Abstract
Aims
We aimed to document the drug management of patients at high cardiovascular risk in daily practice, with the special focus on lipid-lowering treatment.
Methods and results
In this prospective noninterventional study in 2387 outpatient centers throughout Germany, a total of 13,942 high-risk patients (mean age 65.7 years, 61.6% males) were treated with simvastatin 40 mg/day at entry as monotherapy. All patients were followed up for 12 months in terms of drug utilization, laboratory values, target attainment, and clinical events (including death, hospitalization, vascular events, and dialysis). Patients had coronary heart disease in 35.0%, diabetes mellitus in 24.4%, and the combination of coronary heart disease plus diabetes mellitus in 25.7%. In 21% of patients, a cholesterol absorption inhibitor was added to statin therapy at the entry visit, and in 23%, this was added at the follow up visit 6 months later. The target values for low-density lipoprotein-cholesterol (<2.6 mmol/L) were reached by 31.8% of patients at entry and by 50.0% at the end of this registry after 12 months. Mean blood pressure decreased (from 135.9/80.5 mmHg at baseline) by 3.1/1.9 mmHg after 12 months. In patients with documented diabetes, the targeted glycated hemoglobin (HbA1c <6.5%) was reached by 33.5% at baseline and by 40.0% after 12 months. Clinical events occurred in 11.7% of patients between baseline and month 6, and in 12.0% between months 6 and 12.
Conclusion
In patients at high risk for cardiovascular events, comprehensive management under daily practice conditions leads to improvement of lipid, glucose, and blood pressure parameters. There is a need to improve secondary prevention among high-risk patients.
Introduction
In current mortality statistics, cardiovascular (CV) diseases are still among the leading causes of death.Citation1,Citation2 Primary and secondary prevention of myocardial infarction and stroke have a central role in the management of patients at increased CV risk.Citation3–Citation5 Current guidelines highlight the importance of lowering elevated lipid levels, in particular low-density lipoprotein cholesterol (LDL-C). Patients at high CV risk, eg, those with manifest coronary heart disease (CHD) or diabetes mellitus (DM), should meet the LDL-C target value of < 2.6 mmol/L (<100 mg/dL), and those at very high CV risk should aim at even lower levels.Citation5
According to current prescription statistics, the great majority of patients with high lipid levels receive statins and a smaller proportion receive other drugs, such as cholesterol absorption inhibitors (CAI), fibrates, nicotinic acid, or others.Citation6 However, current data on the day-to-day management of patients at high CV risk indicate that their use is not consistent, and part of this inconsistency may be due to clinical inertia, physician misconception about drug efficacy, or other factors.Citation7,Citation8
The current study (Leitliniengerechte Lipidtherapie und Zielwerterreichung bei Risikopatienten im klinischen Alltag, LIMA) aimed to examine the utilization of the most commonly used statin in Germany, simvastatin, in the daily treatment of high–CV risk patients, in terms of patient characteristics, risk factor management, lipid target attainment, and outcomes, during a 12-month follow-up period. We focused on lipid-lowering drug treatment, and analyzed patients in the total cohort and in three subgroups, namely with CHD alone, DM alone, or the combination of both conditions.
Methods
Design and conduct
This was a prospective, noninterventional study in 2387 centers all over Germany, performed between September 2007 and March 2009. The design and basic results of the study have been reported in the German language recently.Citation9,Citation10
Office-based family physicians, general practitioners, and cardiologists/diabetologists in family care treated patients within the framework of either statutory health insurance program or private insurance. The observational plan and the case report form of the study were approved by the Ethics Committee of the Bavarian Federal Medical Council. The study was conducted in accordance with the applicable laws and regulations in Germany. It followed Good Epidemiological Practice (GEP) guidelines.Citation11 All patients gave written, informed consent. The study was registered in the database of the German Association of Research-based Pharmaceutical Companies (http://www.VfA.de). The study protocol did not stipulate any specific treatment or interventions, and physicians were requested not to change their typical practice in treating patients.
Patients
Adult patients were eligible for documentation, if they were at high risk for CV events and had received 40 mg simvastatin as monotherapy once daily without interruption for at least four weeks at the time of recruitment. “High risk” for CV events was defined as an established coronary heart disease (CHD), and/or diabetes mellitus (DM), and/or peripheral arterial disease (PAD). Application of other lipid-lowering therapy was an exclusion criterion. To avoid selection bias, consecutive enrollment of six eligible patients per center was scheduled. Three visits were planned: at baseline, after approximately 6 months, and 12 months.
Documented parameters comprised patient demographics (age, gender, height, and body weight), insurance status, disease-management program (DMP) participation, CV risk factors, concomitant diseases, and concomitant drugs before and at inclusion in the study (with particular focus on lipid-modifying medications). All diagnoses were provided by the treating physicians. CHD included myocardial infarction, angina pectoris, coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI).
The latest available standard laboratory parameters (total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides [TG]) were recorded on standardized case report forms (CRFs) for the objective assessment of lipid goal attainment. Further, physicians were requested to state whether in their opinion, the current treatment led to individual target level attainment (for a subjective assessment of lipid goal attainment). Physicians received feedback from the study management about the target level attainment of their patients after the entry visit and the final visit (). Blood pressure, glycated hemoglobin (HbA1c) (in diabetic patients), and fasting blood glucose values were documented, if available.
Figure 1 Study protocol. The figure displays the study flow and interaction between centers and the data center.
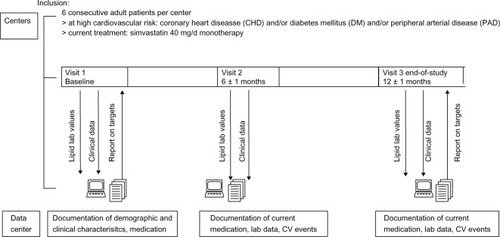
At the two follow-up visits, current medication (with focus on lipid modifying therapy) was recorded, as were current lipid, blood pressure, and glucose values. In addition, the following clinical events were explicitly asked for: hospitalization, death, myocardial infarction, stroke, transient ischemic attacks, coronary angiography, PCI, CABG, newly diagnosed PAD, first-time dialysis, and diabetes-related amputations. The exact date of events was not recorded. Physicians had the choice between internet-based data entry (web-based CRFs) or paper-based data collection. Onsite quality and plausibility checks were performed by onsite monitoring, with source data verification in 50 randomly selected centers.
Statistical analysis
Based on a prespecified statistical plan, analyses on efficacy parameters were performed descriptively, and comparisons were not tested for significance. Continuous numeric variables were expressed as number of evaluable values, mean, standard deviation, median, minimum, and maximum. For categorical variables, frequency counts were applied (absolute and relative frequencies).
Of the 13,942 patients eligible for analysis, 11,998 (87.4%) completed the baseline visit and the visit at 6 months and 10,532 (76.8%) completed all three visits. Patients were categorized according to the presence of three subgroups (CHD alone, DM alone, CHD + DM). Due to likely underreporting of subclinical PAD,Citation12 no subgroup analysis was performed on these patients, but they were included in the total cohort.
Implausible laboratory values were removed from the database (and set as “missing”). Data processing and analysis was performed with SAS Software, release 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
Baseline
Patient characteristics
In the total cohort of 13,942 patients, the mean age was 65.7 years (range: 19–98). Males were more frequent than females (61.6% vs 38.4%). The great majority of patients were covered by statutory health insurance (95.2%); by this, the proportion of patients with private health insurance (which in Germany, is at about 11%) was slightly underrepresented.
Patients with CHD + DM were older and had higher rates of comorbidities and risk factors compared with patients in the CHD and DM groups (). In the presence of CHD, the ratio of males to females was about 2:1.
Table 1 Characteristics of patients at baseline (total cohort and subgroups)
Concomitant diseases and risk factors
CV diseases that qualified patients as “high risk” are shown in . CHD was the leading disease (70.6% of patients), followed by DM (58.2%), and by PAD (14.9%). A substantial proportion of patients had combinations of these CV diseases. Other cardiovascular comorbidities and risk factors were also frequent, with arterial hypertension being the leading condition (87.0%). Overall, the majority of patients (86.1%) were affected by two or more risk factors or concomitant conditions, whereas only 12.5% had one risk factor or concomitant condition and 1.4% had none.
Figure 2 Concomitant diseases and risk factors for cardiovascular disease (total and subgroups).
Abbreviations: CHD, coronary heart disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack.
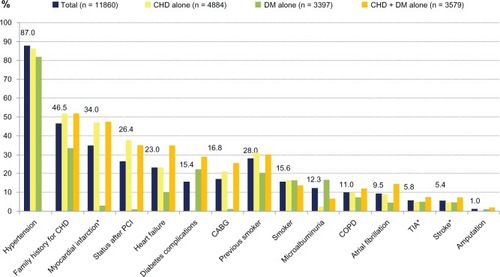
The number of risk factors was higher in the CHD and CHD + DM groups than in the DM group.
Medications
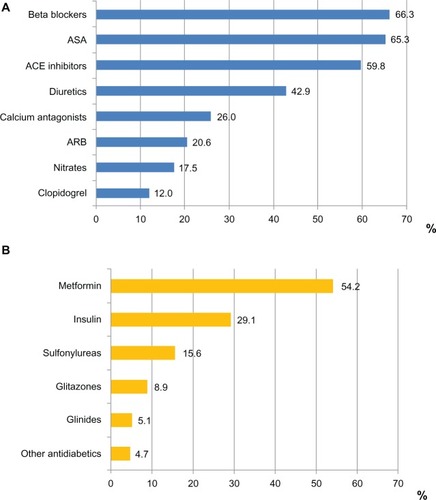
Medications for cardiovascular or metabolic diseases were frequent, as expected in this population (). They most commonly comprised beta blockers (66.3%), acetyl salicylic acid (65.3%), and angiotensin-converting-enzyme (ACE) inhibitors (59.8%). In the subgroup of diabetic patients, metformin was administered in 54.2% and insulin in 29.1%, while sulfonylureas, glitazones, and glinides were used less frequently.
Figure 3 Medications at entry. (A) Concomitant antihypertensive, cardiac and antiplatelet medications at entry; % refer to the total group; (B) Antidiabetic medication at entry; % refer to the patients with diabetes mellitus.
Abbreviations: ASA, acetyl salicylic acid; ARB, angiotensin receptor blockers.
Lipid-modifying medication
In accordance with the respective inclusion criterion (stable after at least 4 weeks on simvastatin 40 mg), patients received this agent at entry (with the exception of 210 patients [1.5%]).
Follow up
Lipid-modifying medication
After the baseline visit, the majority of patients (67.6%) were maintained on simvastatin monotherapy for over 12 months. After baseline, the 80 mg/day dose was used infrequently (3.2%), and the majority of patients (79.4%) remained on the 40 mg/day dose. Further, simvastatin 20 mg/day was used by 8.4%, 10 mg/day by 0.9%, or an unknown dose by 6.5%. In 20.6%, a CAI was added at the baseline visit; and in 23.3%, this was added at the first follow-up visit. A switch to other statins was rare (1.4%). displays the utilization patterns of lipid-modifying drugs during the course of the study.
Table 2 Patterns of lipid-lowering drug utilization at the three visits
Lipid profile and target level attainment
All lipid values improved during the follow-up period (). In the total cohort, TC decreased (from 5.42 mmol/L) by 0.59 mmol/L after 12 months, LDL-C decreased (from 3.19 mmol/L) by 0.49 mmol/L, HDL-C increased (from 1.32 mmol/L) by 0.03 mmol/L, and TG decreased (from 2.15 mmol/L) by 0.23 mmol/L.
Figure 4 Lipid values (mmol/L) at baseline and follow-up visits, in the total cohort and subgroups of patients with CHD, DM, and CHD + DM. (A) Total cohort; (B) Subgroup CHD (n = 4884); (C) Subgroup DM (n = 3397); (D) Subgroup CHD + DM (n = 3579).
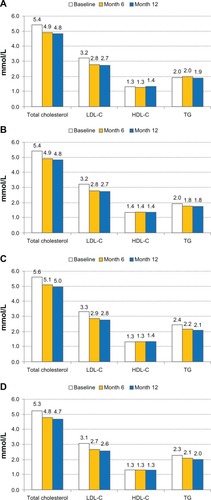
–D display lipid parameters at the three visits in the CHD, DM, and CHD + DM subgroups. Lipid-lowering effects were similar across the three groups, while at all visits, TG was higher in the DM and CHD + DM groups compared with the CHD group.
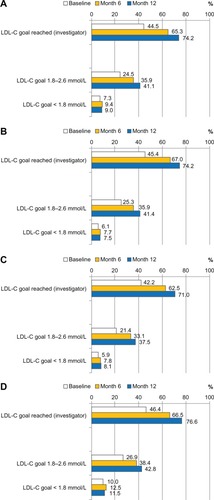
In terms of target level attainment, physicians stated subjectively that LDL-C levels of their patients were achieved in 44.5% of cases at baseline, and in 74.2% after 12 months (). Based on laboratory values, the goal attainment rates (,2.59 mmol/L) were lower, namely 31.8% at baseline and 50.1% after 12 months. Subjective and objective LDL-target achievement rates at month 12 were highest in the CHD + DM group, followed by the CHD and DM groups ().
Figure 5 LDL-C goal achievement (%) as assessed by investigator (subjective) and according to laboratory findings (objective). (A) Total cohort; (B) Subgroup CHD (n = 4884); (C) Subgroup DM (n = 3397); (D) Subgroup CHD + DM (n = 3579).
Abbreviations: CHD, coronary heart disease; DM, diabetes mellitus; LDL-C, low-density lipoprotein cholesterol.
Blood pressure and blood glucose
Mean blood pressure decreased (from 135.9/80.5 mmHg at baseline) by 3.1/1.9 mmHg. The <140/90 mmHg target was met by 53.0% of patients at baseline, and by 65.8% after 12 months. The mean fasting blood glucose was 6.61 mmol/L at entry and 6.44 mmol/L after 12 months. Mean HbA1c (measured in patients with diabetes only) was 6.9% at entry and 6.7% after 12 months; the <6.5% goal was achieved by 33.5% of patients at baseline and by 40.0% after 12 months.
Clinical events
displays events that occurred in the period between baseline and month 6, and in the period between month 6 and month 12. In total, 1407 events (11.7% of patients were affected) were documented between baseline and month 6, and 1268 events (12.0% of patients) between month 6 and month 12. Hospitalization was the most frequently reported event (5.5% of patients at month 6, 6.1% of patients at month 12). Fifty-six patients died during the study (0.5%); among these, myocardial infarction caused death in eight patients, stroke in six patients.
Table 3 Incidence of clinical events during follow-up
Event rates were highest in the CHD + DM groups in both observation periods, mainly driven by hospitalizations as the most frequent event.
Discussion
The present large-scale registry provides insights into the management of patients with lipid disorders who are at high CV risk due to manifest CHD, DM as coronary equivalent, or other vascular diseases.
While at first glance the study criteria seem restrictive, as only patients on 40 mg of simvastatin were eligible, this drug is by far the most frequently administered statin. Due to specific refunding rules, simvastatin is considered the lead statin in Germany. According to an analysis of all prescriptions paid by the statutory health insurance in 2010, of the 1471 million defined daily doses of statins (which allow treatment of 4 million patients, at standard doses), 1277 million were for simvastatin, 91 million were for pravastatin, 62 million for fluvastatin, 18 million for atorvastatin, and 5 million were for lovastatin and rosuvastatin each. The simva statin 40 mg/day entry dose, used as the inclusion criterion in our study, is somewhat higher than in other studies documenting actual care: in the DYSIS cross-sectional study,Citation13 84% of patients treated for lipid disorders received simvastatin, and the mean simvastatin dose (or in case of other statins, the equivalent dose) was 27 mg/day, with no major differences between high-risk patients and other patients.
The LIMA registry only included patients at high risk for CV events. The Third Report of the National Cholesterol Education Program expert panel and Adult Treatment Panel (NCEP ATP III) describes that lipid disorders, in particular dyslipidemia and hyper cholesterolemia, are the most important modifiable risk factors for CV disease.Citation5 The central role of dyslipidemia as a CV risk factor has been highlighted by the global case-control INTERHEART study,Citation14 in which hypercholesterolemia was responsible for 54% of the population-attributable risk for myocardial infarction. Rigorous dyslipidemia treatment goals have been set for patients with manifest CHD and/or type 2 DM.Citation5 These primarily focus on LDL-C (and total cholesterol), because, according to the meta-analysis by Baigent et alCitation15 of 14 prospective studies, for every 1 mmol/L (39 mg/dL) decrease in LDL-C, the risk of major cardiovascular events is reduced by 21%. With respect to the substantial residual cardiovascular risk of statin-treated patients,Citation16 elevated triglyceride levels should be lowered as well, and low HDL-C values should be raised.Citation17
In our study, lipid target level attainment rates improved over the course of the trial. The objective target level attainment rates (ie, LDL-C <2.59 mmol/L; <100 mg/dL) were of the same order as was seen in other recent studies; for example, in the 2L registry performed in 2006, office-based cardiologists treated a high-risk population similar to ours and achieved target LDL-C levels in 36.2% (CHD + another coronary equivalent), in 39.7% (CHD only), and in 27.2% (coronary equivalent only) of patients.Citation18 In both studies, 2L and LIMA, physicians claimed substantially higher target attainment rates when asked for their subjective assessment. This provides the impression that physicians, at least in a subset of patients, deviate from the guidelines’ recommendations. Several explanations may account for this finding. First, physicians may underestimate the individual risk to their patient. Sager et alCitation19 used logistic regression to analyze whether 907 primary care physicians utilized risk factors and comorbidities appropriately for the assignment of correct LDL-C target values. They found that overall, physicians estimated recommended LDL-C target values correctly in only about half of their 25,000 patients (55.1% of male vs 49.1% of female patients); poor perception of CV risk resulted in lower rates of objective LDL-C target achievement. Second, the treat-to-target paradigm and the stipulated LDL-C targets in the NCEP ATP III current guidelines have been challenged by some experts.Citation20 Third, enduring discussions about whether DM is a coronary equivalent or not (challenging the stringent LDL-C targets set by NCEP ATP III)Citation21–Citation23 could have contributed to noncompliance with guidelines. However, in a population-based observational study in the entire Danish population of 3.3 million people, Schramm et alCitation24 found that patients requiring glucose-lowering therapy who were at least 30 years of age exhibited a CV risk (composite of myocardial infarction, stroke, and CV death) comparable to nondiabetics with a prior myocardial infarction, regardless of sex and diabetes type, supporting the concept of diabetes as a CHD equivalent.
Few physicians prescribed statins at high doses, even if lipid goals were not met. This can be explained, among other factors, by the fear of increased rates of side effects.Citation16 In the recent SEARCH study, the simvastatin 80 mg/day dose was associated with an increased rate of myopathy (0.9% vs 0.03% in the simvastatin 20 mg/day group).Citation25 This finding formed the basis for the US FDA recommendation to avoid starting treatment with the 80 mg/day dose and to continue use of this strength only in patients who have already been taking it for 12 months or longer without negative effect.Citation26 Furthermore, the 80 mg/day dose lowers the LDL-C only slightly, by an additional decrease of roughly 6% over simvastatin 40 mg/day (in agreement with the “rule of six”).Citation27,Citation28
In addition, special German reimbursement rules prefer the prescription of simvastatin. All these together might explain why physicians preferred to use combination therapy with a CAI on top of simvastatin, if needed, to achieve treatment target. In 25% of cases, a CAI was added to simvastatin. According to the meta -analysis by Mikhailidis et alCitation29 of 13 studies including 5080 patients, the add-on of a CAI is significantly more effective than doubling the statin dose (odds ratio 2.5; P = 0.007) for the attainment of LDL-C targets.
With respect to outcomes, at first glance the LIMA registry shows the high incidence of events that patients suffer in this high-risk population, which is predominantly due to the frequent hospitalization events. The rather low death rate (n = 56; 0.5%) in LIMA is remarkable and substantially below the event rates observed in other registries. In addition, the rates of myocardial infarction and stroke are lower than one might expect from other registries. For example, in the current 3A registry focusing on patients with hypertension (often with multiple comorbidities), the death rate after 12 months was 0.8%, and the combined rate of death, myocardial infarction, and stroke was 1.3%.Citation30 Further, in the German cohort of the Reduction of Atherothrombosis for Continued Health Registry (REACH), after 12 months, 2.1% of the patients with symptomatic atherothrombotic disease (CHD, cerebrovascular disease, and/or PAD) and 1.5% of patients with at least three CV risk factors had died of a CV event, and 5.8% of symptomatic atherothrombotic patients and 2.5% of patients with risk factors had reached a combined endpoint consisting of CV death, myocardial infarction, and stroke.Citation31 The reasons for the low event rates in LIMA remain unclear. Possibly the reporting of LDL-C achievement rates to the treating physicians resulted in more aggressive lipid-lowering treatment in patients with missed targets. However, it is not possible to quantify this effect. While it appears that the lipid-lowering treatment was safe and effective overall, the additional LDL-C-lowering effect due to CAI was modest (about 10%) and far below the effect achieved in controlled clinical trials.Citation29,Citation32 This effect may be attributed to insufficient compliance, among other factors.Citation33
In the analysis by subgroup, patients with CHD + DM had more risk factors and individual comorbidities compared with the other two groups. In the presence of CHD, LDL-C-control rates were higher compared with the DM group. Various studies have reported that the treatment intensity of physicians increases at a late stage, once complications have occurred.Citation34,Citation35
Further methodological issues have to be taken into account when interpreting the results of the present registry. Participating physicians and patients may well differ from those refusing to participate, as they may represent a positive selection of a physician sample, with better outcomes. Further, the study was nonrandomized, did not include a control group, and was not blinded, which may have introduced bias that confounded the indication or overestimation of treatment effects.Citation36 Diet and physical activity were not assessed. Clinical events were collected according to commonly accepted registry rules, but were not adjudicated. No safety information was gathered in the context of this study.
Conclusion
The present LIMA registry shows that by intensifying lipid-lowering treatment, physicians were able to substantially increase the number of patients at LDL-C target and to improve other lipid parameters as well as blood pressure and glucose levels. However, LDL-C control rates are not yet optimal. Treatment effects were consistent across the CHD, DM, and CHD + DM subgroups, but event rates were highest in the latter group. Combination therapy of statin plus CAI offers additional patients the chance to achieve targets.
Author contributions
Concept/design: JRS, KB, AKG, FS, AW, CJ. Data analysis/ interpretation: JRS, KB, AKG, FS, AW, BK, D P. Drafting of article: D P, KB. Critical revision of article: JRS, AKG, CJ, KB. Approval of article: all. Funding secured by: CJ, BK, KB.
Acknowledgments
The registry was supported by MSD SHARP and DOHME GmbH, Haar, Germany.
Disclosure
JRS serves as a consultant for MSD/ESSEX and received lecture fees by MSD/ESSEX, Roche, Sanofi, Genzyme and B Braun. DP has received consultancy honoraria from MSD, Sanofi, Pfizer and Novartis. KB is a former employee of MSD; CJ and BK are current, full-time employees of MSD. Apart from this, the authors report no conflicts of interest.
References
- World Health OrganizationThe Global Burden of Disease: 2004 UpdateGenevaWorld Health Organization2008 Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.htmlAccessed September 13, 2012
- Centers for Disease Control and Prevention (CDC)QuickStats: Age-adjusted death rates for heart disease and cancer – United States, 1999–2009*MMWR [series on the Internet]620116021713 Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6021a6.htm?s_cid=mm6021a6_wAccessed November 28, 2012
- GoldsteinLBAdamsRAlbertsMJPrimary prevention of ischemic stroke. A guideline from the American Heart Association/American Stroke Association Stroke Council:Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guidelineStroke20063761583163316675728
- GreenlandPAlpertJSBellerGA2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: Executive summary. A report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic ResonanceJ Am Coll Cardiol2010562521822199
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III)JAMA2001285192486249711368702
- KloseGSchwabeULipidsenkende Mittel. [Lipid lowering drugs]SchwabeUPaffrathDArzneiverordnungs-Report 2011BerlinSpringer2010683698 German.
- PhillipsLSBranchWTCookCBClinical inertiaAnn Intern Med2001135982583411694107
- CabanaMDRandCSPoweNRWhy don’t physicians follow clinical practice guidelines? A framework for improvementJAMA1999282151458146510535437
- BestehornKSchäferJGittALeitliniengerechte Lipidtherapie und Zielwerterreichung bei Risikopatienten im klinischen Alltag. Rationale, Ziele und Design des LIMA-registers [LIMA-register: rationale, aims and design]MMW Fortschr Med2008150Suppl 3S135S141 German.
- SonntagFSchaeferJRGittAKLipidtherapie im Alltag (LIMA): Leitliniengerechtes Lipidmanagement bei Patienten mit hohem kardiovaskulären Risiko in der klinischen Praxis (LIMA Register) [Lipid therapy in daily routine. Guidelines compatible lipid management of patients with high cardiovascular risk in clinical practice (LIMA registry)]Dtsch med Wochenschr20121374020472052 German.23023622
- Good Epidemiological Practice (GEP): Proper conduct in epidemiologic researchUpdated 2007. International Epidemiological Association2007[updated April 24, 2010] Available from: http://ieaweb.org/2010/04/good-epidemiological-practice-gep/Accessed November 28, 2012
- NorgrenLHiattWRDormandyJATASC II Working GroupInter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II)Eur J Vasc Endovasc Surg200733Suppl 1S1S7517140820
- GittAKJüngerCSmolkaWBestehornKPrevalence and overlap of different lipid abnormalities in statin-treated patients at high cardiovascular risk in clinical practice in GermanyClin Res Cardiol2010991172373320521058
- YusufSHawkenSOunpuuSINTERHEART Study InvestigatorsEffect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control studyLancet2004364943893795215364185
- BaigentCKeechAKearneyPMCholesterol Treatment Trialists’ (CTT) CollaboratorsEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet200536694931267127816214597
- LawMRWaldNJRudnickaARQuantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysisBMJ200332674041423142712829554
- LeeJMSChoudhuryRPProspects for atherosclerosis regression through increase in high-density lipoprotein and other emerging therapeutic targetsHeart200793555956416449520
- GittAKJuengerCJannowitzCKarmannBSengesJBestehornKGuideline-oriented ambulatory lipid-lowering therapy of patients at high risk for cardiovascular events by cardiologists in clinical practice: the 2L cardio registryEur J Cardiovasc Prev Rehabil200916443844419369876
- SagerHBLinsel-NitschkePMayerBPhysicians’ perception of guideline-recommended low-density lipoprotein target values: characteristics of misclassified patientsEur Heart J201031101266127320219745
- HaywardRAKrumholzHMThree reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institute of HealthCirc Cardiovasc Qual Outcomes2012512522253366
- BuykenAEvon EckardsteinASchulteHCullenPAssmannGType 2 diabetes mellitus and risk of coronary heart disease: results of the 10-year follow-up of the PROCAM studyEur J Cardiovasc Prev Rehabil200714223023617446801
- SaelyCHAczelSKochLDiabetes as a coronary artery disease risk equivalent: before a change of paradigm?Eur J Cardiovasc Prev Rehabil2010171949919940780
- BulugahapitiyaUSiyambalapitiyaSSitholeJIdrisIIs diabetes a coronary risk equivalent? Systematic review and meta-analysisDiabet Med200926214214819236616
- SchrammTKGislasonGHKøberLDiabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million peopleCirculation2008117151945195418378618
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative GroupArmitageJBowmanLWallendszusKIntensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trialLancet201037697531658166921067805
- FDA: Limit use of 80 mg simvastatinUS Food and Drug Administration2011[updated June 8, 2011] Available from: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm257884.htmAccessed November 28, 2012
- KnoppRHDrug treatment of lipid disordersN Engl J Med1999341749851110441607
- FDA drug safety communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injuryUS Food and Drug Administration2011[updated June 8, 2011] Available from: http://www.fda.gov/Drugs/DrugSafety/ucm256581.htmAccessed November 28, 2012
- MikhailidisDPLawsonRWMcCormickALComparative efficacy of the addition of ezetimibe to statin vs statin titration in patients with hypercholesterolaemia: systematic review and meta-analysisCurr Med Res Opin20112761191121021473671
- ZeymerUDechendRDeegE3A Registry InvestigatorsAliskiren for the treatment of essential hypertension under real-life practice conditions: design and baseline data of the prospective 3A registryInt J Clin Pract201266325126122321062
- ZeymerUSengesJParhoferKGRötherJRisk factors and event rates in patients with atherothrombotic disease in Germany: Results of the REACH registryDtsch Arztebl Int20081054576977519578408
- IjiomaNRobinsonJGLipid-lowering effects of ezetimibe and simvastatin in combinationExpert Rev Cardiovasc Ther20119213114521453210
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med2005165101147115215911728
- PittrowDStallaGKZeiherAMPrävalenz, medikamentöse Behandlung und Einstellung des Diabetes mellitus in der Hausarzt-praxis. [Prevalence, drug treatment and metabolic control of diabetes mellitus in primary care]Med Klin (Munich)20061018635644 German.16896570
- BestehornKJannowitzCKarmannBPittrowDKirchWCharacteristics, management and attainment of lipid target levels in diabetic and cardiac patients enrolled in Disease Management Program versus those in routine care: LUTZ registryBMC Public Health2009928019653899
- PsatyBMKoepsellTDLinDAssessment and control for confounding by indication in observational studiesJ Am Geriatr Soc199947674975410366179