Abstract
In physiological hemostasis a prompt recruitment of platelets on the vessel damage prevents the bleeding by the rapid formation of a platelet plug. Qualitative and/or quantitative platelet defects promote bleeding, whereas the high residual reactivity of platelets in patients on antiplatelet therapies moves forward thromboembolic complications. The biochemical mechanisms of the different phases of platelet activation – adhesion, shape change, release reaction, and aggregation – have been well delineated, whereas their complete translation into laboratory assays has not been so fulfilled. Laboratory tests of platelet function, such as bleeding time, light transmission platelet aggregation, lumiaggregometry, impedance aggregometry on whole blood, and platelet activation investigated by flow cytometry, are traditionally utilized for diagnosing hemostatic disorders and managing patients with platelet and hemostatic defects, but their use is still limited to specialized laboratories. To date, a point-of-care testing (POCT) dedicated to platelet function, using pertinent devices much simpler to use, has now become available (ie, PFA-100, VerifyNow System, Multiplate Electrode Aggregometry [MEA]). POCT includes new methodologies which may be used in critical clinical settings and also in general laboratories because they are rapid and easy to use, employing whole blood without the necessity of sample processing. Actually, these different platelet methodologies for the evaluation of inherited and acquired bleeding disorders and/or for monitoring antiplatelet therapies are spreading and the study of platelet function is strengthening. In this review, well-tried and innovative platelet function tests and their methodological features and clinical applications are considered.
Introduction
Human platelets are critically involved in both normal hemostasis and pathological bleeding and thrombosis. These particular cells contribute greatly to vessel constriction and repair, host defense, and tumor growth/metastasis.Citation1 In addition, platelets acting together with other cells – white, endothelial, or smooth muscle cells – play a part in inflammation, in related pathologies, and in the promotion of atherosclerosis.Citation2–Citation4 Notwithstanding this multiplicity of roles, the available platelet function tests explore principally the alterations directly related to the hemostatic process.Citation5
Primary hemostasis consists of the interaction between platelets and vessel wall. At the site of damage of a vessel wall, platelets are rapidly engaged in a sequential functional response including various steps in platelet activation: adhesion, spreading, shape change, aggregation, release reaction, exposure of a procoagulant surface, and clot retraction. The rapid progression of these different capacities causes the activated platelets to form a hemostatic plug that occludes the site of injury to prevent blood loss.Citation6 An increased risk of bleeding could be present when platelet count is reduced and/or one of their functions is defective. Conversely, improper thrombus formation could be due to a growth in platelet count or reactivity. In particular, activated platelets adhere and aggregate within atherosclerotic lesions, forming occluding arterial thrombi that may result in thromboembolic disease such as stroke or myocardial infarction, two of the major causes of morbidity and mortality in the Western world.Citation4,Citation7
These different functions of platelets may be reliably detected with a wide spectrum of tests. The inherited or acquired platelet dysfunctions have to be essentially diagnosed because a rapid identification of patients at risk of bleeding is really necessary; the monitoring of antiplatelet therapy is becoming increasingly important for the identification of hypo- or hyperresponder patients at risk of both thrombosis or hemorrhage; the management of perioperative hemostasis and the different aspects of platelet banking (concentrates, transfusions, and evaluation of donor platelet function) is of vital importance; and – last but not least – the physiopathology of platelets should be critically recognized by basic research with these tests.Citation8,Citation9
The history of platelet function testing (PFT) begins with the development of the evaluation of the bleeding time (BT) by the Duke procedure.Citation10 This was the first test for the assessment of the capacity of platelets to form a plug. For many years, it has been the unique screening test to identify both congenital or acquired platelet disorders.Citation11 In the 1960s, the revolutionary platelet aggregation test in platelet-rich plasma (PRP) – ie, light transmission aggregometry (LTA) – according to Born’s studies was the key method used to diagnose platelet function.Citation12 In this test, the ability of platelets to aggregate to each other in response to external aggregating agents – agonists, such as adenosine-diphosphate (ADP), arachidonic acid (AA), collagen, and epinephrine (EPI) – is measured in vitro.Citation13 Since the late 1980s, other PFT methods, such as platelet aggregometry in whole blood (WB), the study of activated platelets ex vivo by flow cytometry, the measurement of specific compounds released by platelets, and the assessment of platelet nucleotides have become available.Citation14–Citation16 Hence, PFT has been narrowly employed for the diagnosis and management of patients suffering from hemorrhagic problems.Citation17 In the last 2 decades, the clinical application of PFT has improved as it has increasingly come to be used for monitoring the antiplatelet treatment of cardiovascular patients at risk of arterial disease.Citation18 However, the increasing number of patients on antiplatelet drugs, with a higher risk of bleeding, especially during trauma and surgical procedures, has also led to the emergence of PFT as a useful tool in presurgical/perioperative settings for the prediction of hemorrhage and for monitoring the efficacy of different prohemostatic therapies. In this scenario, the development of new, simpler instruments for assessing platelet function at the point-of-care (POC) or bedside has led to better prospects of using PFT not only in specialized clinical or research laboratories, but also in general laboratories and in different clinical settings.Citation9,Citation18,Citation19
This review aims to describe the current status of available PFT that employs laboratory methodologies and point-of-care testing (POCT) with the pertinent instrumentation for different clinical settings. Different methodologies are employed both in the diagnosis of inherited and acquired bleeding disorders and in the monitoring of residual platelet reactivity of patients on antiplatelet treatment.
Platelet function methods
In recent years, the assessment of platelet (dys)function has become increasingly necessary in a variety of clinical settings: 1) for the identification of patients with bleeding disorders; 2) for monitoring the response to antiplatelet treatment; 3) in the evaluation of perioperative hemostasis; and 4) in transfusion medicine. Because there are diverse employment fields in which platelet function is studied and the platelets present a plethora of functions, different methodologies have been developed. Indeed, the platelet methods are based on different operating principles, and few assays are able to investigate “all in one device” platelet activation pathways. The different modalities of devices to operate may be based on the assessment of platelet adhesion and aggregation, on the submission of platelets under special shear conditions, on the analysis of physical properties of clot, and on the measurement of platelet compounds.Citation8,Citation9 In addition to the wide set of assays and devices for studying platelet function, because platelets are cells that may be easily activated during the blood sampling, a number of preanalytical variables can produce platelet artifacts affecting the different platelet functions. Therefore, a high degree of experience and expertise is necessary to perform and interpret PFT. In this sense, this review attempts to make a comparison of the various platelet function methodologies on the basis of the same principle that both are laboratory tests or POCT. The different platelet function tests reported in this review are grouped by the various methodological principles in , and the pros and cons of some platelet tests are documented in .
Table 1 Different methodologies for assessment of platelet function
Table 2 Pros and Cons of reviewed platelet function tests
Bleeding time
BT is the first test designed for evaluating in vivo primary hemostasis.Citation10 BT assesses the platelet ability to develop a hemostatic plug by recording the time that the platelets take to occlude an in vivo skin wound for stopping the hemorrhage.Citation20 Although the technique is easy and quick to perform without any WB processing, it is influenced by different variables such as differences in skin thickness and temperature among patients and an incorrect management of the test procedure. Nevertheless, the use of available devices to standardize the test, a lack of accuracy, and unclear association with clinical patient state persist. No study has clearly established the ability of BT evaluation to predict the risk of bleeding in patients, and a study only reported that BT could predict clinical bleeding in patients with acute myocardial infarction undergoing thrombolytic therapy.Citation21,Citation22 Moreover, this test is not used routinely to monitor the effect of antiplatelet therapy.Citation23 Nevertheless, BT is still regarded as a screening test to identify both congenital and acquired disorders of primary hemostasis in those laboratories that do not perform other platelet function tests.
Tests based on platelet aggregation
Light transmission platelet aggregometry on platelet-rich plasma
LTA, designed by Born in the 1960s, is performed by using the PRP as milieu.Citation12,Citation24 It is still deemed the gold standard test for the assessment of the various platelet functions. Indeed, by adding a wide panel of agonists to PRP, a considerable amount of data are obtained about the various pathways of platelet activation.Citation13
This test assesses in vitro the platelet-to-platelet clump formation in a glycoprotein (GP) IIb/IIIa-dependent manner, ie, the aggregation, the most important function of platelets. The assay is based on the measurement of the increase in light transmission through the optically dense sample of PRP after the addition of the exogenous platelet agonist. During the assay, the PRP after the addition of agonist becomes clearer because of the precipitation of platelet aggregates. This determines an increase in light transmission through the plasma sample. The device records the rate and maximal percentage of this increase from 0% (maximal optical density of PRP) to 100% (no optical density of autologous platelet-poor plasma) by a photometer. This signal is converted automatically in a graphic curve that parallels the increase in light transmission during the platelet aggregation (). The available aggregometers are easy-to-use devices equipped with automatic setting (100% and 0%), software for storing results and disposable cuvettes with a stirring bar. The slope of the curve, the maximal extent of aggregation (%), and the latency time (lag phase) are the parameters automatically measured, and the shape change and primary and secondary aggregation may be seen graphically. To the PRP sample, different agonists are added to stimulate different platelet activation pathways, obtaining information about the several features of platelet function.
Figure 1 Light transmission platelet aggregation tracings from a healthy subject in response to different agonists.
Abbreviation: ADP, adenosine-diphosphate.
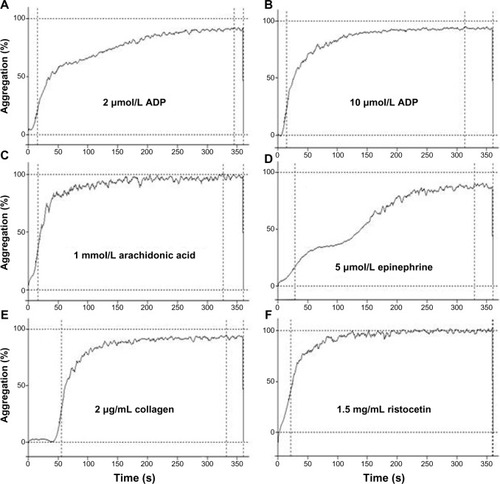
Born’s platelet aggregometry is the most widely employed methodology for detecting platelet function disorders and monitoring antiplatelet therapies. Actually, it is deemed the first step in the flowchart for the study of hemorrhagic patients with inherited or acquired platelet dysfunctions.Citation25–Citation29 At the first sign of congenital/acquired bleeding disorders, in addition to the most commonly used agonists ADP, AA, collagen, and epinephrine, other agonists should be employed: thrombin receptor activating peptide (TRAP), Thromboxane A2 (TXA2) mimetic U46619, calcium ionophore A23187. The antibiotic ristocetin is another agent used as agonist in the assessment of platelet function. It facilitates the binding of Von Willebrand Factor (VWF) to the glycoprotein Ib/IX/V complex. Different concentrations of ristocetin are used: this way of dose–response allows one to investigate both increased and decreased sensitivity to ristocetin. For a normal result, both functional VWF and normal glycoprotein Ib/IX/V complex must be present. Hence, ristocetin-induced platelet aggregation is considered a test that can detect both VW disease and platelet dysfunctions such as Bernard–Soulier syndrome.Citation29
Monitoring antiplatelet therapies (eg, aspirin and thienopyridine) by using LTA has permitted the prediction of major adverse cardiovascular events (MACE) in cardiovascular patients at high risk. The rate of residual platelet reactivity defined by ADP-, AA-LTA, or both has been associated with the development of ischemic events both in acute coronary syndrome (ACS) patients and in those with stable coronary artery disease. Not only the previous evaluation of degree of platelet inhibition in stable cardiovascular artery disease (CAD) patients, but also the laboratory and clinical research of a cut-off value for ADP and/or AA-induced platelet aggregation have been instrumental in identifying those ACS patients who are nonresponders to antiplatelet agents and are at high risk of MACE.Citation30–Citation33
Although LTA is accepted as the most important and complete assay that clinical laboratories can perform to diagnose platelet function disorders, this assay presents some peculiar problems. Indeed, the technique may be affected by different preanalytical conditions (ie, type of anticoagulant, lipid plasma, hemolysis, or low platelet count), and by different procedural conditions (ie, PRP preparation, use of different concentrations of agonists), and the laboratory staff should have a high degree of skill, experience, and expertise in performing and interpreting platelet function. Hence, this method is constantly verified by an ongoing standardization process,Citation25,Citation34,Citation35 and recent specific guidelines for LTA aimed at stabilizing/normalizing the correct procedure have been published.Citation36–Citation39
Finally, LTA test is the first diagnostic step in the evaluation of platelet disorders as reported by different studies.Citation25,Citation28 Because platelet dysfunction may be caused by a plethora of factors, for achieving a diagnostic hypothesis, the results obtained by LTA should be confirmed by further specific tests. By using flow cytometry analysis for the identification of expression of specific platelet components and lumiaggregometry for the identification of impaired platelet secretion or the Platelet Function Analyzer-100 (PFA-100) system, performed as a second diagnostic, LTA as a first-step diagnostic tool can be specifically confirmed.Citation28
Impedance whole blood aggregometry
Impedance whole blood aggregometry (WBA) allows one to assess platelet function by using the anticoagulated WB as milieu without any sample processing.Citation40 It is based on the principle that activated platelets stick via their surface receptors to artificial surfaces of two electrodes within the WB sample positioned at a determined distance between them. Platelet aggregation is assessed by detecting the increase in electrical impedance generated by the aggregation of other platelets upon those fixed to the electrodes. Hence, by diminishing the current intensity, the electrical impedance increases. The degree of the increase in impedance is recorded in Ohms.Citation14
By the utilization of WB aggregometry, platelet function is assessed under more physiological conditions given that the contributions of other blood elements also may affect platelet function. Another key aspect is that WB aggregometry takes place on surfaces, which enables this assay to be more adherent to the actual capacity of platelets to adhere and aggregate upon a solid phase. Moreover, WBA presents two important advantages: 1) use of a small quantity of WB in which all subpopulations of platelets are present; 2) no manipulation of the sample without activation of platelets, resulting in a rapid analysis of platelet function.
Now, the Multiple Electrode Aggregometry (MEA) is a new methodology by which the WB platelet aggregation can be assessed by using a new pertinent device that allows one to consider this assay as a POCT.Citation41 Recently, this new aggregometer (Multiple Platelet Function Analyzer – Dynabyte – Roche Diagnostics, Mannheim, Germany) has become available across the world. The device presents particular features that make it a valid tool for rapid and complete platelet function evaluation. It is a five-channel computerized instrument equipped with disposable cuvettes ready to use with two independent sensor units and an automated pipetting. The reagents – agonists and diluents – are ready to use. The platelet aggregation is simultaneously measured in duplicate by using each sensor unit separately and calculated automatically as area under curve (AUC). With these important advantages MEA has acquired a high clinical value like that of LTA, and the use of different agonists similar to those used for LTA has made it suitable for the diagnosis of bleeding diathesis and for monitoring antiplatelet therapies, defining cut-off values to discriminate cardiovascular patients with high on-treatment platelet reactivity.Citation42–Citation49 MEA has been used to study the occurrence of VWD in patients with severe aortic stenosis and, on the other hand, the high thrombotic risk due to heparin-induced thrombocytopenia (HIT).Citation50,Citation51
In addition, MEA has not only been able to identify cardiovascular patients not responding to antiplatelet agents and at risk of MACE, but has been considered a skill assay to distinguish those patients with a high inhibition of platelet function and at risk of bleeding.Citation52–Citation54 Ranucci et alCitation55 reported that investigating platelet function by using MEA before cardiac surgery has made it possible to identify the hyper-responder patients at risk of postoperative bleeding. Recently, it was reported that low extent of TRAP-induced platelet aggregation by MEA was defined as a factor independently associated with intramyocardial hemorrhage in patients with myocardial infarction.Citation56 Actually, multiple evidence reveals that MEA might be able to identify pre-/postoperatively those patients at risk of blood loss. Hence, this platelet function test has been proposed as a rapid and useful tool for the management of postoperative severe bleeding.Citation56–Citation58
MEA, overcoming the traps that LTA presents, may allow the rapid assessment of the platelet function without the requirement of a specialized laboratory. This POC system requires minimal technical knowledge and training to perform accurately, the working steps are automatically accomplished and easily controlled, and only interpretation data and judgment are required. Probably, in the future this test will be able to have a similar clinical value as of LTA with the advantage of its use in general laboratories.
Lumiaggregometry
Lumiaggregometry allows simultaneous measurement of the release of adenine nucleotides from platelet granules and platelet aggregation.Citation16,Citation59–Citation61 The method is based on the evaluation of adenosine triphosphate (ATP) released from activated platelets by different agonists by using a luminescence technique in PRP, washed platelets (WP), or WB.Citation62–Citation65 The assay is based on the conversion of ADP, released from the platelet dense granules, to ATP that reacts with the luciferin–luciferase reagent. The light emitted, proportional to the ATP concentration, is quantified by the lumi-aggregometer. This technique is used for evaluating specific deficiencies in the number and content of dense granules: the so-called storage pool defects or specific defects in degranulation.Citation8,Citation9,Citation62–Citation65 As with LTA, this analysis might be used as a first screening test of patients with clinical suspicion of platelet function defects such as abnormalities of platelet granule secretion and/or content, plasma membrane receptor defects, and to evaluate platelet functions during thrombocytopenia.Citation28,Citation66,Citation67
VerifyNow system
The VerifyNow system (ITC, Edison, NJ, USA) is a platelet function-waived POCT consisting of a device that assesses in WB platelet aggregation by a turbidimetric-based optical detection and using a system cartridge containing fibrinogen-coated beads and platelet agonists.Citation68 The method is based on the capacity of activated platelets to bind to fibrinogen: platelets aggregate upon the fibrinogen-coated beads within the assay cartridge in proportion to the number of activated GPIIb/IIIa receptors. The crossing WB optical signal increases as the activated platelets aggregate to the fibrinogen attached to the spheres. The use of this closed system requires no blood manipulation and instrument handling: actually, the methodology is very waived and it is largely employed to monitor antiplatelet therapies in the emergency cardiac operating room without the help of a specialized laboratory.Citation54 Formerly, the method (called Ultegra) was used to verify the inhibitory effect of platelet GPIIb/IIIa antagonists (abciximab or eptifibatide) in cardiovascular patients undergoing percutaneous coronary intervention (PCI): in this assay (VerifyNow IIb/IIIa Test), the thrombin receptor activating peptide (iso-TRAP) is used as agonist to maximally stimulate platelet aggregation.Citation69 The device reports patient results as Platelet Aggregation Units (PAU) calculated as rate and degree of aggregation. Now, two more assays each sensitive to targeted drugs are available: Aspirin Test with AA as agonist (sensitive to aspirin therapy), whose results are expressed as Aspirin Reaction Units (ARU); and PRUTest (sensitive to thienopyridine treatment), whose results are expressed as P2Y12 Reaction Units (PRU), by using ADP as agonist and PGE1 as suppressor of intracellular free calcium levels for diminishing the nonspecific influence of the ADP-binding to P2Y1 receptors. The VerifyNow system showed a moderate agreement with other platelet function tests. The assessment of the sensitivity and specificity of the method and the definition of a cut-off value for classifying patients at high risk of MACE and not responsive to acetylsalicylic acid (ASA) or thienopyridines have been debated and different laboratories and clinical studies have reported the clinical value of this system.Citation70–Citation77 Recently, in surgical settings, the use of the VerifyNow system in patients on antiplatelet agents has been expanding for attempting to predict postoperative hemorrhage.Citation78,Citation79
Plateletworks system
This system is a WB POC assay based on the measurement of platelet count before and after aggregation. This system consists of the Plateletworks aggregation kit formed by EDTA tubes and citrate tubes implemented with agonists (ADP or AA) and the Ichor blood counter (Helena Laboratories, Beaumont, TX, USA).Citation80 The Plateletworks method matches the platelet count assessed in the control sample (EDTA tube) with those obtained after aggregation in citrate blood with ADP or AA (citrate tube plus agonist). Since platelets aggregate, the fall in their count measured in citrate tubes is the extent of activated platelets that have aggregated themselves. This rapid test does not need any manipulation of blood sample, and results are ready in minutes. A disadvantage of the test is that it should be performed within a few minutes of blood sampling. The need for this strict time interval has relegated this analysis to restricted operating areas such as cardiac surgery and cardiac operating room. Relationships between Plateletworks and LTA, VerifyNow system and Thromboelastography have been reported as validation test for monitoring antiplatelet therapy.Citation81–Citation83 In acute care condition, Plateletworks shows the clinical value of giving information about both platelet count and function.Citation84,Citation85 Nevertheless, it is still under consideration and has not been reported to predict clinical outcomes.
Tests based on platelet adhesion under shear stress
The Platelet Function Analyzer – PFA-100/Innovance PFA-200
The Platelet Function Analyzer – PFA-100/Innovance PFA-200 is a PFT that uses a device called the PFA-100 or the new available and updated Innovance PFA-200 (Siemens, Munich, Germany). At the beginning, PFA-100 has been considered as the standardization of BT.Citation86,Citation87 The method is based on the property of platelets to adhere upon shear stress conditions and aggregate in consequence of agonist presence in the system. This methodology is considered a POCT that assesses platelet function in WB using appropriate cartridges in which primary hemostasis is simulated.Citation88–Citation90 Two different test-cartridges containing either collagen (C) plus ADP – CADP cartridge – or collagen plus epinephrine (EPI) – CEPI cartridge – are available. Within the cartridge, the citrated WB is drawn at a high shear stress rate through a capillary that has at its end a collagen-coated membrane, in which a defined microscopic aperture (147 μm) is present, filled with either ADP or EPI. A platelet clot, which fills the hole, occurs because of shear stress and agonists. The time taken by platelets to occlude the orifice and to block the WB flow is defined as closure time (CT), a measure of overall platelet-related hemostasis. The CT is as much prolonged as the platelets are able to act.
The use of two different assay cartridge allows to differentiate platelet defects due to intrinsic or platelet defects (prolonged times of CADP cartridge alone or plus CEPI cartridge) such as VW defects or Bernard–Soulier syndrome or Glanzmann’s thromboasthenia from those due to antiplatelet therapy with ASA (prolonged time of CEPI cartridge).Citation70,Citation90–Citation93 In comparison with BT test, this assay has been shown to be more sensitive,Citation70,Citation89 especially for diagnosis of VWD and platelet function defects.Citation91 Recently, a third diagnostic, the new Innovance cartridge, sensitive to the thienopyridine therapy, has become available, but information on its clinical value is poor and limited.Citation94
The PFA-100 is sensitive to many variables that influence platelet function – prolonging the CT – as well as low platelet count and hematocrit, conditions associated with thrombocytopathies. Thus, the clinicians should take these limitations into account. On the other hand, factors such as high levels of VWF, fibrinogen, or erythrocytes have been reported to shorten CEPI CT.Citation95,Citation96 In addition, a short CEPI CT could reveal high residual platelet reactivity despite aspirin therapy, thereby predicting the risk of thromboembolic events.Citation97,Citation98 Indeed it has been demonstrated in ACS patients on ASA treatment that a high concordance exists between LTA and the PFA-100 CEPI test, giving significant negative predictive value to the PFA system.Citation65 In addition, PFA CEPI CT shortened results were demonstrated to be a significant and independent predictor of MACEs in patients with acute myocardial infarct (AMI) undergoing primary PCI.Citation99–Citation101 In patients undergoing different kinds of elective surgeries, the assessment of platelet dysfunction with PFA-100 may provide useful information for postoperative blood transfusion management.Citation102,Citation103 Especially in cardiac surgery, PFA methodology showed a high predictive value of platelet function for management of intra- and postoperative blood loss.Citation104–Citation107 In patients with biventricular assist device implantation, on treatment with clopidogrel, the use of CADP diagnostics for monitoring the degree of platelet inhibition allowed them to undergo transplantation without major blood loss.Citation108 In addition, prolonged CADP CTs were found be independent risk factors for postpartum hemorrhage (PPH) severity and were significantly related to menorrhagia.Citation109,Citation110 Presurgical prolonged CTs corrected with 1-deamino-8-D-arginine vasopressin (DDAVP), ie, desmopressin treatment, made it possible to keep unchanged the quantity of postoperative blood transfusions with respect to those provided to patients with normal presurgical CTs.Citation102
It has been recommended that the PFA system be employed as a screening tool integrated into a panel of existing tests.Citation28,Citation38 This assay is characterized by a high negative predictive value: when a normal CT result is found in a suspected platelet disease, further PFT should not be performed.Citation5
IMPACT: Cone and Plate(Let) Analyzer
IMPACT: Cone and Plate(Let) Analyzer (Image Analysis Monitoring Platelet Adhesion Cone and Plate Technology) (CPA) (DiaMed, Cressier, Switzerland) is a novel and innovative POC test that fully assesses platelet function by using an automatic and computerized system that evaluates in vitro primary hemostasis.Citation111 The test is based on the in vitro activation and adhesion of platelets that lie all over a standardized plate covered by a substrate of polystyrene. The shear stress force is impressed to platelets by the spinning of a cone on the plate. Citrated WB is used in the system that is formed by the reaction device – cone and plate – an automatic staining device and image analysis software. After automated staining, the percentage of the well surface covered by platelet aggregates – representing platelet adhesion – and the average size of the aggregates (per μm2) – representing platelet aggregation – are measured.
This system is highly dependent on plasma. This instrument methodology could be a consistent system for the diagnosis of platelet defects. Moreover, the addition of the agonists AA and ADP in the system has made it possible to monitor and evaluate the efficacy of dual antiplatelet therapy.Citation112,Citation113 However, additional studies should be undertaken to assess its possible role in the diagnosis of inherited or acquired platelet dysfunctions.
The global thrombosis test
The global thrombosis test (GTT) (Montrose Diagnostics Ltd, London, UK) is a recent test that assesses platelet function in a manner close to physiological conditions because this technique is performed by using native nonanticoagulated WB, without adding agonists.Citation114–Citation116 The principle on which it is based is platelet activation due to high shear stress, opportunely provoked, similar to that in coronary artery stenosis. In this new system, the WB flows into a plastic tube that holds a conical part in which two ceramic balls are placed. The complete occlusion of the tube lumen due to the presence of the spheres is prevented by the flat segment created along the inner wall of the tube. WB flows under the force of gravity through the four narrow gaps of the first ball where high shear stress activates platelets. In the space underlying the first ball, low shear and turbulent flow support platelet aggregation. The platelet plug occludes the gaps at the level with second ball at the end tube and the WB exits always more slowly in the form of drops. In the first phase, the occlusion of the tube system, expressed as occlusion time (OT) in seconds, occurs. The time of interval (d) between two consecutive drops is recorded until an arbitrary endpoint (d ≥15 seconds) is reached. In the second phase, the blood flow resumes at the end of the tube owing to spontaneous lysis of occlusion expressed as lysis time (LT) in seconds. This rapid POC test provides results on the patient’s thrombotic status, and it is thus employed in acute and critical care settings, as well as in more general screening.Citation116–Citation118 The evaluation of GTT results in association with clinical outcomes is still in progress. However, because GTT presents a double clinical value, this test might play a role in the future for the assessment of platelet function.Citation115,Citation119
Platelet function methods in conjunction with viscoelastic methodologies
TEG platelet mapping system and ROTEM platelet test
Thromboelastography and thromboelastometry are methodologies employed for the global assessment of the hemostatic process. These assays investigate the entire development of clot formation and are based on the analysis of WB modifications in viscoelastic forces during the clotting formation. Indeed, the potential of this methodology is related to assessing the extent of platelet count and function, clotting, and fibrinolytic activation.Citation120 In these tests, platelets play all their functional roles in hemostasis: thrombin generation, clot formation, clot retraction, and lysis.
At present, three principal systems are available: Thromboelastography, performed on “old” renewed devices (TEG; Haemoscope, IL, USA), Thromboelastometry, formerly called Rotational Thromboelastography, performed on a new device (ROTEM; TEM Int, Munich, Germany), and Sonoclot analysis performed on a new device (Sonoclot Signature; Sienco, CO, USA). The principle underlying the method for TEG and ROTEM consists in a rotating system inclusive of a pin suspended by a torsion wire in a cup. In the WB sample, owing to the addition of reagents and the shear stress of the rotating system, the forming clot entraps the pin promoting a motion that increases as the clot strengthens and decreases as the clot lyses. In the Sonoclot device in the cup, the pin is moved up and down at an ultrasonic rate.Citation121
The in vitro addition of specific reagents and activator to WB provokes the hemostatic activation followed by modifications in hematic viscoelastic forces: the entire process is instantly recorded during all clotting formation and also visualized in a typical curve. Different tests are available depending on the reagent used: tissue factor for extrinsic pathway, different activators (glass, silica, kaolin, ellagic acid) for intrinsic pathway. Those additional compounds are used for revealing particular aspects of hemostasis: heparinase for evaluating the degree of anticoagulation of heparin, aprotinine for evaluating the activation of fibrinolysis, and antiplatelet agents (cathepsin G or GPIIb/IIIa antagonists) for eliminating the contribution of platelets to clotting formation. For each test, different parameters, indicating the different steps of hemostasis, are shown after the recognition and computation by the integrated software detection: K index (TEG) and Clot Formation Time (CFT, ROTEM) recognizes platelet function, Angle alpha (for both) indicates the rate of fibrin-formation, Maximal Amplitude (MA, TEG) and Maximum Clot Firmness (MCF, ROTEM) indexes show the platelet contribution to clot formation. In addition, by the selective activation of extrinsic pathway without and with antiplatelet agents (ie, clotting by fibrinogen alone), it is possible to evaluate the extent of platelet contribution to the clot formation. Because all this instrumentation promptly delivers a graphic representation of clot formation and lysis, they are now used as POC devices in different clinical settings such as cardiac surgery, liver transplantation, obstetrical emergency, and trauma center.Citation122–Citation135 To date, the clinical value of this technology has consisted in offering a predictive evaluation of the risk of increased postoperative bleeding. For surgical and emergency settings, different studies have documented flow charts in which are reported when to use these systems and how to interpret their results in patients at risk of bleeding, such as those on antiplatelet treatment. Actually, it has now been identified how the use of the different parameters provided by these tests may be predictors of both postoperative bleeding and blood product use.Citation136–Citation138
Currently, new applications for the study of platelet function are available. The TEG platelet mapping system is a modification of the original TEG and has been developed for monitoring antiplatelet therapy.Citation139–Citation142 First, a kaolin-activated test is performed to evaluate the maximal hemostatic activity. Second, a test in the presence of reptilase and Factor XIIIa (activator F) is used to produce a cross-linked fibrin clot. The addition of ADP or AA stimulates differentially the role of platelet ADP or TXA2 receptors in clot formation. The effect of therapy with aspirin (AA addition) or thienopyridines (ADP addition) is evaluated by comparing the TEG kaolin-activated test curve with the AA or ADP-stimulated TEG curve. The method has been shown to be reliable with low analytical variation.Citation143,Citation144 However, further large prospective studies should be performed in order to define the possible role of these devices in monitoring antiplatelet therapy.
On the other hand, the ROTEM platelet system is a new module that could be added to the ROTEM. A WB aggregometer as an integrated module has been added to the ROTEM system. This allows a deeper analysis of the primary hemostasis of the patient along with that of coagulation. The measurement is based on true impedance aggregometry (such as the MEA system), which allows the measurement of platelet aggregation in WB samples. The software for the analysis data is the same as of the ROTEM system. In this manner, the platelet function analysis is visualized together with the thromboelastometric measurements, altogether on the same screen; in fact, the two different assays (both thromboelastometry and platelet aggregometry) can be run together in the same time on the same system. Some characteristics of the ROTEM platelet are similar to those of MEA, such as single-use cuvettes, time of test, measured parameters, and agonists used. Because the availability of this assay is very recent – it goes back to a few months ago – only a few data are currently available about its laboratory and clinical value.Citation145
Platelet analysis based on flow cytometry
Flow cytometry (FC) is a technique of rapidly measuring specific characteristics of many different cells such as platelets, measuring the cell size, and granularity. FC analysis of platelets may offer information on their functional status in vivo, and this technique encompasses multiple assays for several purposes such as the assessment of the activation state (platelet membrane-associated IgG), evaluation of thrombopoiesis, diagnosis of specific disorders, and antiplatelet agent monitoring.Citation146–Citation148
This analysis is based on the optical and fluorescence evaluation of physical – such as size and internal complexity – and antigenic properties of platelets, ie, determination of surface receptors including conformational changes related to the receptor activation, platelet granule secretion, presence of platelet aggregates, and leukocyte–platelet aggregates.Citation149 It consists of the measurement of antibodies conjugated to fluorescent dyes that may bind specific proteins on cell membranes or inside cells, revealing their presence. A light source, crossing labeled platelets, excites to a higher energy state the fluorescent molecules of dyes. When the fluorochromes return to a resting state, they emit light energy at different wavelengths. The use of multiple fluorochromes with similar excitation wavelengths, but different emission wavelengths (or “colors”), allows several cell properties to be measured simultaneously. Different fluorochromes such as fluorescein isothiocyanate (FITC) or phycoerythrin (PE) can be used, but also a specie-specific secondary antibody coupled to a fluorochrome can be employed to recognize a primary antibody linked to surface antigens.Citation15 In the WB, the use of a double labeling binding allows the identification of platelets, platelet microparticles, or mixed cell aggregates.Citation150–Citation152 The results of FC are represented in the form of histograms with mean fluorescence intensity (MFI) plotted against cell number.
A plethora of platelet defects and features can be investigated by FC. Indeed, it can be a useful tool for the diagnosis of inherited (ie, Bernard–Soulier syndrome, Glanzmann’s thrombasthenia) or acquired platelet dysfunctions (ie, HIT) and storage pool disease. As this analysis is able to assess platelet function defects in patients with very low platelet count, it is considered the test of choice to define the presence of thrombocytopathy in a thrombocytopenic patient.Citation9 In this regard, an FC for the diagnosis of thrombocytopenic disorders has been developed to measure reticulated platelets.Citation153 In addition, FC allows one to recognize the procoagulant capacity and activation state of platelets (eg, in the setting of acute coronary syndromes or cardiopulmonary bypass), by highlighting the expression of phosphatidylserine on activated platelet membranes using labeled annexin V.Citation154
Different steps in intracellular signal transduction can be investigated by FC. Indeed, the intracellular quantification of Vasodilator Stimulated Phosphoprotein (VASP) phosphorylation by FC is the most used method for monitoring the efficacy of antiplatelet drugs.Citation155,Citation156 Finally, FC is used to evaluate the state of stored platelets in blood banks. For example, it is possible to assess the platelet activation state and identify leukocyte contamination of platelet concentrates for the evaluation of the efficacy of platelet transfusion.Citation157–Citation159
FC has many advantages, such as the use of small volumes of blood samples and the analysis of platelets in physiological milieu (WB). On the other hand, FC analysis is an expensive test, requiring specialized operators and devices, and the preanalytical phase can be affected by the delicate preparation prone to artifact unless carefully prepared.Citation8,Citation9
Evaluation of Thromboxane metabolites
In physiological conditions, when platelets are stimulated by agonists, TXA2 is rapidly synthesized and released from platelets at the site of vascular injury. TXA2 amplifies the platelet activation, recruits additional platelets to the site of clot formation, and induces vasoconstriction, accelerating the hemostasis process.Citation160 TXA2 is the major product of the platelet metabolism of AA. AA, released from platelet membrane phospholipids via phospholipase A2 (PLA2), is converted in the endoperoxides, prostaglandin G2 (PGG2) and PGH2, via cyclooxygenase-1 (COX-1) and finally transformed into TXA2 by thromboxane synthase. TXA2 is rapidly transformed by hydrolysis into TXB2, a biologically inactive and stable product.Citation160,Citation161 TXB2 is excreted unchanged in urine in a small percentage being prevalently transformed into two major metabolites: 2,3-dinor-TXB2 and in 11-dehydro-TXB2 by β-oxidation and dehydration, respectively. These metabolites circulate in the plasma at low concentrations and are excreted in urine, and it has been established that 11-dehydro-TXB2 is more abundant in urine than 2,3-dinor-TXB2.Citation162,Citation163 In inflammatory conditions, TXA2 can be produced by other biochemical pathways independently from platelets and extraplatelet sources of TXA2 can become relevant.Citation164 Conversely, ASA interfering with COX-1 activity causes a decrease in the concentration of serum TXB2 and urinary 11-dehydro-TXB2.Citation165,Citation166
The measurement of TXA2 metabolites allows evaluation of the activation state of platelets.Citation8,Citation9 Serum and urine TXB2 metabolites concentrations are related to the entity of TXA2 biosynthesis, and their dosage may be useful to assess platelet function in different diseases, to detect defects of thromboxane production, and to monitor aspirin therapy.Citation8,Citation9,Citation167,Citation168 Different ligand-binding assays based on the binding of the analyte to specific receptors by using different detection methods can be used to assess serum TXB2 and urinary 11-dehydro-TXB2 concentration, such as: 1) the “old” radioimmunoassays (RIA) or immunoradiometric assays (IRMA) that are now outdates;Citation169–Citation171 2) enzyme-linked immunoassays (ELISA) that are now the most commonly utilized assays.Citation172,Citation173
Future perspective
Different methodologies for the assessment of platelet (dys) function in order to screen different idiopathic or acquired pathological conditions – hemorrhagic and/or prothrombotic status – are currently available. At present, platelet tests are mostly used, thanks to the work put into the standardization of these tests, in the drawing up flowchart and to the introduction of primary hemostasis POCT. These available tests allow either the study of global platelet function or the evaluation of particular aspects of platelet activation. Primary hemostasis POC tests simultaneously may assess in vitro platelet adhesion and aggregation; platelet aggregation analysis is inclusive of platelet secretion and aggregation phenomena. In particular, in WB platelet aggregation, the role of other blood cells is considered; viscoelastic methods analyzing global hemostasis are able to individuate platelet dysfunction ( and ). Despite this versatility and their important clinical value, platelet assays pertaining to the same category (eg, those based on platelet aggregometry) are again affected by a wide range of methodological aspects. The numerous conditions involved in them cause an interlaboratory variability of results and can delay a clear standardized procedure. This major gap is partly overcome by the introduction of platelet function POCT, which presents a basilar standardized procedure. In light of this, a new platelet adhesion test using microfluidic devices, for example, a biochip containing several different adhesions, is under evaluation.Citation174,Citation175 Now, platelet spreading tests, using fluorescence microscopy or scanning electron microscopy, are frequently employed.Citation176 On activation, platelets are able to release or express several compounds such as ATP, ADP, 5-HT, CXCL12, CD34, P-selectine, or thromboxanes, which may be assessed by using different methodologies such as immunological assay, High-performance liquid chromatography, fluorescence microscopy, or flow cytometry.Citation28,Citation177,Citation178 The major drawback of the application of these assays in clinical practice is the scarcity and high variability of clinical and laboratory data and the absence of clear indications or guidelines for the correct use of such tests. Either for the study of global or single step of platelet function or for the definition of the clinical value of new platelet biomarkers, new or renewed platelet assays with high sensitivity and specificity are considered necessary, especially for routine laboratory analysis. In the future, it is desirable that specific, standardized, more rapid and easy tests – whose clinical value has been well defined – are available.
Conclusion
To date, the effort of translating the different PFTs as diagnostic tools for evaluating bleeding disorders and monitoring antiplatelet therapies is in progress. Actually, the available platelet function POCT is making possible the institution of platelet tests in laboratories and intensive care units, allowing their use in different clinical settings such as inherited bleeding disorders, cardiovascular intensive care, trauma coagulopathy, liver transplantation, and obstetric care for the prediction of bleeding. Similarly, the use of these POC tests could be extended not only at the bedside in critical areas outside of the specialized laboratory, but also in centralized and satellite laboratories. Indeed, the upgrading of the WB test, such as MEA, or platelet mapping or the new ROTEM platelet device, may guide clinicians in making the correct diagnosis of bleeding risk or in properly tailoring the antiplatelet therapy directly in pre-/postoperative care.
Nevertheless, for the amelioration of the ongoing standardization of LTA, new procedures were reported in 2010, 2011, and 2013.Citation37–Citation39 For this, PRP or WB platelet aggregometry by using new systems might be realized into routine laboratories. Because the availability of platelet tests is an important improvement, the drafting of validation procedures and the study of reliability and quality control testing of PFT are becoming an increasingly important issue.
In conclusion, the new POCTs available may be considered useful supplements to the existing well-known platelet function tests, but further prospective studies are necessary to define the applications of these tests. In the future, the improvements in the study of platelet genome and proteome may encompass knowledge of platelet function testing with a noteworthy impact on the diagnosis and management of patients affected by hemorrhagic or thrombotic defects.
Author contributions
Paniccia R conceived and designed the review, prepared the manuscript, and surveyed the literature; Priora R contributed to the literature review and preparation of the manuscript; Alessandrello Liotta A contributed to the preparation of the manuscript; and Abbate R approved the version to be published. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- GeorgeJNPlateletsLancet20003551531153910801186
- KatzJNKolappaKPBeckerRCBeyond thrombosis: the versatile platelet in critical illnessChest201113965866821362652
- CaudrillierAKessenbrockKGillissBMPlatelets induce neutrophil extracellular traps in transfusion-related acute lung injuryJ Clin Invest20121222661267122684106
- BadimonLVilahurGThrombosis formation on atherosclerotic lesions and plaque ruptureJ Intern Med20142766618632doi: 10.1111/joim.12296.25156650
- HarrisonPAssessment of platelet function in the laboratoryHamostaseologie200929253119151842
- MichelsonADHow platelets work: platelet function and dysfunctionJ Thromb Thrombolysis20031671214760205
- RuggeriZMPlatelets in atherothrombosisNat Med200281227123412411949
- PakalaRWaksmanRCurrently available methods for platelet function analysis: advantages and disadvantagesCardiovasc Revasc Med20111231232221036109
- KehrelBEBroddeMFState of the art in platelet function testingTransfus Med Hemother201340738623653569
- DukeWWThe relation of blood platelets to hemorrhagic disease. Description of a method for determining the bleeding time and the coagulation time and report of three cases of hemorrahagic disease relieved by blood transfusionJAMA19105511851192
- RodgersRPLevinJA critical reappraisal of the bleeding timeSemin Thromb Hemost1990161202406907
- BornGVAggregation of blood platelets by adenosine diphosphate and its reversalNature196219492792913871375
- ZhouLSchmaierAHPlatelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the fieldAm J Clin Pathol200512317218315842039
- CardinalDCFlowerRJThe electronic aggregometer: a novel device for assessing platelet behavior in bloodJ Pharmacol Methods198031351587392654
- MichelsonADEvaluation of platelet function by flow cytometryPathophysiol Haemost Thromb200635678216855350
- HolmsenHHolmsenIBernhardsenAMicrodetermination of adenosine diphosphate and adenosine triphosphate in plasma with the firefly luciferase systemAnal Biochem1966174564735965982
- ColletJPMontalescotGPlatelet function testing and implications for clinical practiceJ Cardiovasc Pharmacol Ther20091415716919721130
- BonelloLTantryUSMarcucciRConsensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphateJ Am Coll Cardiol20105691993320828644
- DarlingCESala MercadoJAQuiroga-CastroWPoint-of-care assessment of platelet reactivity in the emergency department may facilitate rapid rule-out of acute coronary syndromes: a prospective cohort pilot feasibility studyBMJ Open201441e003883
- QuickAJThe bleeding time as a test of hemostatic functionAm J Clin Pathol19756487941080353
- PetersonPHayesTEArkinCFThe preoperative bleeding time test lacks clinical benefit: College of American Pathologists’ and American Society of Clinical Pathologists’ position articleArch Surg19981331341399484723
- GimpleLWGoldHKLeinbachRCCorrelation between template bleeding times and spontaneous bleeding during treatment of acute myocardial infarction with recombinant tissue-type plasminogen activatorCirculation1989805815882504511
- JakubowskiJAMatsushimaNAsaiFA multiple dose study of prasugrel (CS-747), a novel thienopyridine P2Y12 inhibitor, compared with clopidogrel in healthy humansBr J Clin Pharmacol20076342143017076696
- O’BrienJMPlatelet aggregation. II. Some results from a new method of studyJ Clin Pathol19621545245816810986
- Kottke-MarchantKCorcoranGThe laboratory diagnosis of platelet disordersArch Pathol Lab Med200212613314611825107
- HaywardCPDiagnostic approach to platelet function disordersTransfus Apher Sci200838657618291723
- HaywardCPPaiMLiuYDiagnostic utility of light transmission platelet aggregometry: results from a prospective study of individuals referred for bleeding disorder assessmentsJ Thromb Haemost2009767668419143930
- PoddaGFemiaEAPuglianoMCattaneoMCongenital defects of platelets functionPlatelets20122355256323020613
- GadisseurAHermansCBernemanZSchroyensWDeckmynHMichielsJJLaboratory diagnosis and molecular classification of von Willebrand diseaseActa Haematol2009121718419506352
- BreetNJvan WerkumJWBoumanHJComparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantationJAMA201030375476220179285
- BuonamiciPMarcucciRMiglioriniAImpact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosisJ Am Coll Cardiol2007492312231717572245
- ChenWHChengXLeePYAspirin resistance and adverse clinical events in patients with coronary artery diseaseAm J Med200712063163517602938
- LevEIAspirin resistance transient laboratory finding or important clinical entity?J Am Coll Cardiol20095367868019232900
- PanicciaRAntonucciEMagginiNLight transmittance aggregometry induced by different concentrations of adenosine diphosphate to monitor clopidogrel therapy: a methodological studyTher Drug Monit201133949821192314
- FemiaEAPuglianoMPoddaGCattaneoMComparison of different procedures to prepare platelet-rich plasma for studies of platelet aggregation by light transmission aggregometryPlatelets20122371021770862
- ChristieDJAvariTCarringtonLRPlatelet Function Testing by Aggregometry: Approved GuidelineWayne, PAClinical and Laboratory Standards Institute2008145
- HaywardCPMoffatKARabyADevelopment of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometryAm J Clin Pathol201013495596321088160
- HarrisonPMackieIMumfordAGuidelines for the laboratory investigation of heritable disorders of platelet functionBr J Haematol2011155304421790527
- CattaneoMCerlettiCHarrisonPRecommendations for the standardization of light transmission aggregometry: a consensus of the working party from the Platelet Physiology Subcommittee of SSC/ISTHJ Thromb Haemost20131111831189
- MackieIJJonesRMachinSJPlatelet impedance aggregation in whole blood and its inhibition by antiplatelet drugsJ Clin Pathol1984378748786206096
- TóthOCalatzisAPenzSLosonczyHSiessWMultiple electrode aggregometry: a new device to measure platelet aggregation in whole bloodThromb Haemost200696678178817139373
- PanicciaRAntonucciEMagginiNAssessment of platelet function on whole blood by multiple electrode aggregometry in high-risk patients with coronary artery disease receiving antiplatelet therapyAm J Clin Pathol200913183484219461090
- PanicciaRAntonucciEMagginiNComparison of methods for monitoring residual platelet reactivity after clopidogrel by point-of-care tests on whole blood in high-risk patientsThromb Haemost201010428729220458439
- SibbingDBraunSMorathTPlatelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosisJ Am Coll Cardiol20095384985619264241
- SibbingDMorathTBraunSClopidogrel response status assessed with Multiplate point-of-care analysis and the incidence and timing of stent thrombosis over six months following coronary stentingThromb Haemost201010315115920062919
- BeynonCSakowitzOWUnterbergAWMultiple electrode aggregometry in antiplatelet-related intracerebral haemorrhageJ Clin Neurosci2013201805180623830689
- WürtzMHvasAMChristensenKHRubakPKristensenSDGroveELRapid evaluation of platelet function using the Multiplate® AnalyzerPlatelets201425862863324246241
- ParkYJeongYHKimISThe concordance and correlation of measurements by multiple electrode and light transmittance aggregometries based on the pre-defined cutoffs of high and low on-treatment platelet reactivityPlatelets20122329029821942752
- KongRTrimmingsAHutchinsonNConsensus recommendations for using the Multiplate® for platelet function monitoring before cardiac surgeryInt J Lab Hematol Epub7232014
- BolligerDDell-KusterSSeebergerMDImpact of loss of high-molecular-weight von Willebrand factor multimers on blood loss after aortic valve replacementBr J Anaesth201210875476222311365
- Morel-KoppMCAboudMTanCWKulathilakeCWardCWhole blood impedance aggregometry detects heparin-induced thrombocytopenia antibodiesThromb Res2010125e234e23920053425
- SibbingDSchulzSBraunSAntiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placementJ Thromb Haemost2010825025619943882
- SolomonCHartmannJOsthausAPlatelet concentrates transfusion in cardiac surgery in relation to preoperative point-of-care assessment of platelet adhesion and aggregationPlatelets20102122122820158381
- TantryUSBonelloLAradiDConsensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleedingJ Am Coll Cardiol2013622261227324076493
- RanucciMBaryshnikovaESoroGBallottaADe BenedettiDContiDSurgical and Clinical Outcome Research (SCORE) GroupMultiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridinesAnn Thorac Surg20119112312921172499
- MalekLAKlopotowskiMSpiewakMPlatelet reactivity and intramyocardial hemorrhage in patients with ST-segment elevation myocardial infarctionClin Appl Thromb Hemost20132055355823344994
- Rahe-MeyerNWinterhalterMBodenAPlatelet concentrates transfusion in cardiac surgery and platelet function assessment by multiple electrode aggregometryActa Anaesthesiol Scand20095316817519175576
- GörlingerKShore-LessersonLDirkmannDHankeAARahe-MeyerNTanakaKAManagement of hemorrhage in cardiothoracic surgeryJ Cardiothorac Vasc Anesth201327Suppl 4S20S3423910533
- FritsmaGAPlatelet function testing: aggregometry and lumiaggregometryClin Lab Sci200720323717361966
- WhiteMMFoustJTMauerAMRobertsonJTJenningsLKAssessment of lumiaggregometry for research and clinical laboratoriesThromb Haemost1992675725771519217
- CattaneoMLight transmission aggregometry and ATP release for the diagnostic assessment of platelet functionSemin Thromb Hemost20093515816719408189
- PaiMWangGMoffatKADiagnostic usefulness of a lumiaggregometer adenosine triphosphate release assay for the assessment of platelet function disordersAm J Clin Pathol201113635035821846909
- HaywardCPRaoAKCattaneoMCongenital platelet disorders: overview of their mechanisms, diagnostic evaluation and treatmentHaemophilia200612Suppl 312813616684008
- PaiMHaywardCPDiagnostic assessment of platelet disorders: what are the challenges to standardization?Semin Thromb Hemost20093513113819408186
- McGlassonDLFritsmaGAWhole blood platelet aggregometry and platelet function testingSemin Thromb Hemost20093516818019408190
- BuyukasikYGokerHBuyukasikNSSayinalpNHaznedarogluICOzcebeOIEffect of platelet count on secretion capacity: formulization and use of the formulae for evaluation of platelet secretion in thrombo-cytopenic patientsBlood Coagul Fibrinolysis20081963363718832902
- GlembotskyACBluteauDEspasandinYRMechanisms underlying platelet function defect in a pedigree with familial platelet disorder with a predisposition to acute myelogenous leukemia: potential role for candidate RUNX1 targetsJ Thromb Haemost20141276177224606315
- SmithJWSteinhublSRLincoffAMRapid platelet-function assay: an automated and quantitative cartridge-based methodCirculation1999996206259950658
- SteinhublSRTalleyJDBradenGAPoint-of-care measured platelet inhibition correlates with a reduced risk of an adverse cardiac event after percutaneous coronary intervention: results of the GOLD (AU-Assessing Ultegra) multicenter studyCirculation2001103212572257811382726
- HarrisonPSegalHBlasberyKFurtadoCSilverLRothwellPMScreening for aspirin responsiveness after transient ischemic attack and stroke: comparison of 2 point-of-care platelet function tests with optical aggregometryStroke2005361001100515817896
- PanicciaRAntonucciEGoriAMComparison of different methods to evaluate the effect of aspirin on platelet function in high-risk patients with ischemic heart disease receiving dual antiplatelet treatmentAm J Clin Pathol200712814314917580282
- PanicciaRAntonucciEGoriAMDifferent methodologies for evaluating the effect of clopidogrel on platelet function in high-risk coronary artery disease patientsJ Thromb Haemost200751839184717723123
- BreetNJvan WerkumJWBoumanHJHigh on-treatment platelet reactivity to both aspirin and clopidogrel is associated with the highest risk of adverse events following percutaneous coronary interventionHeart20119798399021478385
- MarcucciRGoriAMPanicciaRCardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-upCirculation200911923724219118249
- PriceMJEndemannSGollapudiRRPrognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantationEur Heart J200829992100018263931
- JeongYHBlidenKPAntoninoMJParkKSTantryUSGurbelPAUsefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapiesAm Heart J2012164354222795280
- AngiolilloDJCurzenNGurbelPPharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: results of the SWAP-2 Study (Switching Anti Platelet-2)J Am Coll Cardiol2014631500150924333493
- RosengartTKRomeiserJLWhiteLJPlatelet activity measured by a rapid turnaround assay identifies coronary artery bypass grafting patients at increased risk for bleeding and transfusion complications after clopidogrel administrationJ Thorac Cardiovasc Surg20131461259126623953984
- YuPJCassiereHADellisSLManettaFSteinJHartmanARP2Y12 platelet function assay for assessment of bleeding risk in coronary artery bypass graftingJ Card Surg20142931231624588751
- CampbellJRidgwayHCarvilleDPlateletworks: a novel point of care platelet function screenMol Diagn Ther20081225325818652521
- LennonMJGibbsNMWeightmanWMMcGuireDMichalopoulosNA comparison of Plateletworks and platelet aggregometry for the assessment of aspirin-related platelet dysfunction in cardiac surgical patientsJ Cardiothorac Vasc Anesth20041813614015073699
- CraftRMChavezJJSniderCCMuenchenRACarrollRCComparison of modified Thrombelastograph and Plateletworks whole blood assays to optical platelet aggregation for monitoring reversal of clopidogrel inhibition in elective surgery patientsJ Lab Clin Med200514530931515976759
- WhiteMMKrishnanRKueterTJJacoskiMVJenningsLKThe use of the point of care Helena ICHOR/Plateletworks and the Accumetrics Ultegra RPFA for assessment of platelet function with GPIIB-IIIa antagonistsJ Thromb Thrombolysis20041816316915815877
- DalénMvan der LindenJLindvallGIvertTCorrelation between point-of-care platelet unction testing and bleeding after coronary artery surgeryScand Cardiovasc J201246323821973169
- van WerkumWKleibeukerMPostmaSA comparison between the Plateletworks™-assay and light transmittance aggregometry for monitoring the inhibitory effects of clopidogrelInt J Cardiol201014012312619091430
- KunduSKHeilmannEJSioRGarciaCDavidsonRMOstgaardRADescription of an in vitro platelet function analyzer – PFA-100Semin Thromb Hemost199521Suppl 2S106S112
- KoesslerJEhrenschwenderMKobsarABrunnerKEvaluation of the new INNOVANCE® PFA P2Y cartridge in patients with impaired primary haemostasisPlatelets20122357157822185369
- FavaloroEJClinical application of the PFA-100Curr Opin Hematol2002940741512172459
- MichelsonADFrelingerAL3rdFurmanMICurrent options in platelet function testingAm J Cardiol2006984N10N
- HarrisonPThe role of PFA-100 testing in the investigation and management of haemostatic defects in children and adultsBr J Haematol200513031015982339
- HaywardCPHarrisonPCattaneoMOrtelTLRaoAKPlatelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet functionJ Thromb Haemost2006431231916420557
- HomoncikMJilmaBHergovichNStohlawetzPPanzerSSpeiserWMonitoring of aspirin (ASA) pharmacodynamics with the platelet function analyzer PFA-100Thromb Haemost20008331632110739392
- MarcucciRPanicciaRAntonucciEResidual platelet reactivity is an independent predictor of myocardial injury in acute myocardial infarction patients on antiaggregant therapyThromb Haemost20079884485117938810
- EdwardsAJakubowskiJARechnerARSugidachiAHarrisonPEvaluation of the INNOVANCE PFA P2Y test cartridge: sensitivity to P2Y(12) blockade and influence of anticoagulantPlatelets20122310611521848368
- ChakrounTGerotziafasGRobertFIn vitro aspirin resistance detected by PFA-100 closure time: pivotal role of plasma von Willebrand factorBr J Haematol2004124808514675411
- ManniniLMarcucciRPanicciaRErythrocyte deformability and white blood cell count are associated with aspirin resistance in high-risk vascular patientsClin Hemorheol Microcirc20063517518116899924
- GianettiJParriMSSbranaSPlatelet activation predicts recurrent ischemic events after percutaneous coronary angioplasty: a 6 months prospective studyThromb Res200611848749316343603
- HovensMMSnoepJDEikenboomJCvan der BomJGMertensBJHuismanMVPrevalence of persistent platelet reactivity despite use of aspirin: a systematic reviewAm Heart J200715317518117239674
- MarcucciRPanicciaRAntonucciEUsefulness of aspirin resistance after percutaneous coronary intervention for acute myocardial infarction in predicting one-year major adverse coronary eventsAm J Cardiol2006981156115917056317
- RenyJLDe MoerloosePDauzatMFontanaPUse of the PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: a systematic review and meta-analysisJ Thromb Haemost2008644445018194417
- CrescenteMDi CastelnuovoAIacovielloLVermylenJCerlettiCde GaetanoGResponse variability to aspirin as assessed by the platelet function analyzer (PFA)-100. A systematic reviewThromb Haemost200899142618217130
- KoscielnyJvon TempelhoffGFZiemerSA practical concept for preoperative management of patients with impaired primary hemostasisClin Appl Thromb Hemost20041015516615094936
- RechnerARPlatelet function testing in clinical diagnosticsHamostaseologie201131798721152677
- RamanSSilvermanNAClinical utility of the platelet function analyzer (PFA-100) in cardiothoracic procedures involving extracorporeal circulationJ Thorac Cardiovasc Surg200112219019111436059
- CammererUDietrichWRampfTBraunSLRichterJAThe predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgeryAnesth Analg200396515712505922
- SuckerCLitmatheJFeindtPZotzRPlatelet function analyzer (PFA-100) as a useful tool for the prediction of transfusion requirements during aortic valve replacementThorac Cardiovasc Surg20115923323621412708
- SteinlechnerBZeidlerPBaseEPatients with severe aortic valve stenosis and impaired platelet function benefit from preoperative desmopressin infusionAnn Thorac Surg2011911420142621439546
- FriesDInnerhoferPStreifWCoagulation monitoring and management of anticoagulation during cardiac assist device supportAnn Thorac Surg2003761593159714602292
- ChauleurCCochery-NouvellonEMercierESome hemostasis variables at the end of the population distributions are risk factors for severe postpartum hemorrhagesJ Thromb Haemost200862067207418826390
- PhilippCSMillerCHFaizAScreening women with menorrhagia for underlying bleeding disorders: the utility of the platelet function analyser and bleeding timeHaemophilia20051149750316128894
- VaronDDardikRShenkmanBA new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditionsThromb Res1997852832949062952
- AnandSXKimMCKamranMComparison of platelet function and morphology in patients undergoing percutaneous coronary intervention receiving bivalirudin versus unfractionated heparin versus clopidogrel pretreatment and bivalirudinAm J Cardiol200710041742417659921
- ShenkmanBMatetzkySFeferPVariable responsiveness to clopidogrel and aspirin among patients with acute coronary syndrome as assessed by platelet function testsThromb Res200812233634518155752
- YamamotoJYamashitaTIkarugiHGörög thrombosis test: a global in-vitro test of platelet function and thrombolysisBlood Coagul Fibrinolysis200314313912544726
- YamamotoJInoueNOtsuiKIshiiHGorogDAGlobal thrombosis test (GTT) can detect major determinants of haemostasis including platelet reactivity, endogenous fibrinolytic and thrombin generating potentialThromb Res201413391992624613697
- SarafSWellstedDSharmaSGorogDAShear-induced global thrombosis test of native blood: pivotal role of ADP allows monitoring of P2Y12 antagonist therapyThromb Res200912444745119476973
- SarafSChristopoulosCSalhaIBStottDJGorogDAImpaired endogenous thrombolysis in acute coronary syndrome patients predicts cardiovascular death and nonfatal myocardial infarctionJ Am Coll Cardiol2010552107211520447533
- GorogDAFusterVPlatelet function tests in clinical cardiology: unfulfilled expectationsJ Am Coll Cardiol2013612115212923541972
- RosserGTricociPMorrowDPAR-1 antagonist vorapaxar favorably improves global thrombotic status in patients with coronary diseaseJ Thromb Thrombolysis20143842342924676931
- LuddingtonRJThrombelastography/thromboelastometryClin Lab Haematol200527819015784122
- GanterMTHoferCKCoagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devicesAnesth Analg20081061366137518420846
- GörlingerKDirkmannDSolomonCHankeAAFast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulabilityBr J Anaesth201311022223023112213
- WelsbyIJJiaoKOrtelTLThe kaolin-activated Thrombelastograph predicts bleeding after cardiac surgeryJ Cardiothorac Vasc Anesth20062053153516884984
- BolligerDTanakaKARoles of thrombelastography and thromboelastometry for patient blood management in cardiac surgeryTransfus Med Rev20132721322024075802
- DirkmannDGörlingerKDusseFKottenbergEPetersJEarly thromboelastometric variables reliably predict maximum clot firmness in patients undergoing cardiac surgery: a step towards earlier decision makingActa Anaesthesiol Scand20135759460323240733
- ArmstrongSFernandoRAshpoleKSimonsRColumbMAssessment of coagulation in the obstetric population using ROTEM® thromboelastometryInt J Obstet Anesth20112029329821835606
- OudghiriMKeitaHKouamouEReference values for rotation thromboelastometry (ROTEM®) parameters following non-haemorrhagic deliveries. Correlations with standard haemostasis parametersThromb Haemost201110617617821475775
- AfshariAWikkelsøABrokJMøllerAMWetterslevJThrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusionCochrane Database Syst Rev20113CD00787121412912
- MallaiahSBarclayPHarrodIChevannesCBhallaAIntroduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in majorobstetric haemorrhageAnaesthesia Epub1072014
- JohanssonPISølbeckSGenetGStensballeJOstrowskiSRCoagulopathy and hemostatic monitoring in cardiac surgery: an updateScand Cardiovasc J20124619420222375889
- JohanssonPIOliveriRSOstrowskiSRHemostatic resuscitation with plasma and platelets in traumaJ Emerg Trauma Shock2012512012522787340
- TanakaKABolligerDVadlamudiRNimmoARotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major traumaJ Cardiothorac Vasc Anesth2012261083109322863406
- SongJGJeongSMJunIGLeeHMHwangGSFive-minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantationBr J Anaesth201411229029724065728
- WangSCShiehJFChangKYThromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trialTransplant Proc2010422590259320832550
- RugeriLLevratADavidJSDiagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastographyJ Thromb Haemost2007528929517109736
- WikkelsoeAJAfshariAWetterslevJBrokJMoellerAMMonitoring patients at risk of massive transfusion with Thrombelastography or Thromboelastometry: a systematic reviewActa Anaesthesiol Scand2011551174118922092122
- AndersonLQuasimISoutarRStevenMMacfieAKorteWAn audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unitTransfus Med200616313916480437
- SpaldingGJHartrumpfMSierigTOesbergNKirschkeCGAlbesJMCost reduction of perioperative coagulation management in cardiac surgery: value of “bedside” thrombelastography (ROTEM)Eur J Cardiothorac Surg2007311052105717398108
- BowbrickVAMikhailidisDPStansbyGValue of thromboelastography in the assessment of platelet functionClin Appl Thromb Hemost2003913714212812383
- TantryUSBlidenKPGurbelPAOverestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulationJ Am Coll Cardiol2005461705170916256872
- HobsonARAgarwalaRASwallowRADawkinsKDCurzenNPThrombelastography: current clinical applications and its potential role in interventional cardiologyPlatelets20061750951817127479
- AgarwalSCoakleyMReddyKRiddellAMallettSQuantifying the effect of antiplatelet therapy: a comparison of the platelet function analyzer (PFA-100) and modified thromboelastography (mTEG) with light transmission platelet aggregometryAnesthesiology200610567668317006064
- BochsenLWiinbergBKjelgaard-HansenMSteinbruchelDAJohanssonPIEvaluation of the TEG platelet mapping assay in blooddonorsThromb J20075317311677
- CattanoDAltamiranoAVKaynakHEPerioperative assessment of platelet function by Thromboelastograph Platelet Mapping in cardiovascular patients undergoing non-cardiac surgeryJ Thromb Thrombolysis201335233022851059
- LangTTollnickMRiekeMEvaluation of the new device ROTEM® plateletPoster presented at: 58th Annual Meeting of Society of Thrombosis and Haemostasis Research (GTH2014)February 12–15; 2014Wien, Austria
- MichelsonADLindenMDBarnardMRFurmanMIFrelingerAL3rdFlow cytometryMichelsonADPlateletsSan Diego, CAElsevier/Academic Press2007545563
- PatiHPJainSFlow cytometry in hematological disordersIndian J Pediatr20138077277823943573
- CarubbiCMasselliEGesiMCytofluorimetric platelet analysisSemin Thromb Hemost201440889824381148
- HarrisonPKeelingDClinical tests of platelet functionMichelsonADPlateletsSan Diego, CAElsevier/Academic Press2007445474
- FurmanMIBarnardMRKruegerLACirculating monocyte-platelet aggregates are an early marker of acute myocardial infarctionJ Am Coll Cardiol2001381002100611583872
- BarnardMRLindenMDFrelingerALEffects of platelet binding on whole blood flow cytometry assays of monocyte and neutrophil procoagulant activityJ Thromb Haemost200532563257016241954
- RobertSLacroixRPonceletPHigh-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles – brief reportArterioscler Thromb Vasc Biol2012321054105822328775
- KienastJSchmitzGFlow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disordersBlood1990751161211688494
- FurmanMIKruegerLAFrelingerAL3rdGPIIb-IIIa antagonist-induced reduction in platelet surface factor V/Va binding and phosphatidylserine expression in whole bloodThromb Haemost20008449249811019977
- AleilBRavanatCCazenaveJPRochouxGHeitzAGachetCFlow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseasesJ Thromb Haemost20053859215634270
- SpurgeonBEJAburimaAOberprielerNGTaskénKNaseemKMMultiplexed phosphospecific flow cytometry enables large-scale signaling profiling and drug screening in blood plateletsJ Thromb Haemost2014121733174325056834
- LindenMDFurmanMIFrelingerALIndices of platelet activation and the stability of coronary artery diseaseJ Thromb Haemost2007576176517371489
- Ferrer-MarinFChavdaCLampaMMichelsonADFrelingerALSola-VisnerMEffects of in vitro adult platelet transfusions on neonatal hemostasisJ Thromb Haemost201191020102821320282
- MiddelburgRARoestMHamJCoccorisMZwagingaJJvan der MeerPFFlow cytometric assessment of agonist-induced P-selectin expression as a measure of platelet quality in stored platelet concentratesTransfusion2013531780178723216254
- GrosserTFriesSFitzGeraldGAThromboxane generationMichelsonADPlateletsSan Diego, CAElsevier/Academic Press2007565574
- MüllerBPharmacology of thromboxane A2, prostacyclin and other eicosanoids in the cardiovascular systemTherapie19914632172211792655
- FitzGeraldGAHealyCDaughertyJThromboxane A2 biosynthesis in human diseaseFed Proc1987461541583100340
- PatronoCBiosynthesis and pharmacological modulation of thromboxane in humansCirculation19908Suppl 1I12I152136814
- DeFilippisAPOloyedeOSAndrikopoulouEThromboxane A(2) generation, in the absence of platelet COX-1 activity, in patients with and without atherothrombotic myocardial infarctionCirc J2013772786279223985963
- HartRGLeonardADTalbertRLAspirin dosage and thromboxane synthesis in patients with vascular diseasePharmacotherapy20032357958412741431
- HedegaardSSHvasAMGroveELOptical platelet aggregation versus thromboxane metabolites in healthy individuals and patients with stable coronary artery disease after low-dose aspirin administrationThromb Res20091249610019215971
- FontanaPZuffereyADaaliYRenyJLAntiplatelet therapy: targeting the TxA2 pathwayJ Cardiovasc Transl Res20147293824353037
- CerlettiCDell’ElbaGManariniSPharmacokinetic and pharmacodynamic differences between two low dosages of aspirin may affect therapeutic outcomesClin Pharmacokinet2003421059107012959636
- ShenRFTaiHHMonoclonal antibodies to thromboxane synthase from porcine lung. Production and application to development of a tandem immunoradiometric assayJ Biol Chem198626111585115913745157
- CiabattoniGMacloufJCatellaFFitzGeraldGAPatronoCRadioimmunoassay of 11-dehydrothromboxane B2 in human plasma and urineBiochim Biophys Acta19879182932973567215
- RogasiPGPanicciaRCoppoMRadioimmunoassay of thromboxane B2 in plasma: methodological modificationsThromb Res1988515335413175991
- MuirARMcMullinMFPattersonCMcKeownPPAssessment of aspirin resistance varies on a temporal basis in patients with ischaemic heart diseaseHeart2009951225122918697805
- GremmelTPerkmannTSeidingerDDifferential impact of inflammation on six laboratory assays measuring residual arachidonic acid-inducible platelet reactivity during dual antiplatelet therapyJ Atheroscler Thromb20132063064523739624
- RoestMReiningerAZwagingaJJKingMRHeemskerkJWBiorheology Subcommittee of the SSC of the ISTH: flow chamber-based assays to measure thrombus formation in vitro: requirements for standardizationJ Thromb Haemost201192322232422947397
- Van KruchtenRCosemansJMHeemskerkJWMeasurement of whole blood thrombus formation using parallel-plate flow chambers – a practical guidePlatelets20122322924222502645
- AslanJEItakuraAGertzJMMcCartyOJPlatelet shape change and spreadingMethods Mol Biol20127889110022130702
- KirchmaierCMPillitteriDDiagnosis and management of inherited platelet disordersTransfus Med Hemother20103723724621113246
- GeSWooEWhiteJGHaynesCLElectrochemical measurement of endogenous serotonin release from human blood plateletsAnal Chem2011832598260421384903
