Abstract
Background
The JET-RANGER study (NCT03206762) was a multicenter (11 US centers) randomized trial, core lab adjudicated, designed to demonstrate the superiority of Jetstream + Paclitaxel coated balloon (JET+PCB) versus angioplasty (PTA) + PCB in treating femoropopliteal (FP) arterial disease. The one-year primary endpoint of JET-RANGER has been recently published. The 2-year outcome data are presented in this report.
Methods
There were 43 patients who completed the 1-year follow-up. Two were lost to follow-up and one died prior to the 2-year follow-up, resulting in 40 patients. Fifteen patients were randomized to PTA+PCB and 25 patients to JET +PCB. Kaplan Meier Survival analysis was performed to estimate the freedom from TLR. Bailout stenting was not considered a TLR in this analysis. Statistical significance was determined by a p-value < 0.05.
Results
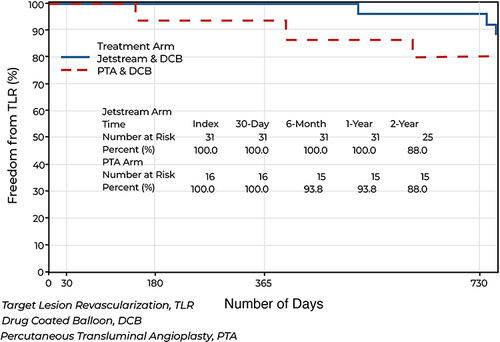
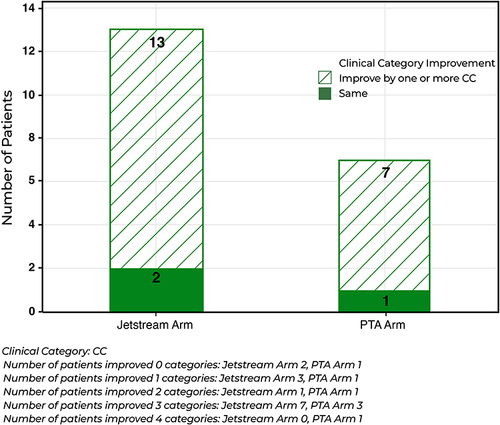
Freedom from TLR was similar between the 2 groups at 2 years. There was also no significant difference in the change of ABI between the PTA + PCB and JET + PCB from baseline at 6-months, (p-value = 0.7890), 1-year (p-value = 0.4070), and 2-year (p-value=0.7410). There was also no statistical difference between the JET + PCB and PTA + PCB arms for RCC improvement by one or more category, (p-value= 1.000). There were no minor or major amputations for either arm throughout the 2-year follow up. One JET + PCB patient died before the 2-year specified window.
Conclusion
JET + PCB had similar freedom from TLR and improvement in ABI and RCC at 2-year follow-up when compared to PTA + PCB with no difference in amputation or mortality between the 2 arms.
Clinical Trial Registration
NCT03206762.
Introduction
Vessel prepping with atherectomy improves technical and procedural outcomes and reduces bailout stenting.Citation1,Citation2 Atherectomy however, has not been demonstrated to alter the long term outcome of femoropopliteal arterial disease treatment when compared to PTA alone.Citation2,Citation3 Smaller randomized and observational data suggest that the combination of atherectomy and PCB is more effective than PCB alone in treating in-stent restenosisCitation4 and possibly complex de novo arterial disease including severe calcium and long lesions.Citation5–8 These data are however not conclusive or powered to answer the question of added long term benefit of atherectomy and PCB when compared to PCB alone.
The Jetstream Atherectomy followed by Paclitaxel-Coated Balloons versus Balloon Angioplasty Followed by Paclitaxel-Coated Balloons (JET-RANGER Study) (NCT03206762) is a randomized trial of Jetstream atherectomy (JET) plus paclitaxel coated balloon (PCB) versus balloon angioplasty (PTA) plus PCB of the femoropopliteal artery (FP). The trial was stopped early because of poor enrolment due to the COVID-19 pandemic and the Food and Drug Administration (FDA) warning of an association of mortality with PCB. The one-year exploratory results were recently publishedCitation9 and demonstrated that bailout stenting was significantly reduced in the JET + PCB arm when compared to the PTA + PCB arm (0 JET + DCB versus 50% PCB, P<0.0001) but target lesion revascularization (TLR) were statistically similar. In this report, we present the 2-year follow-up of the JET RANGER.
Methods
The JET-RANGER design, methods, procedural details and 1-year outcomes have been previously published.Citation9 The study was approved by the central Advarra Independent Ethics Committee (IEC) with additional approval by the local Institutional Review Boards at the research sites when required. The study complies with the Declaration of Helsinki. Informed consent was obtained from the study participants prior to study commencement. Enrolment started in March of 2018 and the last one-year and 2-year patient follow-up were in April of 2021 and April 2022, respectively. The Clinical Events Committee (CEC) reviewed prespecified adverse events.
Secondary outcomes presented in this report include TLR, Change Rutherford Clinical Category (RCC) and Ankle-Brachial Index (ABI) at 2 years when compared to baseline and 1-year follow-up. A change of 0.15 or higher in the ABI was considered significant.
Statistical Methods
Analysis was performed per patient, per procedure, and per lesion. Descriptive analysis on all variables was done. Continuous data was presented as mean ± standard deviation [median]; Categorical data was given as count/sample (percentage). Anderson-Darling test was used to test for normality. Wilcoxon Signed test, Kruskal–Wallis test, one-sample and two-sample t-test, Mood’s Median test, Pearson’s Chi-Square test, and Fisher’s Exact test were used where appropriate. Survival Kaplan Meier Survival analysis was performed to estimate the Freedom from TLR with bailout stenting not considered as TLR. Comparison of Survival Curves was done by Wilcoxon. Statistical significance was determined by a p-value < 0.05. Software used was Minitab 21 (State College Pennsylvania, USA) and Cytel Studio 12 (Cambridge, Massachusetts, USA). Given the overall small number of patients enrolled, the analysis performed is considered exploratory. An intention to treat analysis was performed.
Results
There were 43 patients completed for the 1-year follow-up. Two were lost to follow-up and one died prior to the 2-year follow-up, resulting in 40 patients. Fifteen patients were randomized to PTA+PCB and 25 patients to JET + PCB. A higher procedural success and significantly less bailout stenting were seen in the JET+PCB arm as previously reported. Freedom from TLR, however, was 88% and 80% for the JET+PCB and PTA+PCB arms respectively at 2 years (p=0.3380) (). There was also no significant difference in the change of ABI between the PTA + PCB and JET + PCB from baseline at 6-months, (p-value = 0.7890), 1-year (p-value = 0.4070), and 2-year (p-value=0.7410). There was also no statistical difference between the JET + PCB and PTA + PCB arms for RCC improvement by one or more category, (p-value= 1.000) (). There were no minor or major amputations for either arm throughout the 2-year follow up. One (3.8%) JET + PCB patient died at 432 days post treatment from a cardiopulmonary arrest due to interstitial lung disease and mesenteric ischemia, probably unrelated to the procedure.
Discussion
The 2-year follow up of the JET RANGER continues to show that PTA + PCB of de novo femoropopliteal lesions has similar freedom from TLR to the JET+PCB strategy but at the expense of half the patients in the PTA arm requiring bailout stenting mostly due to a higher residual narrowing.Citation9 Atherectomy reduces the chance of severe dissections and the chance of bailout stenting as it has been demonstrated in several studies.Citation1,Citation2,Citation9 JET appears to be a favorable vessel prepping approach to treat complex de-novo femoropopliteal arterial disease prior to PCB in line of the strategy of leaving the least metal behind while maintaining similar long term outcomes to PTA+PCB. The cost-effectiveness of this approach has to be further studied.
Mortality with PCB has been raised as a concern with Katsanos et alCitation10 and led to an FDA warning about the use of these devices. More recent data indicate that there was no significant increase in all-cause mortality with paclitaxel coated devices.Citation11–15 Given the small number of patients in our study, no definitive conclusions about mortality increase or decrease can be made with the Ranger or In.PACT balloons at 2 years.
In summary, this study shows that the strategy of JET+PCB yields excellent freedom from TLR at 2 years without the need for bailout stenting. Although PTA+PCB showed similar freedom from TLR at 2 years, this came at the cost of very high rate of bailout stenting when treating complex lesions with CTO, moderate to severe calcium and long lesions.
Limitations of the Study
The findings in this study are exploratory because a small number of patients have been evaluated. No definitive conclusions could be made. The study however confirms a high freedom from TLR for both JET+PCB and PTA+PCB at 2-year follow-up offering 2 different strategies to treat femoropopliteal de novo arterial disease. In addition, no safety issues of significance at 2-year follow-up were noted. A large, well-powered randomized trial of JET + PCB versus PTA + PCB with long term follow-up is needed to conclusively evaluate both strategies.
Data Sharing Statement
The authors do not intend to share individual deidentified data unless requested for a specific pre-specified analysis or for auditing purposes by regulatory bodies. Aggregate data will be released on clinicaltrials.gov and part of an indexed publication and will be accessible to public.
Disclosure
Dr Nicolas Shammas receives educational and research grants from Boston Scientific, Angiodynamics, VentureMed group, Phillips, Bard/BD and is on the speaker Bureau of Janssen, Merck, Lilly, Amgen, Esperion, Kiniksa, Boehringer Ingelheim. The other authors report no conflicts of interest in this work.
Acknowledgments
JET-RANGER one-year follow up was published in Vasc Health Risk Manag. 2022 Aug 2;18:603-615.
The following JET-RANGER investigators have contributed to the study by recruiting patients into this clinical trial or participated in the CEC committee or core laboratory:
Investigators
Lawrence Garcia, MD. Steward Saint Elizabeth, Boston MA
Nicholas Petruzzi, MD, Atlantic Medical, New Jersey
Mathew Wooster, MD, Medical University South Carolina, South Carolina
Jack Chamberlin, MD, Alexian Brothers Hospital, Illinois
William B. Eaves, MD, Endovascular Technologies, Louisianna
Richard Kovach, MD, Deborah Heart and Lung, Brown Mills, New Jersey
Mohammad Mehdi Ansari, MD, Texas Tech University Health Science, TX
Esteban Henao, MD, New Mexico Heart Institute, New Mexico
Faisal Latif, MD, VA Oklahoma, OK
April Nedeau, MD, Central Maine Medical Center, Maine
CEC Committee
Jon Robken, MD, Interventional Cardiology
Param Singh, MD, Interventional Cardiology
Vijay Ranjendran, MD, Interventional Cardiology
Additional information
Funding
References
- Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26(8):355–360.
- Shammas NW, Coiner D, Shammas GA, et al. Percutaneous lower-extremity arterial interventions with primary balloon angioplasty versus Silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. J Vasc Interv Radiol. 2011;22(9):1223–1228. doi:10.1016/j.jvir.2011.05.013
- Wu Z, Huang Q, Pu H, et al. Atherectomy combined with balloon angioplasty versus balloon angioplasty alone for de novo femoropopliteal arterial diseases: a systematic review and meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. 2021;62(1):65–73. doi:10.1016/j.ejvs.2021.02.012
- Gandini R, Del Giudice C, Merolla S, et al. Treatment of chronic SFA in-stent occlusion with combined laser atherectomy and drug-eluting balloon angioplasty in patients with critical limb ischemia: a single-center, prospective, randomized study. J Endovasc Ther. 2013;20(6):805–814. doi:10.1583/13-4308MR.1
- Zeller T, Langhoff R, Rocha-Singh KJ, et al. DEFINITIVE AR investigators. directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR Study. Circ Cardiovasc Interv. 2017;10(9):e004848. doi:10.1161/CIRCINTERVENTIONS.116.004848
- Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37(4):898–907. doi:10.1007/s00270-014-0904-3
- Cioppa A, Stabile E, Popusoi G, et al. Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: one-year single centre clinical results. Cardiovasc Revasc Med. 2012;13(4):219–223. doi:10.1016/j.carrev.2012.04.007
- Scheer F, Lüdtke CW, Kamusella P, et al. Combination of rotational atherothrombectomy and Paclitaxel-coated angioplasty for femoropopliteal occlusion. Clin Med Insights Cardiol. 2015;8(Suppl 2):43–48. doi:10.4137/CMC.S15231
- Shammas NW, Purushottam B, Shammas WJ, et al.; for the JET-RANGER Investigators. Jetstream atherectomy followed by paclitaxel-coated balloons versus balloon angioplasty followed by paclitaxel-coated balloons: twelve-month exploratory results of the prospective randomized JET-RANGER study. Vasc Health Risk Manag. 2022;18:603–615. doi:10.2147/VHRM.S371177
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(24):e011245. doi:10.1161/JAHA.118.011245
- Schneider PA, Varcoe RL, Secemsky E, et al. Update on paclitaxel for femoral-popliteal occlusive disease in the 15 months following a summary level meta-analysis demonstrated increased risk of late mortality and dose response to paclitaxel. J Vasc Surg. 2020:S0741–S5214. doi:10.1016/j.jvs.2020.07.093
- Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. 2019;73(20):2550–2563. doi:10.1016/j.jacc.2019.01.013
- Schneider PA, Brodmann M, Mauri L, et al. Paclitaxel exposure: long-term safety and effectiveness of a drug-coated balloon for claudication in pooled randomized trials. Catheter Cardiovasc Interv. 2020;96(5):1087–1099. doi:10.1002/ccd.29152
- Steiner S, Schmidt A, Zeller T, et al. COMPARE: prospective, randomized, non-inferiority trial of high- vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J. 2020;41(27):2541–2552. doi:10.1093/eurheartj/ehaa049
- Schroeder H, Meyer D-R, Lux B, et al. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: outcomes from the ILLUMENATE first-in-human study. Catheter Cardiovasc Interv. 2015;86(2):278–286. doi:10.1002/ccd.25900