Abstract
Background:
Estimation of total cardiovascular risk is useful for developing preventive strategies for individual patients. The POWER (Physicians’ Observational Work on Patient Education According to their Vascular Risk) survey, a 6-month, open-label, multinational, post-marketing observational evaluation of eprosartan, an angiotensin II receptor blocker, was undertaken to assess the efficacy and safety of eprosartan-based therapy in the treatment of high arterial blood pressure in a large population recruited from 16 countries with varying degrees of baseline cardiovascular risk, and the effect of eprosartan-based therapy on total cardiovascular risk, as represented by the SCORE® (Systematic Coronary Risk Assessment) or Framingham risk equations.
Methods:
Participating physicians recruited > 29,000 hypertensive patients whom they considered to be candidates (according to specified criteria) for treatment with eprosartan 600 mg/day, with other drugs added at the discretion of the physician.
Results:
During treatment, systolic blood pressure decreased by 25.8 ± 14.4 mmHg to 134.6 ± 11.4 mmHg (P < 0.001), mean diastolic blood pressure fell by 12.6 ± 9.5 mmHg to 81.1 ± 7.6 mmHg, and pulse pressure fell by 13.2 ± 13.5 mmHg to 53.6 ± 11.4 mmHg (both P < 0.01). Calculated total cardiovascular risk declined in parallel with the reduction in blood pressure.
Conclusion:
The POWER study has demonstrated, in a large and nonselected population, the feasibility and practicability of reducing total cardiovascular risk through systematic management of high blood pressure.
Introduction
Elevated blood pressure is a powerful contributor to total cardiovascular risk.Citation1 However, an estimation of total cardiovascular risk rather than a focus on individual risk factors is preferred as a guide to the optimal preventive strategies for individual patients. A range of risk estimation models has been devised for this purpose, including SCORE® (Systematic Coronary Risk Assessment).Citation2
The POWER (Physicians’ Observational Work on Patient Education According to their Vascular Risk) study, a large post-marketing survey of eprosartan, an angiotensin II receptor blocker, created opportunities to evaluate both the effectiveness and safety of eprosartan-based therapy in the treatment of high arterial blood pressure in a large population recruited in countries with varying degrees of baseline cardiovascular risk, and the effect of eprosartan-based therapy on total cardiovascular risk, as represented by the SCORE or Framingham risk equations. We report here the primary findings of POWER.
Materials and methods
POWER was an open-label, post-marketing observational study with a 6-month treatment phase. The survey was conducted in 16 countries (Bahrain, Belgium, Bulgaria, Canada, Croatia, Greece, South Korea, Kuwait, Poland, Qatar, Russia, Saudi Arabia, South Africa, Sweden, Thailand, and the United Arab Emirates).Citation3
Participating physicians (general physicians or cardiologists) collected data for at least five sequentially recruited patients with newly diagnosed mild-to-moderate hypertension (mean sitting systolic blood pressure > 140 mmHg), which the physicians proposed to treat with eprosartan, or existing hypertension considered insufficiently well controlled by current therapy, or patients who were unable to tolerate other antihypertensive medications.
Initial medication comprised eprosartan 600 mg/day. This could be supplemented with other antihypertensive drugs (preferably hydrochlorothiazide 12.5 mg/day) if the blood pressure response after one month of eprosartan monotherapy was considered insufficient.
The primary efficacy objective of POWER was the absolute change in systolic blood pressure during the period of observation, calculated, if possible, as the mean of two readings obtained at each visit. Blood pressure was measured at baseline, at the intermediate visit scheduled for 1–3 months after the start of eprosartan-based therapy, and at the end of the 6-month period of observation. Blood pressure measurements were made using standard local methods regarded as reliable, accurate, and relevant.
The impact of eprosartan-based therapy on total cardiovascular risk was a specified secondary endpoint.Citation3 The SCORE methodology was used for this purpose in 15 countries. Several methods were used to derive SCORE-based estimates of the absolute change in 10-year risk of fatal cardiovascular disease, as follows:
The “recorded” SCORE risk was obtained directly by physicians using data recorded by them on the case-record forms and applied to the SCORE cardiovascular risk chart appropriate to the cardiovascular risk profile of the country in question (ie, low-risk, high-risk, or country-specific)
The “calculated” SCORE risk was generated centrally using the appropriate SCORE risk chart and individual patient data collected by physicians and recorded on the case-record forms
SCORE values were calculated using country-appropriate formulae and individual patient data collected by physicians, and recorded on the case-record forms.
For the Canadian contingent only, Framingham instruments were used to quantify cardiovascular risk because these were regarded as more appropriate to a North American population. The specific outcome considered was the absolute change in 10-year risk of “hard” coronary heart disease.
The POWER protocol was fully compatible with current rules and guidance for good clinical practice and the ethical conduct of research in humans, including the precepts of informed consent, and was subject to institutional review board and/or ethics committee review and approval as required by local regulations and practice. POWER also conformed to the provisions of the SCOPE initiative for the reporting of observational studies.
Statistical analysis
Given the size of the population, quantitative variables were compared with a one-sample t-test. Blood pressure and laboratory parameters were compared between visits using covariance analysis, with the baseline value as the adjusted variable. Other quantitative variables were compared by variance analysis. All tests were two-sided, with significance specified at the level of 5% probability (ie, 0.05). Qualitative variables were compared using the Chi-squared test or by direct calculation of the degree of significance using Fisher’s Exact test if nominal or by the Wilcoxon test or Kruskal-Wallis test if ordinal.
Results
Patients and demographics
Between May 2005 and October 2009, a total of 29,754 patients were enrolled at 4158 centers. From this population, an intention-to-treat cohort of 26,192 patients was identified, comprising patients aged ≥ 18 years who received at least one dose of study treatment and for whom systolic blood pressure and cardiovascular disease estimates were available at baseline and at least one subsequent visit (). The median duration of treatment was 182 days. The mean age of the intention-to-treat population was 61.3 ± 12.2 years (range 18–99); 13,592 (52.3%) were men, 6592 (25.3%) were smokers at baseline, and mean body mass index was 28.2 ± 4.8 kg/m2. Demographic data are shown in .
Figure 1 CONSORT diagram for the POWER patient population.
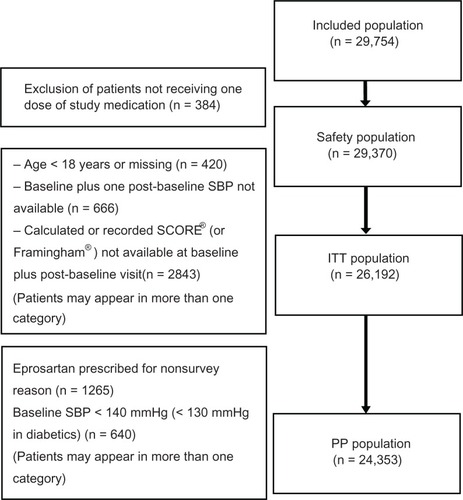
Table 1 Demographic characteristics of the POWER intention-to-treat population (n = 25,078)
The mean ages of men (59.5 ± 12.1 years) and women (63.3 ± 12.0 years) in the intention-to-treat population differed significantly (P < 0.01). Age distribution also varied significantly by gender (P < 0.01), with men representing proportionately more of the population aged 50–59 years (62.7% versus 37.3% women) whereas women represented proportionately more of the population aged > 70 years (58.7% versus men 41.3%). Of 10,437 patients for whom race information was recorded, 6523 (62.5%) were classified as white and 3412 (32.7%) as Asian. Numerically prominent cardiovascular-relevant pathologies included diabetes, left ventricular hypertrophy, and arteriosclerosis (5801 [22.6%], 4987 [19.4%], and 4961 [19.3%], respectively).
Blood pressure data
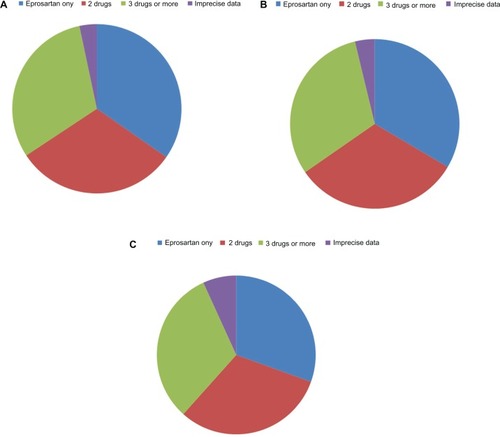
Approximately one-third of patients were assigned each to monotherapy (eprosartan only), to dual therapy, or to multidrug therapy during the survey. Detailed distributions of drug therapy during the survey are presented in . Combination therapy was more often encountered in men, in older or diabetic patients, and in those with a significant cardiovascular history. The proportion of patients with isolated systolic hypertension increased with age, ie, 26.5% (n = 1932) at age 60–69 years and 34.0% (n = 2385) at age > 70 years, compared with 16.0% (n = 691) and 19.6% (n = 1457), respectively, at age < 50 years and 50–59 years.
Figure 2 Patterns of antihypertensive prescribing at (A) baseline, and at (B) 3 and (C) 6months in the POWER survey. Drugs most frequently recorded as supplements to eprosartan at each time point are listed in the notes.
Baseline mean arterial blood pressure in the intention-to-treat population was 160.4 ± 14.3/93.6 ± 9.7 mmHg and mean pulse pressure was 66.9 ± 14.3 mmHg. Systo-diastolic hypertension was documented in 18,741 patients (72.5%) and isolated systolic hypertension in 6429 (24.9%). Systolic blood pressure increased with age (mean systolic blood pressure 161.1 ± 14.7 mmHg at age > 70 years versus 158.9 ± 14.0 mmHg at age ≤ 50 years) whereas diastolic blood pressure decreased with age (91 ± 10.1 mmHg at age > 70 years versus 96.1 ± 9.1 mmHg at age ≤ 50 years). As a consequence, pulse pressure increased with age.
Mean systolic blood pressure in the intention-to-treat population decreased by 25.8 ± 14.4 mmHg during the survey (P < 0.001 versus baseline). Mean diastolic blood pressure decreased by 12.6 ± 9.5 mmHg and mean pulse pressure decreased by 13.2 ± 13.5 mmHg (both P < 0.01 versus baseline). Antihypertensive efficacy was evident in all subsets of the population, including patients with diabetes or other forms of high baseline cardiovascular risk.
Approximately 62% of patients reached the pre-defined target of systolic blood pressure < 140 mmHg plus diastolic blood pressure < 90 mmHg at the end of the observation period; > 90% of patients met the definition of a responder to eprosartan-based therapy (systolic blood pressure < 140 mmHg and/or a change in systolic blood pressure ≥ 15 mmHg or diastolic blood pressure < 90 mmHg and/or change in diastolic blood pressure ≥ 10 mmHg).
Total cardiovascular risk
Some 40% of patients (n = 10,597) had a family history of cardiovascular disease. In all, 4518 patients had documented coronary artery disease and 1416 had a history of congestive heart failure. There were 1305 patients with a history of ischemic stroke and 1029 patients had renal failure. These patients, and others, were excluded from the SCORE calculations because of their secondary prevention status.
Cardiovascular risk was calculated using the SCORE method in all countries except Canada; the mean cardiovascular risk estimate in patients of the 15 countries that used the SCORE method declined by about 40% during the period of observation. In Canada only, cardiovascular risk was calculated using Framingham instruments, and the mean cardiovascular risk score in the Canadian contingent decreased by approximately 27% during the period of observation.
Tolerability and adverse event data
A total of 730 adverse events were recorded in 530 subjects (1.8% of the safety population [n = 29,370]). Of these, 493 incidents were recorded as suspected adverse drug reactions, including 45 reactions that were classified as “severe” and 14 that were classified as “serious” (). Five of the seven deaths recorded were judged unrelated or unlikely to be related to medication use. The other two deaths were classified as suspect because the investigators did not file an assessment of likely causal relation to medication use.
Table 2 Summary of suspected adverse drug reactions in the POWER safety population (n = 29,370)
Discussion
This investigation of 6 months’ duration has demonstrated in a large and nonselected population the feasibility and practicability of eprosartan-based therapy (either as eprosartan monotherapy or in combination regimens) to control arterial blood pressure, especially its systolic component, and, as a result, to reduce total cardiovascular risk. This general effect is in line with expectations of the impact of sustained reduction in systolic blood pressure on overall cardiovascular risk, and is consistent with the findings of an earlier meta-analysis of the effect of angiotensin II receptor blocker treatment on risk of major cardiovascular events.Citation4
The mean systolic blood pressure reduction recorded in POWER was about 26 mmHg. Allowance needs to be made for the lack of placebo correction applied in the POWER survey, but this scale of effect, although large, is not implausible, especially when it is considered that a majority of patients were prescribed eprosartan-based therapy, not eprosartan monotherapy.Citation5,Citation6 Use of combination therapy in order to achieve satisfactory blood pressure control, with a thiazide diuretic being the dominant choice, has been a feature of eprosartan clinical trials, and has been associated with double-digit reductions in mean systolic blood pressure.Citation5,Citation7 Of note, a reduction in mean systolic blood pressure was observed in POWER irrespective of patient gender, age, diabetic status, or cardiovascular history.
Cardiovascular risk status, as represented by mean SCORE values, improved substantially during the period of treatment and observation in POWER. In the absence of structured interventions against other major cardiovascular risk factors, it must be inferred that this effect was substantially, if not entirely, the result of the reduction in systolic blood pressure. Whether or not the reduction in mean SCORE value represents the maximum such effect that can be expected from the achieved degree of systolic blood pressure control remains to be established (see Guallar et alCitation8 for a recent contribution in this area). It remains also to be established if the observed reduction in mean SCORE value represents a broad-based effect in the generality of the POWER population or if it is the result of an effect concentrated in a subset of patients with distinct demographic characteristics. Insights into these matters may be instructive to the development of primary care interventions for reduction of cardiovascular risk.
Eprosartan 600 mg once daily was well tolerated in POWER when used alone or in combination. This experience is consistent with other reports of eprosartan.Citation9,Citation10 The overall profile of antihypertensive efficacy with favorable tolerability seen with once-daily eprosartan in POWER is consistent with, and supplements in circumstances of routine practice, data from controlled trials of angiotensin II receptor blockers, as recently reviewed.Citation11,Citation12 The large number of patients enrolled in the safety population confers power to detect rare adverse drug reactions, and it is noteworthy that no new adverse drug reactions were detected. However, continued and longer-term follow-up is needed to provide fuller assurances on that point.
The very large number of patients involved in this project creates opportunities to examine hypothesis-generating effects in subgroups. Investigations of the effects of eprosartan-based therapy on total cardiovascular risk estimated by SCORE in 15 countries and by Framingham instruments in patients recruited in Canada are in progress, as are explorations of eprosartan-based therapy effects in the large subset of patients with diabetes. Data accrued in POWER may be useful for evaluating the role of risk assessment instruments as aids to resource allocation in cardiovascular prevention.Citation13
Disclosure
The POWER study is supported financially by Abbott Products Operations AG, Switzerland. AG reports funding by Abbott, receipt of an honorarium from Abbott, and reimbursement for attending Abbott symposia. JP-B is an employee of Abbott, sponsor of the POWER observational study. AP reports funding by Abbott, receipt of an honorarium from Abbott, and reimbursement for attending Abbott symposia. Preparation of this report was assisted by Hughes associates, Oxford, UK.
References
- CzernichowSZanchettiATurnbullFThe effects of blood pressure reduction and of different blood pressure-lowering regimens on major cardiovascular events according to baseline blood pressure: meta-analysis of randomized trialsJ Hypertens20112941620881867
- ConroyRMPyöräläKFitzgeraldAPEstimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE projectEur Heart J200324987100312788299
- De BackerGPetrellaJRGoudevARDesign and methodology of POWER, an open-label observation of the effect of primary care interventions on total cardiovascular risk in patients with hypertensionFundam Clin Pharmacol1142011 [Epub ahead of print.].
- Blood Pressure Lowering Treatment Trialists’ CollaborationEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet20033621527153514615107
- AmbrosioniEBombelliMCerasolaGAmbulatory monitoring of systolic hypertension in the elderly: eprosartan/hydrochlorothiazide compared with losartan/hydrochlorothiazide (INSIST trial)Adv Ther20102736538020556561
- ConterHSMcKayDWReizRJEprosartan mesylate effectively reduces systolic and diastolic blood pressure in a Canadian primary care settingCan J Cardiol200420Suppl C6C10C
- SchraderJLüdersSKulschewskiAMorbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES)Stroke2005361218122615879332
- GuallarEBanegasJRBlasco-ColmenaresEExcess risk attributable to traditional cardiovascular risk factors in clinical practice settings across Europe – The EURIKA StudyBMC Public Health20111170421923932
- XuFYYangBShiDLiHZouZShiXYAntihypertensive effects and safety of eprosartan: a meta-analysis of randomized controlled trialsEur J Clin Pharmacol20126819520521881888
- BöhmMSachseASafety and tolerability of eprosartan in combination with hydrochlorothiazideDrug Saf20022559961112113644
- BaumhäkelMBöhmMCardiovascular outcomes with angiotensin II receptor blockers: clinical implications of recent trialsVasc Health Risk Manag2011739139721796253
- FlackJMNasserSABenefits of once-daily therapies in the treatment of hypertensionVasc Health Risk Manag2011777778722241952
- De BackerGThe SCORE model in the POWER study: an attempt to focus the limited resources for prevention on patients with greatest needCurr Med Res Opin200723Suppl 5S19S2418093410