Abstract
Rivaroxaban (Bayer AG, Leverkusen, Germany) is a highly selective direct inhibitor of factor Xa. It has completed Phase III clinical trials evaluating its efficacy and safety against enoxaparin in the prophylaxis against venous thromboembolism (VTE) in orthopedic patients following primary total hip and total knee arthroplasty. Rivaroxaban has been extensively studied worldwide in 12,729 patients in the Regulation of Coagulation in Major Orthopedic Surgery Reducing the Risk of DVT and PE (RECORD) program. Pivotal clinical trials have demonstrated the superior efficacy in reducing total VTE in comparison with both the North American and European regimens of enoxaparin. Safety of the drug was found to be excellent, with no demonstrable cardiovascular or hepatic effects and no statistically significant increase in major bleeding. A pooled analysis of data collected on the patients from the four RECORD trials revealed rivaroxaban to be the first antithrombotic agent to demonstrate superiority over another antithrombotic (enoxaparin) in reducing symptomatic VTE and all-cause mortality. While there was a significant difference in the composite safety endpoint of major and clinically relevant nonmajor bleeding in the pooled analysis with the use of rivaroxaban compared with enoxaparin, there was no significant difference in major bleeding or in any other bleeding.
Introduction
Venous thromboembolism (VTE) is the most frequent serious complication following total hip and total knee arthroplasty. In the absence of thromboprophylaxis, 50% to 60% of these patients will develop either deep vein thrombosis (DVT) or pulmonary embolism (PE). Because patients following total joint arthroplasty are at very highest risk for the development of VTE, the use of routine thromboprophylaxis has been recommended in these patients by a number of nationally and internationally recognized entities including the American College of Chest Physicians, the American Academy of Orthopaedic Surgeons, the International Surgical Thrombosis Forum, and the US Centers for Medicare and Medicaid Services.
Contemporary antithrombotic strategies
Current guideline recommendations for thromboprophylaxis involve the use of vitamin K antagonists (VKA), low-molecular-weight heparins (LMWH), aspirin, or fondaparinux, an indirect inhibitor of factor Xa.Citation1,Citation2,Citation3 While aspirin has been advocated by a number of organizations for thromboprophylaxis,Citation3 this agent functions principally as an inhibitor of platelet aggregation rather than as an antithrombotic agent. The use of VKAs is associated with extremely variable patient-to-patient response, delay in onset of action, extensive food and drug interactions, and the need for monitoring and dose adjustment to target the international normalized ratio value. Citation3 Both LMWHs and fondaparinux require subcutaneous injection for drug administration. This can significantly affect patient acceptance and compliance during extended prophylaxis and in circumstances depending on patient self-administration.Citation4
A new antithrombotic agent
Rivaroxaban is a highly selective direct inhibitor of factor Xa that has completed Phase III clinical trials evaluating its efficacy and safety against enoxaparin in the prophylaxis against VTE in orthopedic patients following primary total hip and total knee arthroplasty. Rivaroxaban has a half-life of approximately 9 hours, with a rapid onset of action, reaching maximum serum concentration in <2 hours. It has very predictable pharmacokinetics and pharmacodynamics, and has no significant food or drug interactions. Approximately one-third of the drug is metabolized by the liver and/or through gastrointestinal transit and eliminated via the fecal route; the remaining two-thirds is cleared via renal excretion.Citation5–Citation7 Rivaroxaban is administered orally at a fixed dose, unmonitored, once daily. As such, the use of the drug provides a very simple regimen for both the patient and the physician, with a route of administration that is readily embraced by the patient, reducing compliance concerns associated with parenteral drug administration.
Clinical trial results
Rivaroxaban has been extensively studied in 12,729 patients worldwide in the RECORD Program (REgulation of Coagulation in major Orthopaedic surgery reducing the Risk of DVT and PE). This represents the largest orthopedic clinical development program ever conducted. Four Phase III pivotal trials evaluated the safety and efficacy of rivaroxaban against the active comparator, enoxaparin. All four clinical trials shared the same basic, prospective, randomized, double-blind study design template, as well as the same blinded, independent adjudication committee for outcomes. These trials also shared the same primary efficacy endpoint, which was a composite of symptomatic DVT, nonfatal PE, and all-cause mortality. All four trials also shared common safety endpoints for major bleeding, major bleeding including the surgical site requiring reoperation, major and clinically relevant nonmajor bleeding, and any bleeding. This allowed a pooled data analysis from the >12,000 patients of the four pivotal RECORD trials to be performed.
Two of these trials (RECORD1 and 2) dealt with patients following total hip arthroplasty, and two (RECORD3 and 4) dealt with patients following total knee arthroplasty. RECORD1 compared rivaroxaban 10 mg once daily for 5 weeks against enoxaparin 40 mg once daily also for 5 weeks.Citation8 RECORD2 evaluated the safety and efficacy of rivaroxaban 10 mg once daily for 5 weeks against enoxaparin 40 mg once daily for 10–14 days followed by oral placebo.Citation9 In RECORD3, rivaroxaban 10 mg once daily for 10–14 days was compared against enoxaparin 40 mg once daily also for 10–14 days.Citation10 In RECORD4, rivaroxaban 10 mg once daily for 10–14 days was compared against enoxaparin 30 mg twice daily also for 10–14 days.Citation11 In all four RECORD trials, rivaroxaban was found to be statistically significantly superior to enoxaparin in the reduction of total VTE (the primary efficacy endpoint). Additionally, in RECORD1, 2, and 3, rivaroxaban was superior in reducing major VTE (a composite of proximal DVT, symptomatic PE, and all-cause mortality), and in RECORD2 and 3, superior in reducing symptomatic VTE (a composite of symptomatic DVT, symptomatic PE, and VTE-related death). RECORD4 is notable in that it represents the only clinical trial ever to demonstrate superior efficacy of any antithrombotic agent in comparison with the North American regimen of enoxaparin (30 mg subcutaneously twice daily). The superior efficacy of rivaroxaban was achieved without a statistically significant increase in major bleeding, which was <0.7% in all trials. summarizes the efficacy results from the four RECORD Trials.Citation8–Citation11 Summarizes key primary as well as composite safety endpoints. There was no statistically significant difference in major bleeding, or difference in clinically relevant nonmajor bleeding.Citation12
Table 1 Summary table RECORD trials: RRR (%) rivaroxaban vs enoxaparin
Table 2 Safety data at follow-up day 12 ± 2 days
Pooled analysis
A pooled analysis of the data from RECORD1, 2, 3, and 4 was performed to compare rivaroxaban with enoxaparin regimens in reducing the incidence of efficacy and safety-related endpoints across the four pivot trials. A pre-specified integrated analysis strategy for symptomatic event composites across the RECORD1–4 studies was utilized. Because the number of VTE events was expected to be low in any of the individual RECORD studies, pooling was utilized to increase statistical precision, and to allow for a better assessment of clinically relevant endpoints. Because the RECORD trials differed in the duration of drug administration, treatment duration and total follow-up duration, three population pools were evaluated. The Active Treatment Pool represented that group of patients receiving drug administration up to 12 ± 2 days. The Total Treatment Duration Pool included all patients during their treatment periods (from day 12 ± 2 days to day 35 ± 4 days). The total study duration pool represented all patients up to and including the final follow-up (from day 42 to 65). illustrates the pooled analysis study design.Citation13
Figure 1 Pooled analysis design.
Data from Kwong LM presentation.Citation13
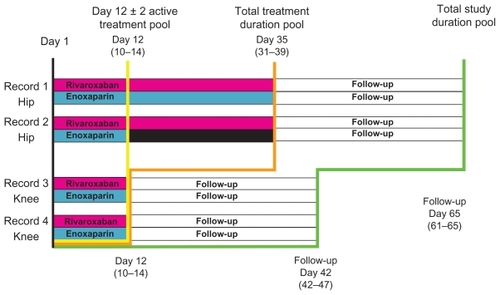
The prespecified primary efficacy outcome in this pooled analysis was a composite of symptomatic DVT and nonfatal PE, as well as all-cause mortality. All symptomatic events were confirmed and centrally adjudicated. Other outcomes evaluated included all-cause mortality; a post-hoc analysis of a composite of PE and all-cause mortality; and a post-hoc analysis of a composite of all major clinical outcomes (death, myocardial infarction, stroke, symptomatic VTE, and major bleeding). Safety outcomes consisted of the prespecified treatment-emergent bleeding endpoints of major bleeding: major bleeding including surgical site bleeding, major bleeding and clinically relevant nonmajor bleeding, and any bleeding. Other safety endpoints included adverse events, including cardiovascular events.
Rivaroxaban demonstrated a 58% reduction in the primary efficacy outcome (symptomatic VTE and all-cause mortality) for the Total Treatment Duration Pool compared with enoxaparin in the primary population for analysis (). In the Active Treatment Pool, there was a 52% reduction in the primary efficacy outcome with rivaroxaban compared with enoxaparin (hazard ratio [HR] 0.48; 95% confidence interval [CI] 0.31–0.75) (). Rivaroxaban was also found to significantly reduce the incidence of symptomatic VTE and all-cause mortality in the Total Study Duration Pool up to and including final follow-up (HR 0.49; 95% CI 0.35–0.69) (). Additionally, rivaroxaban significantly improved the composite outcome of symptomatic VTE, cardiovascular events, all-cause mortality, and major bleeding compared with enoxaparin regimens. There were no demonstrable adverse effects on hepatic function (). There was no statistically significant difference in any bleeding, major bleeding, or major plus clinically relevant nonmajor (CRNM) bleeding at day 12 ± 2 days and at follow-up. There was a significant difference in major plus CRNM at day 35 (including the placebo phase of RECORD2) favoring the use of enoxaparin in the safety analysis of composite bleeding endpoints (rivaroxaban 158/12,383 [2.55%]; enoxaparin 197/12,383 [3.19%], P = 0.039 [analyzed using Cox regression model]).Citation12
Figure 2 Total treatment duration pool efficacy outcome.
Abbreviations: VTE, venous thromboembolism; RRR, relative risk reduction; HR; hazard ratio.
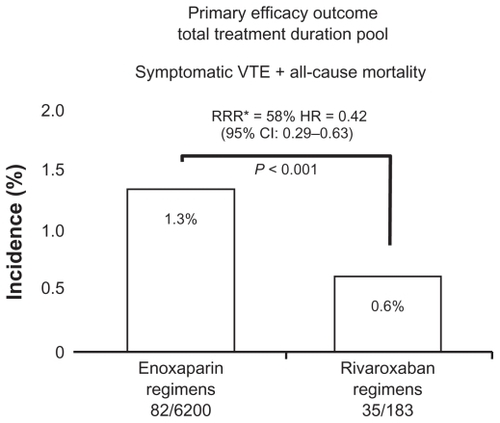
Figure 3 Active treatment pool efficacy outcome.
Abbreviations: VTE, venous thromboembolism; RRR, relative risk reduction; HR; hazard ratio.
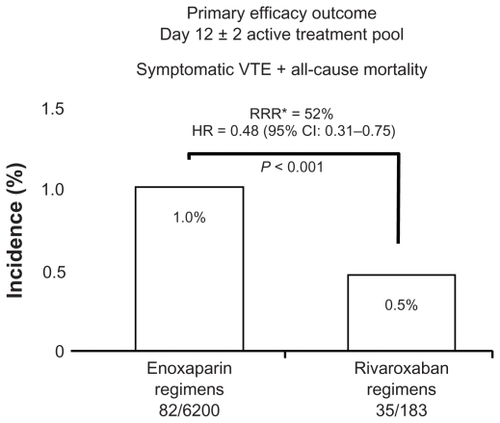
Figure 4 Total study duration efficacy outcome.
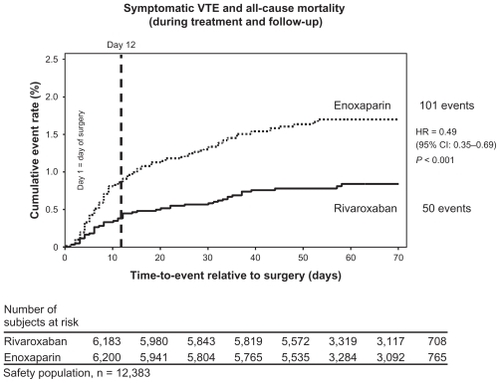
Table 3 Liver safety: total duration study pool
Discussion
Rivaroxaban is a new oral antithrombotic agent that is a highly selective direct inhibitor of factor Xa. It has been designed with a simple once-a-day regimen to be used across a broad spectrum of the population at a fixed dose, unmonitored. Pivotal clinical trials have demonstrated the superior efficacy in reducing total VTE in comparison with both the North American and European regimens of enoxaparin. Safety of the drug was found to be excellent with no demonstrable cardiovascular or hepatic effects, and no statistically significant increase in major bleeding or in any bleeding. Although a statistically significant difference was found in major plus CRNM at day 35 favoring the use of enoxaparin in the safety analysis of composite bleeding endpoints, in the clinical setting, this finding may not represent a relevant factor for the surgeon in terms of surgical site complications (no statistically significant differences were found in bleeding leading to reoperation, prolonged wound drainage, hemarthrosis, or wound dehiscence) and no statistically significant difference was found in postoperative infection rate between the rivaroxaban and enoxaparin groups.Citation12 The use of pharmacologic thromboprophylaxis as a risk factor for postoperative bleeding and prolonged wound drainage leading to infection is a concern of some orthopedic surgeons, although a causal relationship has never been established, and a recent review of the peer-reviewed literature also does not support this association.Citation14 A pooled analysis of the 12,729 patients from the four RECORD trials revealed rivaroxaban to be the first antithrombotic agent to demonstrate superiority over another antithrombotic (enoxaparin) in reducing symptomatic VTE and all-cause mortality. A pharmacoeconomic analysis of the efficacy and safety of rivaroxaban compared with the use of enoxaparin indicates that rivaroxaban has the potential to be cost effective in reducing costs associated with the prophylaxis and treatment of thromboembolic events in postoperative hip and knee replacement patients.Citation15,Citation16
In summary, rivaroxaban appears to have the potential to provide a safe, convenient, and effective method for prophylaxis against VTE in the high-risk group of orthopedic patients following total hip and knee arthroplasty.
Disclosures
Dr Kwong has received research grants from Astellas, Bayer, GlaxoSmithKline, Pfizer, sanofi-aventis, and Takeda. He is a consultant for Zimmer and also receives royalties for product development.
References
- GeertsWHBergqvistDPineoGFPrevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed)Chest2008133381S453S18574271
- JohansonNALachiewiczPFLeibermanJRAAOS Physician Volunteer Work GroupAmerican Academy of Orthopaedic Surgeons clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty: summary of recommendationsJ Am Acad Orthop Surg200917318319619264711
- EllisRFStephensMASharpGBEvaluation of a pharmacy-managed warfarin-monitoring service to coordinate inpatient and outpatient therapyAm J Hosp Pharm19924923873941554003
- WattsACHowieCRSimpsonAAssessment of a self-administration protocol for extended subcutaneous thromboprophylaxis in lower limb arthroplastyJ Bone Joint Surg2006881107110
- RoehrigSStraubAPohlmannJDiscovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitorJ Med Chem200548195900590816161994
- PerzbornEKubitzaDMisselwitzFRivaroxaban. A novel, oral, direct factor Xa inhibitor in clinical development for the prevention and treatment of thromboembolic disordersHamostaseologie20072728228917938768
- KubitzaDBeckaMWensingGVoithBZuehlsdorfMSafety, pharmacodynamics, and pharmacokinetics of BAY 59-7939-an oral, direct factor Xa inhibitor-after multiple dosing in healthy male subjectsEur J Clin Pharmacol2005611287388016328318
- ErikssonBIBorrisLCFriedmanRJRivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplastyN Engl J Med2008358262765277518579811
- KakkarAKBrennerBDahlOEExtended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trialLancet20083729632313918582928
- LassenMRAgenoWBorrisLCRivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplastyN Engl J Med2008358262776278618579812
- TurpieAGLassenMRDavidsonBLRivaroxaban Versus Enoxaparin for Thromboprophylaxis After Total Knee Arthroplasty (RECORD4): a randomised trialLancet200937396761673168019411100
- LassenMRTurpieAGKakkarAKErikssonBIGentMPotential impact of rivaroxaban on surgical safety outcomes after total hip or knee replacement surgeryAnnual Meeting of the International Society on Thrombosis and Haemostasis. PP-WE-404July 2009
- KwongLMOnce-daily, oral rivaroxaban for the prevention of venous thromboembolism (VTE) after total hip or knee replacement (THR/TKR) surgeryWestern Orthopaedic Association Annual Meeting Podium presentation: PM SessionSeattle, WAAugust 1, 2009
- KwongLMBleeding and Thrombo. Poster presentation # 462011Mid- America Orthopaedic Association Annual MeetingApril 7–8, 2011
- KwongLMCost-effectiveness of rivaroxaban after total hip or knee arthroplastyAm J Manag Care201117SupplS222621517652
- KwongLMDuranADiamantopoulosASenguptaNLeesMCost- effectiveness of rivaroxaban for prevention of venous thromboemebolism (VTE) after total hip or knee replacement (THR, TKR) in the US. [Abstract]J Thromb Haemost20097Suppl 2PP-WE-473