of rivaroxaban 10 mg once daily 6–8 hours postoperatively for the prevention of venous thromboembolism after elective hip and knee arthroplasty has been established in the four randomized controlled trials of the REgulation of Coagulation in ORthopedic Surgery to Prevent DVT and PE (RECORD) study program ().Citation5,Citation18–Citation20 In these studies, rivaroxaban was either compared with enoxaparin 40 mg once dailyCitation5,Citation18,Citation20 or with 30 mg twice daily. Citation19 Rivaroxaban was more effective for the prevention of venous thromboembolism in all individual RECORD studies with no significant differences in major bleeding. A pooled analysis of data from RECORD 1–3 (n = 9581) showed that rivaroxaban was more effective than enoxaparin in reducing the incidence of the composite of symptomatic venous thromboembolism and all-cause mortality at two weeks (0.4% versus 0.8%, respectively, odds ratio [OR] 0.44; 95% confidence interval [CI] 0.23–0.79; P = 0.005), and at the end of the planned treatment period (0.5% versus 1.3%, respectively; OR 0.38; 95% CI 0.22–0.62; P < 0.001).Citation21 Based on these results, rivaroxaban is currently approved for the prophylaxis of venous thromboembolism in patients undergoing hip or knee arthroplasty in more than 100 countries worldwide, and with a broad label for major orthopedic surgery to the lower limbs in some countries.
Table 1 Results of the RECORD study program on hip and knee arthroplasty
Rivaroxaban has the potential to overcome the limitation of current prophylaxis regimens for venous thromboembolism. It is orally available (resulting in a potential increase in compliance), has no propensity for development of heparin-induced thrombocytopenia, comes as a fixed dose independent of bodyweight, age, or gender, and has a broad therapeutic window. It should be used with caution in patients with severe impairment of renal function (creatinine clearance 15–30 mL/minute) and is not recommended in patients with a creatinine clearance below 15 mL/minute. With rivaroxaban, no routine monitoring of coagulation factors or platelet counts is necessary. A perceived limitation of the new oral anticoagulants is the lack of specific antidotes. In a recent study in healthy volunteers, prothrombin complex concentrate has been shown to reverse the anticoagulant effect of rivaroxaban immediately and completely after application,Citation22 but as promising as the results of this study are, the utility of prothrombin complex concentrate to reverse the action of rivaroxaban in the clinical setting will need to be evaluated in future studies in patients who are at risk of bleeding.
Study design
XAMOS (Xarelto® in the prophylaxis of post-surgical venous thromboembolism after elective major orthopedic surgery of hip or knee) is an international, noninterventional, open-label, controlled cohort study to document the effectiveness and safety of rivaroxaban in daily clinical practice, involving approximately 7500 patients treated with rivaroxaban and approximately 7500 using current standards in the prophylaxis of venous thromboembolism (low molecular weight heparins, fondaparinux, and vitamin K antagonists). The study will be done in accordance with Good Epidemiologic Practice guidance. It was registered with clinicaltrials. gov and received the identifier NCT00831714. The study was approved by the appropriate ethics committees prior to commencement in all countries, where an independent ethics committee or an independent review board was required. XAMOS is part of a risk management plan, agreed upon with the European Medicines Agency (EMA).
Objectives
The main objectives of this study are to collect real-life data on uncommon adverse events, bleeding events, symptomatic thromboembolic events, and all-cause mortality in patients treated with rivaroxaban or standard therapy. Further measures to be evaluated are treatment convenience, patient compliance, health care resource use, use in special patient populations, such as renal impairment, and the use of certain concomitant medications, eg, cytochrome P450 (CYP) 3A4-metabolized drugs and or inducers of CYP 3A4 or P-glycoprotein. In countries where the labeling is for major orthopedic surgery, data on patients receiving rivaroxaban for prophylaxis after hip fracture surgery will also be collected.
Physician and patient selection
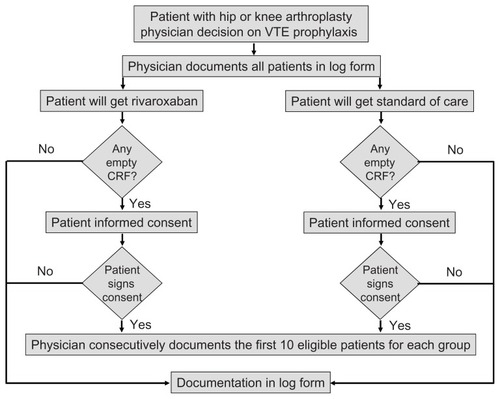
Approximately 250 centers where more than 80 total hip or knee replacement surgeries per year are performed were invited to participate. The conduct of the study is supervised by an independent steering committee. Consecutive patients of either gender being at least 18 years old undergoing hip or knee arthroplasty (or hip fracture surgery where appropriate) and in whom a decision on pharmacologic venous thromboembolism prophylaxis has already been made will be documented. Patients have to provide written informed consent where necessary. To assess the representativeness of patients enrolled against the total population with hip or knee arthroplasty, a patient log will be completed by the surgeon that documents all patients receiving venous thromboembolism prophylaxis at that site and their study status (eg, enrolled, declined participation, or excluded). Basic information will be collected in case of nonparticipation ().
Drug administration and initiation
Because this is a noninterventional study, the decision on the type, duration, and dose of drug used for venous thromboembolism prophylaxis is solely at the discretion of the attending physician, and the specific prophylaxis is determined before patients enter the study.
Data acquisition
Data will be collected at the start of the venous thromboembolism prophylaxis, at hospital discharge, one week after completion of venous thromboembolism prophylaxis, and 3 months after surgery (see ). Serious adverse events will be followed up until a final outcome is available. The participating centers collect the information using an electronic data capture system or a paper case report form collected by fax or mail. At collection, paper-based case report forms will be checked for completeness and missing information requested of the participating physician. After data entry, missing or implausible data will be queried and 5% of sites will be monitored in accordance with local regulatory provisions.
Table 2 Scheduled procedures
Statistical considerations
The main analysis is the comparison of the incidence rates of uncommon adverse events, bleeding events, symptomatic thromboembolic events, and all-cause mortality between the rivaroxaban group and the standard of care group comprising all nonrivaroxaban prophylactic venous thromboembolism drug regimens. A secondary analysis is a comparison between the rivaroxaban group and patients receiving low molecular weight heparins. For all adverse events and for each outcome of interest, crude cumulative incidence rates will be calculated, together with 95% CI. Study subjects taking at least one dose of a venous thromboembolism prophylactic drug will be included in the safety analysis. This study population also includes patients treated with a prophylactic drug for venous thromboembolism but where surgery was cancelled after enrolment of the patient.
Sample size calculation
For the evaluation of the incidence of uncommon adverse events (which implies incidence rates between 0.1% and 1%) the corresponding 95% CI range from 0.164 to 0.465 percent points for 7500 patients (scenario A in ). These values mark the minimum and maximum precisions which can be achieved. They are deemed to be sufficiently small to describe the incidence of uncommon adverse events in the study population. For subgroup analyses, the CI broaden because subgroups comprise fewer patients. However, for the expected subgroup sizes and incidences between 0.1% and 1%, the corresponding 95% CI are also considered as reasonably narrow (scenario B in ). In addition, the planned number of patients allows the detection with 95% power of a two-fold (or greater) increase of adverse event incidence in the rivaroxaban group compared with the standard of care drug therapy group of 0.55% (or higher) if the incidence rate is 0.55% (or higher) in the standard of care group. Uncommon adverse events with an incidence rate between 0.1% and 1% can be described with a 95% CI ranging from 0.164 to 0.465. This precision appears reasonably narrow, both in the overall study group, as well as in subgroup analyses.
Table 3 Width of 95% confidence intervals in relation to incidence rate
Handling of confounders
In addition to reporting crude estimates, measures will be adjusted for baseline characteristics, such as age, gender, weight, smoking, alcohol use, and others. In addition, covariate adjustment using propensity score analysis will be performed.Citation23 The propensity score will be computed for each subject by means of a logistic regression model, with treatment group as the dependent variable and all pretreatment baseline characteristics as independent variables by type of intervention, ie, elective major surgery of hip or knee. Propensity scores are usually estimated using a large number of measured pretreatment covariates in a multivariate logistic regression model to predict exposure. The resulting summary of each study subject’s pretreatment covariates yields the expected individual’s propensity score, ie, the probability of receiving rivaroxaban or reference exposure. However, propensity scores can only be generated on covariates collected. Although the study will collect some relevant pretreatment information, it will not be sufficient to provide risk estimates without confounding in association with the exposure of interest.
Outcomes assessment
All adverse events, including symptomatic thromboembolic and bleeding events will be reported on the adverse event report form and coded using the latest release of the standardized Medical Dictionary for Regulatory Activities. An adverse event is considered as treatment-emergent when it starts on or after the day of the first dose of a venous thromboembolism prophylactic drug and up to two days after the last dose. In case of a switch of the venous thromboembolism prophylactic treatment during the study course, any adverse event occurring within two days after the switch will be counted towards the previous treatment.
Thromboembolic events
Symptomatic thromboembolic events are identified using the Maintenance and Support Services Organization (MSSO) Standardized MedDRA Queries (SMQ) “thrombotic and embolic events.” For additional analyses, the events will be grouped into venous and arterial events. Given the noninterventional nature of this study, no additional diagnostic measures for the detection or confirmation of thromboembolic events are mandated by the protocol.
Bleeding events
If a bleeding event occurs, an additional bleeding questionnaire for each reported event will be completed. Based on the data provided in the case report form and in the bleeding questionnaire the events will be adjudicated and differentiated as major and nonmajor bleeding events using the same adjudication rules used within the RECORD program. Additionally, bleeding events will be adjudicated according to the European Medicines Agency guideline on clinical investigations of medicinal products for prophylaxis of high intraoperative and postoperative venous thromboembolic risk.Citation24
Discussion
XAMOS is a large noninterventional, open-label, observational study to document the real-life use of rivaroxaban in daily clinical practice in patients undergoing hip and knee arthroplasty. The choice of the noninterventional study design was based on several factors related to the specific aims of this study. First, rivaroxaban has been compared exclusively with enoxaparin, a current standard of care, in four large Phase III clinical trials (RECORD).Citation5,Citation18–Citation20 Thus, the clinical efficacy of rivaroxaban and the positive benefit-risk ratio in comparison with enoxaparin is well established. Although having recruited more than 12,000 patients into the RECORD study program, the clinical experience is still limited compared with the established prophylaxis modalities. Randomized controlled trials use strict inclusion and exclusion criteria, which narrow the population that can be studied in those trials, influencing the generalizability of the study results to the real world. Observational studies like XAMOS are specifically designed to be representative of the broad patient population treated in everyday care.
Furthermore, reporting of adverse events is highly reliable in clinical trials but, is less so in clinical practice, making an organized and well structured gathering of information necessary to obtain further information on the clinical relevance of potential drug–drug interactions with regard to adverse events. Lastly, measuring compliance in Phase III clinical trials is usually misleading due to the tight nature of the study protocol and frequent monitoring. Vitamin K antagonists are a good example of how INR adjustments and bleeding complications may differ from clinical trials to daily practice.Citation25 With this respect, the noninterventional study type used for XAMOS is suited to obtain data in practice on the actual use, dose regimen used, duration of use, and effectiveness and number of complications in routine care.
Limitations of study design
Because of the noninterventional, open-label study design and limitations inherent to observational studies,Citation26 this study will not generate unbiased relative risk estimates or absolute incidence rates. It is acknowledged that biases of channeling and confounding by indication are present in observational studies, due to the lack of randomization and the open-label design, despite more advanced study designs and analytical methods, such as propensity score matching or adjustment for multiple covariates associated with drug use and the clinical outcome.Citation27 Propensity scores are estimated using a large number of measured pretreatment covariates in a multivariate logistic regression model to predict exposure. The resulting summary of each study subject’s pretreatment covariates yields the expected individual’s propensity score, ie, the probability of receiving rivaroxaban or reference exposure. Propensity score pairs suggest that pairs of, eg, rivaroxaban and low molecular weight heparin, mimic a randomized trial. Although the study will collect relevant pretreatment information, it will be insufficient to provide risk estimates without confounding in association with the exposure of interest. Furthermore, the number of different outcomes cannot be anticipated. Even though propensity score can balance observed baseline covariates between exposure groups, they do nothing to balance unmeasured characteristics and confounders. Therefore, as with all observational, noninterventional studies, and unlike randomized controlled trials, propensity score analyses have the limitation that remaining unmeasured confounding may still be present.
Another important factor that must be considered is the so called “Weber effect.” This effect was first defined in 1984 by Weber,Citation28 describing an increased adverse event reporting rate within the first 12–24 months after the introduction of a new drug to the market, due to increasing patient exposure and higher interest in the new drug, followed by a later fall in reporting when physicians become familiar with the new compound and the respective adverse event profile. A comparison of a new drug, like rivaroxaban, with older drugs with stable reporting and physicians who are experienced with their use in an open-label study might be misleading.
Conclusion
XAMOS is a large international, noninterventional study on the effectiveness, safety, and tolerability of rivaroxaban in daily clinical practice. This real-life data will be a valuable supplement to the data obtained during the four large Phase III trials in the RECORD program in which rivaroxaban has been shown to be superior to enoxaparin. The study was started in early 2009 and completed recruitment at the end of 2011. First results of this study will be available in 2012.
Acknowledgments
Participants in this study were from different locations in Australia, Austria, Belgium, Bosnia and Herzegovina, Canada, China, Colombia, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Hong Kong, Hungary, Italy, Korea, Lebanon, Lithuania, Republic of Macedonia, Netherlands, Norway, Philippines, Portugal, Serbia, Singapore, Slovakia, Spain, Sweden, Switzerland, United Arab Emirates, United Kingdom, and Venezuela. The information contained in this paper is presented on behalf of the XAMOS investigators.
Disclosure
This study is funded by Bayer Healthcare and Johnson and Johnson Pharmaceutical Research and Development. ACS and WJ are employees of Bayer Healthcare.
References
- GeertsWHPineoGFHeitJAPrevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic TherapyChest20041263 Suppl338S400S15383478
- BjornaraBTGudmundsenTEDahlOEFrequency and timing of clinical venous thromboembolism after major joint surgeryJ Bone Joint Surg Br200688338639116498018
- SeagroattVTanHSGoldacreMBulstrodeCNugentIGillLElective total hip replacement: incidence, emergency readmission rate, and postoperative mortalityBMJ19913036815143114351773147
- GeertsWHBergqvistDPineoGFPrevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)Chest20081336 Suppl381S453S18574271
- KakkarAKBrennerBDahlOEExtended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trialLancet20083729632313918582928
- WarkentinTEMaurerBTAsterRHHeparin-induced thrombocytopenia associated with fondaparinuxN Engl J Med2007356252653265517582083
- WarkentinTEChongBHGreinacherAHeparin-induced thrombocytopenia: towards consensusThromb Haemost1998791179459312
- WarkentinTELevineMNHirshJHeparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparinN Engl J Med199533220133013357715641
- ElalamyITriboutBCan heparin-induced thrombocytopenia be associated with fondaparinux use? A rebuttalJ Thromb Haemost2008671242124319330935
- SpyropoulosACMagnusonSKohSKThe use of fondaparinux for the treatment of venous thromboembolism in a patient with heparin-induced thombocytopenia and thrombosis caused by heparin flushesTher Clin Risk Manag20084365365718827864
- MismettiPLaporteSZuffereyPEpinatMDecoususHCucheratMPrevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: a meta-analysisJ Thromb Haemost2004271058107015219187
- MeskoJWBrandRAIorioRVenous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: a survey of the AAHKS membershipJ Arthroplasty200116667968811547365
- GageBFEbyCJohnsonJAUse of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarinClin Pharmacol Ther200884332633118305455
- FreedmanKBBrookenthalKRFitzgeraldRHJrWilliamsSLonnerJHA meta-analysis of thromboembolic prophylaxis following elective total hip arthroplastyJ Bone Joint Surg Am200082-A792993810901307
- AsnisPDGardnerMJRanawatALeitzesAHPetersonMGBassARThe effectiveness of warfarin dosing from a nomogram compared with house staff dosingJ Arthroplasty200722221321817275636
- KubitzaDBeckaMVoithBZuehlsdorfMWensingGSafety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitorClin Pharmacol Ther200578441242116198660
- KubitzaDBeckaMWensingGVoithBZuehlsdorfMSafety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 – an oral, direct Factor Xa inhibitor – after multiple dosing in healthy male subjectsEur J Clin Pharmacol2005611287388016328318
- ErikssonBIBorrisLCFriedmanRJRivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplastyN Engl J Med2008358262765277518579811
- TurpieAGLassenMRDavidsonBLRivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD 4): a randomised trialLancet200937396761673168019411100
- LassenMRAgenoWBorrisLCRivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplastyN Engl J Med2008358262776278618579812
- ErikssonBIKakkarAKTurpieAGOral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacementJ Bone Joint Surg Br200991563664419407299
- EerenbergESKamphuisenPWSijpkensMKMeijersJCBullerHRLeviMReversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjectsCirculation2011124141573157921900088
- D’AgostinoRBJrPropensity score methods for bias reduction in the comparison of a treatment to a non-randomized control groupStat Med19981719226522819802183
- EMEAGuideline on clinical investigation of medicinal products for prophylaxis of high intra- and postoperative venous thromboembolic risk2007 Available from: http://www.emea.europa.eu/pdfs/human/ewp/70798en_fin.pdfAccessed May 26, 2009
- SamsaGPMatcharDBGoldsteinLBQuality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communitiesArch Intern Med2000160796797310761962
- BrownMLGershBJHolmesDRBaileyKRSundtTMIIIFrom randomized trials to registry studies: translating data into clinical informationNat Clin Pract Cardiovasc Med200851061362018679381
- SchneeweissSDevelopments in post-marketing comparative effectiveness researchClin Pharmacol Ther200782214315617554243
- WeberJCPEpidemiology of adverse reactions to nonsteroidal antiinflammatory drugsNew YorkRaven Press1984