Abstract
Multiple lines of evidence confirm that the cumulative burden of low-density lipoprotein cholesterol (LDL-C) is causally related to the development of atherosclerotic cardiovascular disease (ASCVD). As such, lowering LDL-C is a central tenet in all ASCVD prevention guidelines, which recommend matching the intensity of LDL-C lowering with the absolute risk of the patient. Unfortunately, issues such as difficulty with long-term adherence to statin therapy and inability to achieve desired LDL-C thresholds with statins alone results in residual elevated ASCVD risk. Non-statin therapies generally provide similar risk reduction per mmol/L of LDL-C reduction and are included by major society guidelines as part of the treatment algorithm for managing LDL-C. Per the 2022 American College of Cardiology Expert Consensus Decision Pathway, patients with ASCVD are recommended to achieve both an LDL-C reduction ≥50% and an LDL-C threshold of <55 mg/dL in patients at very high-risk and <70 mg/dL in those not at very high risk. Patients with familial hypercholesterolemia (FH) but without ASCVD should lower LDL-C to <100 mg/dL. For patients who remain above LDL-C thresholds with maximally tolerated statin therapy plus lifestyle changes, non-statin therapy warrants strong consideration. While several non-statin therapies have been granted FDA approval for managing hypercholesterolemia (eg, ezetimibe, Proprotein Convertase Subtilisin/Kexin 9 [PCSK9] monoclonal antibodies, and bempedoic acid), the focus of the current review is on inclisiran, a novel small interfering RNA therapy that inhibits the production of the PCSK9 protein. Inclisiran is currently FDA approved as an adjunct to statin therapy in patients with clinical ASCVD or heterozygous FH who require additional LDL-lowering. The drug is administered by subcutaneous injection twice a year, after an initial baseline and 3 month dose. In this review, we sought to provide an overview of the use of inclisiran, review current trial data, and outline an approach to potential patient selection.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of death in the United States (U.S.)Citation1 and globally.Citation2 ASCVD affects approximately 24 million Americans, or 1 in 10 persons older than 21 years,Citation3 and ASCVD death rates have been increasing in the US since 2010.Citation1 There is compelling evidence from genetic studies, observational data, and interventional studies that low density lipoprotein cholesterol (LDL-C) is causally related to ASCVD.Citation4 As such, lowering LDL-C remains the cornerstone for ASCVD prevention across all major society guidelines.Citation5,Citation6 The cumulative burden of LDL-C, meaning the magnitude of LDL-C elevation times the years of exposure to that level, nicknamed “cholesterol-years”, is directly related to ASCVD risk.Citation7,Citation8 Atherosclerotic plaques progress over time proportional to a person’s cumulative exposure to atherogenic lipids such as LDL-C and other apolipoprotein B (apoB) containing lipoproteins;Citation8 thus, it is important to achieve lower LDL-C earlier in life and maintain lower levels for longer periods of time.
In addition to healthy lifestyle measures, statins remain the first line pharmacotherapy for managing LDL-C for the purposes of ASCVD prevention.Citation5,Citation6,Citation9 Data from the Cholesterol Treatment Trialist’s meta-analysis of statin trials suggest that for every 1 mmol/L reduction in LDL-C, there is an approximate 20–25% reduction in vascular events.Citation10 Furthermore, the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial demonstrated a 44% relative risk reduction (RRR) [absolute risk reduction (ARR) 1.2%; number needed to treat (NNT) 169 over a median 1.9 years] in first major adverse cardiovascular events (MACE) in primary prevention patients with elevated high-sensitivity C-reactive protein (≥2 mg/L) and LDL-C levels of <130 mg/dL treated with rosuvastatin.Citation11 This data confirmed a benefit for further LDL-C lowering in higher risk primary prevention patients even when the LDL-C was not classically high.
Unfortunately, ASCVD events still occur in statin-treated patients, a phenomenon called “residual risk”,Citation12 and many high-risk patients do not achieve recommended LDL-C targets with statin treatment alone.Citation13,Citation14 Non-statin therapies targeting LDL-C, such as ezetimibe and the Proprotein Convertase Subtilisin/Kexin 9 (PCSK9) monoclonal antibody (mAb) inhibitors provide similar ASCVD risk reduction per mmol/L of LDL-C reduction and are currently recommended as second line agents for additional lipid lowering.Citation15–18 In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), ezetimibe added to moderate intensity statin compared to statin monotherapy in high-risk patients conferred a ~2% ARR in MACE with NNT of 50.Citation16 For the mAbs, evolocumab conferred a 15% RRR (1.5% ARR; NNT of 67) in the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk [FOURIER] trial and alirocumab conferred a 15% RRR (1.6% ARR; NNT of 63) in the ODYSSEY Outcomes trial for MACE reduction when added to background statin therapy in patients with ASCVD over a relatively short 2–3 year follow-up.Citation17,Citation18
Furthermore, there does not appear to be a threshold of LDL-C below which benefits cease to exist. Recent studies evaluating achievement of very low LDL-C levels (<30 mg/dL) show a significant reduction in cardiovascular death, unstable angina, myocardial infarction (MI), coronary revascularization, and ischemic stroke compared to patients with residual LDL-C values >30 mg/dL.Citation19,Citation20 The FOURIER trial demonstrated that patients with a baseline LDL-C of ≥70 mg/dL (median LDL-C of 92 mg/dL) who started lipid-lowering therapy with evolocumab (a PCSK9 mAb inhibitor) experienced cardiovascular benefit with further lowering of LDL-C levels, with no safety concerns even with LDL-C below 10 mg/dL.Citation21 Additionally, during the FOURIER open-label extension study, participants who were originally assigned to the placebo group during the trial but were then switched over to evolocumab in the extension study (median treatment time of 5 years) still had higher rates of MACE compared to those originally assigned to evolocumab in the trial and who were maintained on evolocumab during the extension (maximum treatment time of 8.4 years).Citation22 This was despite the original placebo group achieving a median LDL-C level of 30 mg/dL when starting evolocumab in the extension period, reinforcing the incremental benefit for those in the continuous evolocumab group who achieved lower LDL-C values earlier and for a longer period of time.
The PCSK9 mAb inhibitors (evolocumab and alirocumab) reduce LDL-C by ~60% and are administered either once or twice monthly by subcutaneous (SQ) injection.Citation23 Inclisiran is a novel small interfering RNA (siRNA) therapy that inhibits the production of the PCSK9 protein. It is administered by SQ injection twice a year, after the initial baseline and 3 month doses, and can reduce LDL-C by ~50%. Inclisiran is a useful adjunct to maximally tolerated statin therapy in patients with clinical ASCVD or heterozygous familial hypercholesterolemia (HeFH) who require additional LDL-lowering, as an alternative to treatment with PCSK9 mAb therapy. In this review, we provide an overview of the use of inclisiran, review the current trial data, and outline an approach to patient selection and consideration for this therapy.
Current Cholesterol Guideline and Consensus Statement Recommendations
For both primary and secondary ASCVD prevention, guidelines have recommended matching the intensity of lipid-lowering therapy with the absolute risk of the patient. In 2018, the American Heart Association (AHA)/American College of Cardiology (ACC)/Multi-society (MS) Cholesterol Guideline recommended that patients with ASCVD at very high risk achieve a reduction of LDL-C of ≥50% with high-intensity statin therapy, and if patients remained above an LDL-C threshold of ≥70 mg/dL despite maximally tolerated statin, the non-statin agents of ezetimibe and/or PCSK9 mAb inhibitors (evolocumab or alirocumab) could be added.Citation5 The definition of “very high risk” includes patients with 2 major ASCVD events (recent acute coronary syndrome within past 12 months, history of MI, stroke, or clinical peripheral arterial disease), or 1 major ASCVD event with more than 1 high-risk feature (such as age ≥65 years, chronic kidney disease (CKD), prior revascularization, current smoking, diabetes, HeFH, hypertension, heart failure or residual LDL-C ≥100 mg/dL despite maximally tolerated statin).Citation5,Citation24
The 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) Dyslipidemia Guideline recommended that in addition to achieving a reduction of LDL-C ≥50%, patients at high risk and those at very high risk for ASCVD should also achieve an LDL-C target of <70 and <55 mg/dL, respectively.Citation6 In order to achieve such intensive targets, many patients will need to utilize combination therapy (statins with non-statin therapy) with prioritization of starting combination therapy early.Citation25
Since the publication of the 2018 AHA/ACC/MS Cholesterol Guideline, three new additional agents were approved by the US Food and Drug Administration (FDA) for LDL-C lowering; these include evinacumab (for homozygous familial hypercholesterolemia [HoFH] only), bempedoic acid, and inclisiran.Citation24 As such, the newest 2022 ACC Expert Consensus Decision Pathway (ECDP) was developed to provide guidance on the use of newer non-statin therapies for additional LDL-C lowering for various patient risk groups.Citation24
The 2022 ACC ECDP, in closer alignment to the 2019 ESC/EAS guideline, again emphasized dual LDL-C targets for patients at elevated ASCVD risk, with the first being the achievement of an LDL-C reduction of ≥50%, and the second being the achievement of risk-specific LDL-C thresholds on maximally tolerated statin therapy.Citation24 These thresholds include 1) LDL-C of <55 mg/dL (or non-HDL-C goal of <85 mg/dL) for patients with ASCVD at very high risk or ASCVD with FH, 2) LDL-C <70 mg/dL (or non-HDL-C goal of <100 mg/dL) for patients with ASCVD not at very high risk, and 3) LDL-C <100 mg/dL (or non-HDL-C goal of <130 mg/dL) for persons with baseline LDL-C ≥190 mg/dL without clinical ASCVD. If these LDL-C thresholds are not reached with maximally tolerated statin, then ezetimibe and/or the PCSK9 mAbs should be initiated.
Alternatively, bempedoic acid (an oral medication that inhibits cholesterol synthesis in the same pathway as statinsCitation26) or inclisiran may be considered, with inclisiran being an alternate option to the PCSK9 mAbs.Citation24 Patients with HoFH may be considered for evinacumab, lomitapide, and/or LDL apheresis under the care of a lipid specialist. For all patients, once the achieved LDL-C level is below the threshold, there should be continual monitoring for adherence to lifestyle modifications, medications, and LDL-C response to therapy. If the LDL-C remains above the recommended threshold, referral to a lipid specialist and a registered dietician is recommended. In sum, in addition to now striving for even lower LDL-C thresholds that those previously endorsed in older guidelines, the concomitant emergence of newer nonstatin therapies increases the need for greater familiarity when prescribing these agents.
Mechanism of Action
PCSK9 is a circulating protein primarily produced and secreted by hepatocytes that regulates LDL receptor half-life in the liver. It functions primarily by altering LDL receptor recycling via binding the receptor and facilitating its transport into lysosomes for degradation.Citation27 Degradation of the LDL receptor leads to an increase in serum LDL-C. Thus, by inhibiting LDL receptor degradation, there is increased concentration of LDL receptors on the surface of the liver and increased clearance of LDL-C from circulation. Therefore, much attention has been given to PCSK9 as a therapeutic targeting for LDL-C lowering and ASCVD risk reduction.
Inclisiran is a novel siRNA that inhibits the translation of the PCSK9 protein in hepatocytes.Citation28 Small interfering ribonucleic acid molecules are double-stranded RNA molecules consisting of both a guide strand, which carries the genetic sequence complementary to the target mRNA, and a passenger strand which is configured for loading the siRNA into the RNA-induced silencing complex (RISC).Citation29 When siRNA molecules enter a cell, they are loaded into the RISC and undergo separation of the guide and passenger strands. This process is facilitated via interaction of the guide strand with the Argonaute protein. After separation, the passenger strand is discarded and the guide strand/RISC complex travels to the target mRNA where it cleaves the PCSK9 specific mRNA, preventing translation of the target protein.Citation29 The guide strand then remains in the RISC and is able to continue to bind and degrade mRNA for an extended period of time.Citation30 Destruction of the PCSK9-specific mRNA eliminates the production of PCSK9, a protein responsible for the breakdown of LDL receptors present on hepatocytes. This leads to prolonged presence of the LDL receptor on the surface of hepatocytes, an increase in the uptake of LDL-C from circulation, and resultant lowering of serum LDL-C levels ().
Figure 1 Mechanism of Action of Inclisiran. Adapted from Di Fusco SA, Colivicchi F, Maggioni AP et al. Inclisiran: A New Pharmacological Approach for Hypercholesterolemia. Rev Cardiovasc Med. 2022;23(11):375. Creative Commons.Citation31
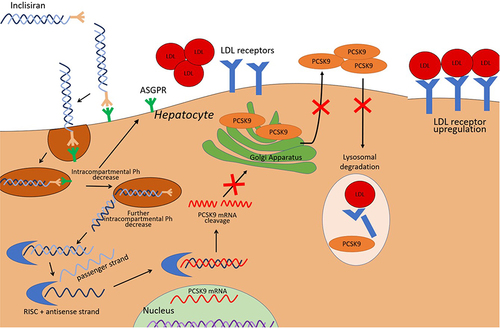
Inclisiran is unique in that it is conjugated to the carbohydrate moiety triantennary N-acetylgalactosamine (GalNAc), which binds specifically to asialoglycoprotein receptors on hepatocytes, leading to targeted uptake by the liver.Citation32 This targeted uptake has numerous benefits. Hepatocyte specificity theoretically leads to a reduction in off-target interactions, thus decreasing the potential for unwanted side effects. The unique mechanism of action and targeted uptake also allows for lower and less frequent dosing than previous PCSK9 mAbs.Citation33
Dosing and Administration
Inclisiran is given subcutaneously as a 284 mg injection on day 1, followed by a second injection on day 90, and then an additional injection every 6 months. This dosing schedule was based on the results of the ORION-1 trial, which found a sustained near 50% reduction in LDL-C levels at 180 and 240 days after receiving two doses (day 1 and day 90).Citation34 This dosing regimen has been used in each of the subsequent Phase 3 trials evaluating the efficacy of inclisiran with similar results.Citation35,Citation36 Although the LDL-C lowering effect persists for the duration of the twice annual dosing schedule, the terminal elimination half-life is 9 hours with concentrations reaching undetectable levels 24–48 hours after administration.Citation37 The rapid and targeted uptake into hepatocytes leads to a short serum half-life; however, the guide strand remains in the RISC, allowing for a half-life in hepatocytes lasting weeks.Citation30 Additionally, unlike the PCSK9 mAbs alirocumab and evolocumab which are self-administered injections, inclisiran is recommended to be administered by a healthcare professional. There are several potential benefits to this for patients.
First, twice yearly administration by a healthcare professional may help to reduce medication non-adherence for patients who have difficulty with daily oral medications and/or a self-injectable medication. Second, in-office administration also influences cost and availability. Medicare Part B fee-for-service patients will have 80% of the cost of administration covered, and those with supplemental insurance may have the additional 20% covered.Citation37 Administration of the injection by a healthcare professional also allows for the reimbursement of the therapy to be directed through the medical benefit pathway, which may facilitate easier access and reduced co-pays for eligible patients. Under this pathway, inclisiran is acquired and administered via a “Buy and Bill” method.
Clinicians order the product through an authorized distributor, administer the therapy in office, and collect co-pays and submit claims for reimbursement.Citation37 For Medicare Advantage and commercially insured patients, clinicians may also send the prescription to specialty pharmacies, who will then contact the patient to collect co-pays as well as work with the healthcare professional on the prior authorization and appeals process prior to dispensing the medication for administration in the clinic. Given the increased complexity of this process, patients with commercial insurance who meet specific eligibility criteria may also receive two free doses of inclisiran if coverage is delayed or denied.Citation37
Lastly, for clinicians who are not comfortable acquiring inclisiran through “Buy and Bill”, those for whom in-office administration is not feasible, or if patient benefits dictate coverage based on the site of administration, there are alternate injection centers (AICs) in which clinicians may refer patients to receive their injection at another clinical site.Citation37 It is for these reasons that healthcare administration should theoretically allow for increased adherence, ease of access, and affordability.
Clinical Efficacy and Safety
The efficacy of inclisiran has been demonstrated in several clinical trials known as the ORION studies. In ORION-1, a Phase 2 trial that sought to evaluate the efficacy of different dosing schedules for inclisiran, patients receiving two 300mg doses (day 1 and day 90) had an average of 52.6% (48.1% to 57.1%) reduction in LDL-C after 180 days.Citation34 At day 240, LDL-C sustained a mean reduction of 47.2%. PCSK9 levels were also found to be reduced by an average of 69.1 ± 12.1% at day 180, with reductions sustained to greater than 40% at day 240. One year follow-up of the ORION-1 participants demonstrated sustained LDL-C reduction of 31.4% at 360 days, signifying a sustained but waning effect over time.Citation38
The inclisiran Phase III trials of ORION-9, 10, and 11 are summarized in .Citation35,Citation36 ORION-9 sought to evaluate the efficacy of inclisiran in adult patients with HeFH on a background of maximally tolerated statin therapy ± ezetimibe with a baseline LDL-C ≥100 mg/dL.Citation35 Patients received 300 mg of inclisiran on day 1, day 90, day 270, and finally day 450. Between day 1 and day 510, there was an average LDL-C reduction of 47.9% (95% CI −53.5 to −42.73; P < 0.001) when compared to placebo. In addition, the average LDL-C reduction at day 510 ranged from 41.2% to 52.1% across all the included prespecified genetic variants (LDL receptor, APOB, and PCSK9 variants). Inclisiran also demonstrated reduced levels of non-HDL cholesterol, apoB, triglycerides, and lipoprotein(a) [Lp(a)] when compared to placebo.
Table 1 Phase 3 Trials Evaluating the Efficacy of Inclisiran
ORION-10 and 11 evaluated the efficacy of inclisiran in patients with ASCVD and LDL-C ≥70 mg/dl.Citation36 In addition, ORION-11 included patients with an ASCVD risk equivalent such as diabetes, HeFH, or 10-year ASCVD risk ≥20% and LDL-C level ≥100 mg/dL. The inclisiran dosing was the same as in ORION-9. Most patients in both trials were on background statin therapy, and approximately 68% and 78.6% were on high-intensity statin therapy in ORION-10 and 11, respectively. The primary endpoint, placebo-corrected percent change in LDL-C from day 1 to day 510, was −52.3% (95% CI −55.7 to −48.8%; P < 0.001) in the inclisiran group. In addition, the time-adjusted reduction in LDL-C between inclisiran and placebo was 53.8% (95% CI −56.2 to −51.3; P < 0.001).
ORION-11 demonstrated a similar LDL-C reduction, with a placebo-corrected reduction of 49.9% (95% CI −53.1 to −46.6; P < 0.001). The inclisiran group also had a time-adjusted reduction in LDL-C of 49.2% (95% CI −51.6 to −46.8%; P < 0.001). Like ORION-9, inclisiran in both trials was shown to lower levels of non-HDL cholesterol, apoB, and Lp(a). Specifically, the placebo-corrected reduction in Lp(a) with inclisiran was 26% in the ORION-10 trial and 19% in ORION-11.Citation36 It should be noted that while the ORION trials included secondary prevention and high-risk primary prevention patients (such as those with FH), the absolute benefit of aggressive lipid-lowering therapy in patients for the purposes of primary prevention among individuals without FH or other high-risk features is attenuated.
The safety of inclisiran was evaluated in each of these phase 3 clinical trials.Citation39 Overall, safety data were collected on 3655 patients between the 3 studies, equating to 5274 patient-years of follow-up. Adverse events resulting in discontinuation of therapy occurred in 2.5% of patients receiving inclisiran and 1.9% of patients receiving placebo. The most common adverse effects included injection site reactions (5% in the inclisiran vs 0.7% in placebo group; risk ratio 7.54) and bronchitis (4.3% for inclisiran vs 2.7% placebo; risk ratio 1.55). The majority of injection site reactions and bronchitis cases were considered mild and self-limited, with no reactions that were considered severe. Other notable adverse effects (which did not differ between treatment and placebo groups) included hypertension (5.7% for inclisiran vs 5.7% for placebo), arthralgia (5.0% for inclisiran vs 4.0% for placebo), back pain (4.5% for inclisiran vs 4.2% for placebo), urinary tract infection (4.4% for inclisiran vs 3.6% for placebo), and increase in serum creatine phosphokinase (2.3% in inclisiran vs 3.2% in placebo). There was no difference in rates of upper respiratory infections, nasopharyngitis, liver function tests, renal function tests, or platelet levels.
Recently, data from the 4-year open label extension of the ORION-3 study were published.Citation40 The 4-year averaged LDL-C reduction was 44%, with continued demonstration of tolerability and safety out to 4 years of extended exposure, with injection site reactions being the most common adverse event in 14% of patients. Longer term safety data beyond this are not currently available.
Cardiovascular Outcomes Data
To date, there have been no completed dedicated cardiovascular outcome trials (CVOTs) for inclisiran. The ORION-4 (NCT03705234) and VICTORIAN-2P (NCT05030428) are ongoing CVOTs with completion dates anticipated in 2026 and 2027, respectively. ORION-4 is a randomized, parallel-group, placebo-controlled trial of approximately 15,000 participants with a history of ASCVD. The primary outcome is time to first occurrence of a MACE, including death from coronary heart disease, MI, fatal or non-fatal ischemic stroke or urgent coronary revascularization. The study is following patients for a median of 5 years. Similarly, VICTORIAN-2P is also a placebo-controlled trial of approximately 15,000 patients with history of ASCVD and on high-intensity statin therapy. The primary outcome is time to first occurrence of 3-point MACE, consisting of cardiovascular death, non-fatal MI or non-fatal ischemic stroke. The study is event-driven with up to 72 months of follow-up. Finally VICTORIAN-1P (NCT05739383) is evaluating inclisiran for MACE reduction among high-risk primary prevention patients such as those with elevated coronary artery calcium scores or non-obstructive coronary plaque seen on a coronary computed tomography angiogram.
While the results of the CVOTs are ongoing, a recently published pooled patient-level analysis of ORION-9, 10, and 11 provides some insight into the possible cardiovascular benefits of treatment with inclisiran.Citation41 This analysis used adverse event data reported in the 3 trials to determine the number of reported episodes of MACE, MI, or stroke. The total number of patients included in the analysis was 3655 and over 18 months, there were 8.3% MACE events overall. The inclisiran-treated group achieved a placebo-corrected LDL-C reduction of 51%. When compared to placebo, the incidence of MACE (131 vs 172 events; hazard ratio (HR) 0.75, 95% CI 0.60–0.94), fatal/non-fatal MI (33 vs 41 events; HR 0.81, 95% CI 0.51–1.29), and fatal/non-fatal stroke (13 vs 15 events; HR 0.80, 95% CI 0.39–1.67) were all lower in the inclisiran group.Citation41 While the HRs for MI and stroke did not achieve statistical significance, they favored treatment with inclisiran in this smaller patient-level analysis. It should be noted that this analysis is likely not sufficiently powered to detect a meaningful difference in these outcomes. While preliminary, these data are encouraging and gives early insight into the ASCVD reduction potential associated with inclisiran, while awaiting the confirmatory CVOTs.
Approach to Patient Selection
Inclisiran is approved by the US FDA for use as an adjunct to diet and maximally tolerated statin therapy for adult patients with HeFH or ASCVD who require further LDL-C lowering.Citation42 While CVOT data are still pending, inclisiran’s ability to lower LDL-C through PCSK9 inhibition suggests that ASCVD outcome data will likely be favorable.
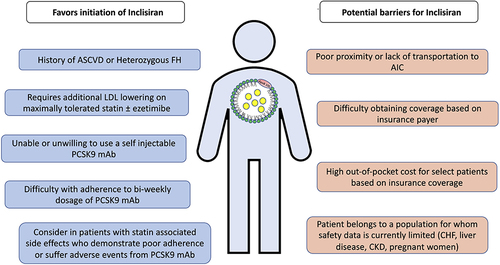
There are several factors to consider when approaching patient selection for inclisiran in clinical practice (). First, as mentioned above the 2022 ACC ECDP on the role of nonstatin therapies provides updated recommendations for more aggressive LDL-C management since the 2018 AHA/ACC/MS Blood Cholesterol Guideline.Citation24 Inclisiran may be considered as an alternative to PCSK9 mAB for patients with clinical ASCVD and/or HeFH who have not achieved their LDL-C targets on maximally tolerated statin ± ezetimibe.
Inclisiran may be particularly appropriate in this scenario for patients who require additional LDL-C reduction who are not willing to or are unable to use a self-injectable PCSK9 mAb, who find difficulty with adherence to a 2-weekly/monthly PCSK9 mAb, or who have Medicare with supplement insurance where the costs may be better covered as compared to the PCSK9 mAbs. Inclisiran may also be considered (though an off-label indication) as second-line therapy in patients with statin-associated side effects who demonstrate poor adherence to or who have adverse effects from statin therapy.Citation24
Special Patient Populations
Trials evaluating the safety and efficacy of inclisiran in patients with homozygous FH (HoFH) are ongoing. ORION-8 (NCT03814187) is an extension trial of ORION-9, 10, and 11 and includes patients with HoFH. Further information on the effectiveness of inclisiran in patients with HoFH will be available upon completion of this trial.
Patients with CKD and those on hemodialysis are at elevated risk for the development of ASCVD. The plasma concentration of inclisiran levels are greater in patients with kidney dysfunction; however, the half-life remains short (5–10 hours) across varying degrees of kidney impairment.Citation43 Further, despite higher plasma concentrations patients with reduced kidney function do not demonstrate any significant change in the amount of LDL-C lowering or greater reduction in PCSK9.Citation43 Finally, there are no notable differences in the rate of adverse events in patients with CKD. These data suggest that inclisiran is safe in patients with CKD; however, outcome data from phase 3 trials in this patient population are not yet available.
Thus far, there has not been studies evaluating safety and efficacy of inclisiran in patients with severe hepatic dysfunction. Therefore, there is insufficient evidence to recommend for or against its use in this population.
Lastly, women who are or may become pregnant or those who are breastfeeding represent a challenging group for lipid management. Currently, there are no data available regarding the use of inclisiran in patients who are pregnant. However, based on the mechanism of action of inclisiran, it may cause fetal harm if administered.Citation44 At this time, there are no data available regarding the association between inclisiran use and birth defects, miscarriage, or adverse outcomes for the mother or fetus.Citation44 It is recommended that inclisiran be discontinued before pregnancy; however, clinicians may choose to consider the individual needs of the patient prior to discontinuation. There are currently no data on the effects of inclisiran in women who are breastfeeding.
Access and Cost Considerations
Clinicians should consider sociodemographic factors such as geographic proximity to AICs or specialty pharmacies, availability based on insurance payer type, and the out-of-pocket cost when selecting nonstatin therapies such as inclisiran. Detailed instructions for finding and referring patients to AICs are available on the company’s website for inclisiran.Citation45
A study of patients referred to AICs from a lipid clinic at the University of California San Diego demonstrated that using AICs was highly effective in providing patients with access to inclisiran.Citation46 This study also retrospectively reviewed patient data to determine patterns in access to therapy.Citation46 In their experience, coverage for inclisiran was variable based on the patient’s payer type. During the study period, 88% of those with Medicare were able to obtain insurance approval and 100% of patients with Medicare were able to access inclisiran. Of the Medicare patients, the majority had traditional Medicare while only 1 had Medicare advantage. Patients with traditional Medicare required no prior authorization, and those with supplemental insurance had no out-of-pocket cost. Those with non-Medicare insurance were approved much less frequently (25%). Given that patients are not eligible for Medicare until age of 65 years, this study suggests younger patients may have more difficulty obtaining coverage for inclisiran. For those unable to obtain insurance coverage, the medication currently costs approximately $3250 US dollars per dose.
Conclusion
Inclisiran, a novel siRNA therapy that inhibits the production of PCSK9, is a useful adjunct to maximally tolerated statin therapy ± ezetimibe in patients with clinical ASCVD or HeFH who require additional LDL-lowering. Off-label, inclisiran may be an effective option in patients with statin associated side effects given its unique mechanism of targeted uptake by hepatocytes. Despite its effectiveness in lowering LDL-C and PCSK9 levels in phase 3 clinical trials, data from a dedicated CVOT are not currently available but forthcoming. However, as evidence accumulates, inclisiran has the potential to represent a new paradigm in the management of hypercholesterolemia. Sociodemographic factors, insurance status and out-of-pocket costs should be considered when deciding on additional nonstatin therapies such as inclisiran. The administration of a twice-yearly LDL-C lowering injection that can be given with nearly the same frequency as an annual influenza vaccine presents an opportunity to redesign models of care and the way we approach ASCVD prevention.
Disclosure
Dr Pam Taub reports personal fees from Novartis, during the conduct of the study; personal fees from Amgen and Esperion, outside the submitted work. Dr Seth S Martin reports personal fees from Novartis, Amgen, Sanofi, AstraZeneca, NewAmsterdam, Novo Nordisk, and 89bio, outside the submitted work. Dr Erin D Michos reports personal fees from Novartis, during the conduct of the study; personal fees from Amgen, Amarin, Bayer, Boehringer Ingelheim, Edwards LifeScience, Esperion, Medtronic, Novartis, Novo Nordisk, and Pfizer, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi:10.1161/CIR.0000000000001123
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–2371. doi:10.1016/j.jacc.2022.11.005
- Gu J, Sanchez R, Chauhan A, Fazio S, Wong N. Lipid treatment status and goal attainment among patients with atherosclerotic cardiovascular disease in the United States: a 2019 update. Am J Prev Cardiol. 2022;10:100336. doi:10.1016/j.ajpc.2022.100336
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi:10.1093/eurheartj/ehx144
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1046–e1081. doi:10.1161/CIR.0000000000000624
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi:10.1093/eurheartj/ehz455
- Shapiro MD, Bhatt DL. ”Cholesterol-Years” for ASCVD risk prediction and treatment. J Am Coll Cardiol. 2020;76:1517–1520. doi:10.1016/j.jacc.2020.08.004
- Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol. 2018;72:1141–1156. doi:10.1016/j.jacc.2018.06.046
- Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381:1557–1567. doi:10.1056/NEJMra1806939
- Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi:10.1016/S0140-6736(10)61350-5
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi:10.1056/NEJMoa0807646
- Patel KV, Pandey A, de Lemos JA. Conceptual framework for addressing residual atherosclerotic cardiovascular disease risk in the era of precision medicine. Circulation. 2018;137:2551–2553. doi:10.1161/CIRCULATIONAHA.118.035289
- Cannon CP, de Lemos JA, Rosenson RS, et al. Use of lipid-lowering therapies over 2 Years in GOULD, a Registry of Patients With Atherosclerotic Cardiovascular Disease in the US. JAMA Cardiol. 2021;6:1060. doi:10.1001/jamacardio.2021.1810
- Ray KK, Haq I, Bilitou A, Catapano AL; Investigators TS. Treatment of high- and very high-risk patients for the prevention of cardiovascular events in Europe: baseline demographics from the multinational observational SANTORINI study. Eur Heart J. 2021:42. doi:10.1093/eurheartj/ehab724.2580
- Khan SU, Khan MU, Virani SS, et al. Efficacy and safety for the achievement of guideline-recommended lower low-density lipoprotein cholesterol levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;28:2001–2009. doi:10.1093/eurjpc/zwaa093
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi:10.1056/NEJMoa1410489
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi:10.1056/NEJMoa1615664
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi:10.1056/NEJMoa1801174
- Giugliano RP, Wiviott SD, Blazing MA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017;2:547–555. doi:10.1001/jamacardio.2017.0083
- Wiviott SD, Cannon CP, Morrow DA, Ray KK, Pfeffer MA, Braunwald E. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46:1411–1416. doi:10.1016/j.jacc.2005.04.064
- Karagiannis AD, Mehta A, Dhindsa DS, et al. How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol. Eur Heart J. 2021;42:2154–2169. doi:10.1093/eurheartj/ehaa1080
- O’Donoghue ML, Giugliano RP, Wiviott SD, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022;146:1109–1119. doi:10.1161/CIRCULATIONAHA.122.061620
- Gaine SP, Quispe R, Patel J, Michos ED. New strategies for lowering low-density lipoprotein cholesterol for cardiovascular disease prevention. Curr Cardiovasc Risk Rep. 2022;16:69–78. doi:10.1007/s12170-022-00694-y
- Writing C, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80:1366–1418. doi:10.1016/j.jacc.2022.07.006
- Michos ED, Ferdinand KC. Lipid-lowering for the prevention of cardiovascular disease in the new era: a practical approach to combination therapy. EAJ. 2022;1:eaj_22005.
- Agarwala A, Quispe R, Goldberg AC, Michos ED. Bempedoic acid for heterozygous familial hypercholesterolemia: from bench to bedside. Drug Des Devel Ther. 2021;15:1955–1963. doi:10.2147/DDDT.S251865
- Warden BA, Duell PB. Inclisiran: a novel agent for lowering apolipoprotein B–containing lipoproteins. J Cardiovasc Pharmacol. 2021;78:e157–e174. doi:10.1097/fjc.0000000000001053
- Soffer D, Stoekenbroek R, Plakogiannis R. Small interfering ribonucleic acid for cholesterol lowering - inclisiran: inclisiran for cholesterol lowering. J Clin Lipidol. 2022;16:574–582. doi:10.1016/j.jacl.2022.06.009
- Alshaer W, Zureigat H, Al Karaki A, et al. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021;905:174178. doi:10.1016/j.ejphar.2021.174178
- Katzmann JL, Packard CJ, Chapman MJ, Katzmann I, Laufs U. Targeting RNA with antisense oligonucleotides and small interfering RNA in dyslipidemias: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:563–579. doi:10.1016/j.jacc.2020.05.070
- Di Fusco SA, Colivicchi F, Maggioni AP, et al. Inclisiran: a new pharmacological approach for hypercholesterolemia. Rev Cardiovasc Med. 2022;23:375. doi:10.31083/j.rcm2311375
- Levin AA. Targeting Therapeutic Oligonucleotides. N Engl J Med. 2017;376:86–88. doi:10.1056/NEJMcibr1613559
- German CA, Shapiro MD. Small interfering RNA therapeutic inclisiran: a new approach to targeting PCSK9. BioDrugs. 2020;34:1–9. doi:10.1007/s40259-019-00399-6
- Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430–1440. doi:10.1056/NEJMoa1615758
- Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382:1520–1530. doi:10.1056/NEJMoa1913805
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi:10.1056/NEJMoa1912387
- Novartis Pharmaceuticals Corporation. Leqvio Access and Reimbursement;. 2022. Available from: https://www.leqvio-access.com/coveragetypes. Accessed June 27, 2023.
- Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. 2019;4:1067–1075. doi:10.1001/jamacardio.2019.3502
- Wright RS, Ray KK, Raal FJ, et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol. 2021;77:1182–1193. doi:10.1016/j.jacc.2020.12.058
- Ray KK, Troquay RPT, Visseren FLJ, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023;11:109–119. doi:10.1016/S2213-8587(22)00353-9
- Ray KK, Raal FJ, Kallend DG, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44:129–138. doi:10.1093/eurheartj/ehac594
- US Food & Drug Administration. Drugs@FDA - Leqvio (inclisiran). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214012Orig1s000TOC.cfm. Accessed June 27, 2023.
- Wright RS, Collins MG, Stoekenbroek RM, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin Proc. 2020;95:77–89. doi:10.1016/j.mayocp.2019.08.021
- Novartis. LEQVIO (inclisiran) [package insert]. Available from: https://www.novartis.com/us-en/sites/novartis_us/files/leqvio.pdf. Accessed November 24, 2022.
- Novartis. Alternate injection center step-by-step guide. Available from: https://www.leqvio-access.com/assets/pdf/LEQVIO-US-Alternate-Injection-Center-Guide_168613.pdf. Accessed November 24, 2022.
- Chiou TT, Tomasi K, Taub PR, Wilkinson MJ. Inclisiran creates unique opportunities and challenges for patient access to therapy: early experience in a United States Lipid Clinic. J Clin Lipidol. 2022;17:73–77. doi:10.1016/j.jacl.2022.10.009