Abstract
Statins lower serum cholesterol and are employed for primary and secondary prevention of cardiovascular events. Clinical evidence from observational studies, retrospective data, and post hoc analyses of data from large statin trials in various cardiovascular conditions, as well as small scale randomized trials, suggest survival and other outcome benefits for heart failure. Two recent large randomized controlled trials, however, appear to suggest statins do not have beneficial effects in heart failure. In addition to lowering cholesterol, statins are believed to have many pleotropic effects which could possibly influence the pathophysiology of heart failure. Following the two large trials, evidence from recent studies appears to support the use of statins in heart failure. This review discusses the role of statins in the pathophysiology of heart failure, current evidence for statin use in heart failure, and suggests directions for future research.
Introduction
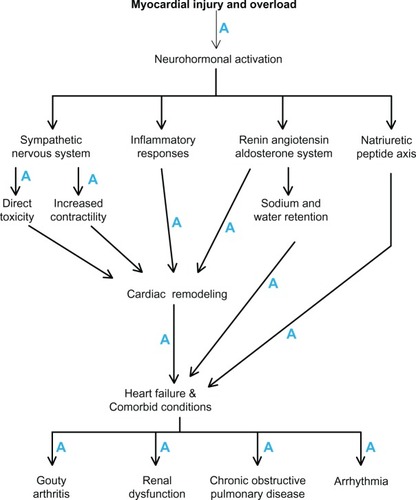
Heart failure (HF) is a complex clinical syndrome which results from structural and functional disorders of the heart associated with a variety of cardiovascular diseases. HF is mainly characterized by a condition in which the heart cannot pump enough blood to the rest of the body. With an increasing number of patients, HF is becoming a major worldwide public health problem which requires a global response. In recent decades, significant strides have been made in the treatment of HF with the appearance of angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers (ARB), β-blockers, aldosterone antagonists, and device therapies.Citation1 However, mortality and morbidity is still high and further strategies are needed to avert or reduce adverse outcomes. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly called statins, are one of the novel but affordable pharmacological agents that have been investigated in patients with HF in recent years.
Statins are a class of drugs that have become one of the most important lipid lowering medications with proven efficacy in treatment of hyperlipidemia. Lovastatin was the first statin introduced into clinical practice in 1987. Now there are seven different statins available for clinical use. Statins are grouped into two main categories according to their origin: (1) naturally occurring statins of fungal origin or semisynthetic analogs, such as lovastatin, pravastatin, and simvastatin; or (2) synthetic statins including fluvastatin, atorvastatin, rosuvastatin, and pitavastatin.Citation2 Generally, statins are regarded as a remarkably safe and well-tolerated class of drugs, despite the withdrawal of cerivastatin in 2001.Citation3 Statins lower plasma cholesterol levels by competitive inhibition of the rate-determining enzyme HMG-CoA reductase in the mevalonate pathway. It is well-established that statins reduce morbidity and mortality in patients with coronary artery disease (CAD)Citation4,Citation5 and prevent its progression to HF.Citation6 The mevalonate pathway also produces isoprenoids (farnesyl pyrophosphate and geranylgeranyl phosphate) as intermediatesCitation7 which mediate the activation of various signaling molecules via the prenylation of small guanosine triphosphate (GTP) binding proteins: Rho, Ras, and Rac. Rho is involved in the activation of inflammatory cytokines and the formation of the actin cytoskeleton which affects intracellular transport, messenger ribonucleic acid (mRNA) stability, and gene transcription.Citation8,Citation9 The Ras proteins regulate cell proliferation and hypertrophy, whereas Rac are involved in reactive oxygen species (ROS) generation via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation. By inhibiting HMG-CoA reductase, statins decrease isoprenoid production and consequently downregulate Rho, Ras, and Rac mediated signaling pathways.Citation7 In addition to lowering cholesterol, statins exert cholesterol-independent effects through mevalonate inhibition; these include the enhancement of endothelial function, reduction of neurohormonal activation, decrease in proinflammatory cytokines, and the attenuation of ventricular remodeling – all of which play a critical role in HF progression and prognosis.
Clinical evidence from observational studies and retrospective and post hoc analyses of data from randomized trials in various cardiovascular conditions suggest the survival benefit of statins for HF.Citation4,Citation10–Citation14 Statins appear to have many pleiotropic effects believed to influence the pathophysiology to confer survival and further outcome benefits in HF. Notwithstanding these observations, two large scale randomized trials – the Controlled Rosuvastatin Multinational Study in Heart failure (CORONA)Citation15 and Gruppo Italiano per lo Studio della Sopravvivenza Nell’Insufficienza Cardiaca Heart Failure (GISSI-HF)Citation16 – which randomized patients to one type of statin at a low dose (rosuvastatin 10 mg) or a matching placebo, did not show improved survival in patients with HF. Clinicians, therefore, withhold statins due to reports of potential harmful effects and lack of substantial clinical trial data to support their use in HF.Citation17 Moreover, recent studies have not confirmed the detrimental effects of statins in HF reported in the CORONA and GISSI-HF trials.Citation18,Citation19 The lack of clarity surrounding the effect of statins in HF raises important clinical questions. This review discusses the role of statins in the pathophysiology of HF, current evidence for statin use in heart failure, as well as possible future research directions.
Potential mechanisms for beneficial effects of statins in the pathophysiology of HF
HF is a complex syndrome typified by hemodynamic and metabolic alterations, elevation of inflammatory and oxidative stress markers, endothelial dysfunction (ED), neurohormonal activation, plaque instability, and adverse cardiac remodeling. Statins show various favorable lipid-dependent and lipid-independent effects which are believed to alter the pathophysiological mechanisms and may bring about clinical benefits in HF.
Endothelial function
The endothelium is a monolayer of cells lining the innermost surface of blood vessels; it serves as a functional and structural barrier between blood and the vessel wall to prevent platelet and leucocyte aggregation, control the permeability of plasma constituents, and regulate blood flow. The endothelium also regulates vascular tone through balanced production of vasodilators and vasoconstrictors in response to various stimuli.Citation20 Endothelium produces nitric oxide (NO) – a prime mediator of normal vascular function which dilates smooth muscles and relaxes myofibrils in response to endogenous (bradykinin, acetylcholine, and catecholamines) and exogenous (ischemia, shear stress, and temperature changes) stimulation.Citation21 The normal endothelium also provides anti-inflammatory and antiproliferative actions, and modulates fibrinolysis and coagulation pathways to maintain the hemostatic properties of blood vessels.Citation22 ED is characterized by decreased NO bioavailability due to impaired NO production by the endothelium and/or increased NO inactivation by ROS. The after effect is impaired vasodilatation, increased vasoconstriction, platelet aggregation, cytokine release, smooth muscle cell proliferation, and increased oxidative stress.Citation23 HF severity is associated with NO imbalance and the ensuing ED.Citation24 Decreased NO mediated vasodilation is common in ED, and appears to impair myocardial perfusion, reduce coronary blood flow,Citation25 and worsen ventricular function in HF.Citation24 ED also contributes to increased vascular stiffness and impairs the ability of arteries to distend, resulting in myocardial damage.Citation26 Furthermore, NO imbalance in ED alters matrix metalloproteinase (MMP) which affects cell migration, cardiac hypertrophy, and atherosclerotic plaque stability.Citation27 In HF, elevated levels of endothelin-1 (ET-1) – a potent vasoconstrictor – cause increased vasoconstriction, matrix production, and smooth muscle cell growth to worsen endothelial function and accelerate HF progression.Citation27 Reduced blood flow coupled with increased lactate production from NO downregulation-mediated catabolism of free fatty acids partly accounts for exercise intolerance seen in patients with HF.Citation28
Statins appear to induce NO synthetase (eNOS) – an enzyme which catalyzes the production of NO expression in human endothelial cells.Citation29 Statins have been shown to prevent the expression of caveolin, which is a negative regulator of eNOS.Citation30 Atorvastatin and simvastatin also inhibit ET-1 mRNA expression and reduce plasma levels of ET-1.Citation31–Citation33 The protein kinase Akt has been identified as a major regulator of cell growth, survival,Citation34 and eNOS activity. Simvastatin induces Akt-mediated phosphorylation of eNOS, which gives rise to heightened NO production and endothelial cell survival.Citation35 Thus, statins’ modulation of Akt activity may partly explain improvement in endothelial function, enhanced tissue perfusion, and reduced cardiovascular events seen in treated patients. Statins further inhibit Rho activation to increase endothelial NO production.Citation29 Experimental animal models confirm the beneficial effects of statins in ED;Citation36,Citation37 likewise, clinical studies have demonstrated improved endothelial function with high, optimal, and low doses of statins.Citation38–Citation40
Inflammation
HF is characterized by worsened inflammation due to the activation of proinflammatory cytokines, cell adhesion molecules, endothelial cells, cardiac myocytes,Citation41,Citation42 the complement system, and cardiac autoantibodies,Citation18 which are produced by activated macrophages. Elevated proinflammatory cytokines – tumor necrosis factor α (TNF-α), interleukin (IL)-1, IL-6, IL-10, and C-reactive protein (CRP) – have been implicated in HF morbidity and mortality.Citation41 These mediators are associated with left ventricular remodeling, enhanced cardiac myocyte apoptosis, ED, and incidence of anorexia and cachexiaCitation41,Citation43 in HF. Statins reduce plasma concentrations of proinflammatory cytokinesCitation44,Citation45 and have been shown in in vitro mononuclear cell cultures of normal human subjects.Citation46 A couple of in vitro studies suggest statins show anti-inflammatory properties through the inhibition of isoprenoid intermediates which serve as ligand attachments for intracellular signaling molecules in the mevalonate pathway.Citation45,Citation46 Statins ameliorate inflammation in HF by favorably modulating many signaling pathways which include endothelial NO synthase, tissue-type plasminogen activator, ET-1, and plasminogen activator inhibitor I.Citation33
Further, statins have been shown to reduce activation of the transcription factor NFκB,Citation47 which is associated with the production of acute-phase proteins such as angiotensinogen, adhesion molecules, and cytokines,Citation48 and are all known to play an important role in the progression of HF.
Myocardium remodeling
Myocardial remodeling is a genomic expression that leads to cellular, molecular, and interstitial changes in the heart characterized by change in size, shape, and function. HF progression is closely associated with ventricular remodeling which manifests as myocyte hypertrophy and ventricular dilation. Angiotensin II mediated mechanisms via the angiotensin II type 1 (AT1) receptor stimulation downstream of the mevalonate pathway account for ventricular remodeling in HF.Citation7 Statins downregulate AT1 receptor mediated negative effects such as enhanced sympathetic activation, vasoconstriction, sodium retention, ROS formation, and cardiac hypertrophy,Citation49 and thus prevent and/or attenuate myocardial remodeling.
Statins may also prevent remodeling via modulation of the effects of MMPs – protein-degrading enzymes involved in the breakdown and remodeling of tissues and organs including the myocardium. MMPs promote extracellular matrix degradation and remodeling whereas endogenous tissue inhibitors of MMP (TIMP) inhibit these effects.
An imbalance between activated MMP and TIMP promotes extracellular matrix degradation and myocardial remodeling in the development of HF.Citation50 Myocardial MMP levels increase in dilated cardiomyopathy.Citation51 Statins suppress expression of MMP-9, MMP-3, and MMP-1 while upregulating the expression of TIMP – a mechanism that could limit extracellular breakdown,Citation52,Citation53 thereby inhibiting myocardial fibrosis and remodeling.
Studies in human and animal models have confirmed that statins attenuate myocardial remodeling by reducing cardiac myocyte hypertrophy, activation of MMP, fibrosis, and myocardial cell apoptosis.Citation54,Citation55 A recent study reported that simvastatin inhibits TNF-α induced myofibroblast proliferation and MMP-9 secretion in a concentration dependent fashion in HF.Citation56 Statins also promote endothelial function and inhibit platelet activationCitation57 to reduce ventricular remodeling after acute myocardial infarction in coronary artery ligation animal models,Citation58 and these cardioprotective effects may be attributed to the statins’ activation of the protein kinase Akt. The protein kinase Akt acts downstream of the mevalonate pathway to promote production of vascular endothelial growth factor and angiopoietinCitation59 that stimulate endothelial cell survival and promote angiogenesis.
There is some evidence to suggest that statins promote regression of left ventricular mass in patients with angina pectorisCitation60 and produce antiarrhythmic effects in high-risk patients with HF through downregulation of potential unfavorable effects of AT1 receptor stimulation. Moreover, Krum et alCitation61 showed that statin therapy has no effect on left ventricular remodeling in patients with New York Heart Association (NYHA) functional class II or III ischemic or nonischemic HF when randomized to either rosuvastatin 40 mg or a placebo in addition to standard therapy for 6 months. The highest effective dose of rosuvastatin (40 mg) used achieved a remarkable 57% reduction in plasma low density lipoprotein (LDL) levels but failed to improve markers of cardiac remodeling. In another study, low dose statins exhibited enhanced endothelial cell proliferation, migration, and differentiation, but the effect was inhibited at high doses after acute coronary syndrome,Citation62 suggesting a biphasic effect requiring further investigations. Cerivastatin reduces collagen I and fibronectin deposition to prevent ventricular hypertrophy in a transgenic rat model and may ameliorate angiotensin II-induced cardiac hypertrophy, fibrosis, and remodeling, independent of plasma cholesterol levels.Citation49
Galectin-3 is secreted by activated macrophages and a member of a family of proteins including soluble β-galactoside-binding lectins that have regulatory roles in fibrogenesis, inflammation, tissue repair, and cell proliferation. In HF, galectin-3 promotes myocardial fibrosis and inflammation, which are involved in myocardial remodeling.Citation63 Recent studies have reported an association between elevated circulating galectin-3 and poor clinical outcomes in patients with HF.Citation64–Citation66 A sub-study of the CORONA trial has reported older patients with systolic HF of ischemic origin, receiving modern pharmacotherapy, who have low levels of galectin-3 may benefit from rosuvastatin treatment.Citation67
Neurohormonal activation
HF severity and mortality is linked to sympathetic nervous system activation, which is characterized by the upregulation of the renin-angiotensin-aldosterone system (RAAS), elevated plasma norepinephrine levels,Citation68,Citation69 and enhanced natriuretic peptide concentration from the myocardium.Citation70 Statins, by downregulating AT1 receptor activation,Citation46 reduce sympathetic nerve activityCitation45 and modulate the vascular functions of ET-1 receptorsCitation71 to attenuate ED and myocardial remodeling in HF.
Further, statins inhibit the RAAS in the vasculatureCitation57 and myocardiumCitation58 to ameliorate angiotensin II mediated cardiac hypertrophy.Citation59,Citation72 Simvastatin decreases plasma norepinephrine levels and renal sympathetic nerve activity, and normalizes baroreceptor responses in rat HF models.Citation73
The natriuretic peptide axis is another important neurohormonal pathway that plays a fundamental role in HF. Plasma concentrations of natriuretic peptides aid in diagnosis and predict HF severity and prognosis.Citation74,Citation75 Plasma brain natriuretic peptide (BNP) levels are elevated in ventricular dysfunction.Citation76,Citation77 Elevated BNP levels are also associated with reduced functional capacity and impaired oxygen uptake at peak exercise in HF.Citation78 Recently, statins have been shown to modulate plasma BNP and its precursor amino-terminal pro-BNP (NT-proBNP) levels,Citation79 thus providing evidence for the neurohormonal downregulating effects of statins in HF.
Ischemia
Recurrent ischemia is associated with the progression of ischemic cardiomyopathy.Citation73 Ischemia may lead to elevated extracellular matrix collagen reduction, cardiomyocyte necrosis, and apoptosis resulting in HF.Citation80 Inhibition of cholesterol synthesis reduces macrophage activation, foam cell formation, and plaque thrombogenicity, thus altering the lipid to cell ratio of the atherosclerotic lesion, making the plaque less liable to rupture. Statins promote atherosclerotic plaque stabilization by inhibiting inflammatory macrophages, depleting the lipid core, and strengthening the fibrous cap. Pravastatin inhibits macrophage cholesterol metabolism in in vivo and in vitro studies.Citation81 Indeed, statins have been shown to reduce atherothrombotic coronary events in patients with low LDL cholesterol levels,Citation82 thus extending anti-ischemic effects beyond plaque stabilization. Laboratory evidence demonstrates that statins reduce the extent of myocardial necrosis, preserve myocardial viability, and improve ventricular function in models of myocardial ischemia.Citation83 Statins improve coronary endothelial function and promote angiogenesis, thus reducing ischemia in HF.Citation35,Citation84 Statins have also been shown to decrease the incidence of HF in patients with hyperlipidemia through the inhibition of cholesterol biosynthesis and other mechanisms which prevent recurrent ischemia.Citation85
Arrhythmia
Cardiac arrhythmias are common in HF and associated structural heart diseases. Atrial fibrillation (AF) coexists in a third of chronic HF patients and may represent either a cause or a consequence of HF.Citation86 AF is more common with the increasing severity of HF. Ventricular arrhythmia, which is often commonly seen in HF, is a major cause of sudden cardiac death. Clinical evidence suggests beneficial effects of statins in atrial and ventricular arrhythmia in HF.Citation87,Citation88 Data from a multicenter registry of patients with left ventricular systolic dysfunction have shown statin therapy to be associated with a significant reduction in the incidence of AF.Citation87 The cumulative rate of ventricular arrhythmia or sudden cardiac death was significantly reduced with statin therapy in ischemic cardiomyopathy patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial-II (MADIT).Citation89
Statins reduce the incidence of arrhythmia through plaque stabilization, but a significant reduction in sudden death was reported in nonischemic cardiomyopathy,Citation90 suggesting non-cholesterol-lowering effects in decreasing arrhythmic events. Aside from anti-ischemic properties, other proposed mechanisms for the observed lowered incidence of arrhythmias with statin therapy in HFCitation18 are membrane-stabilizing and anti-inflammatory properties as well as improved autonomic function. Further, statins appear to improve left ventricular function and prevent remodeling, thereby decreasing the incidence of ventricular arrhythmias.Citation91 In addition, recent experimental data suggest Rac-1 guanosine triphosphatase (GTPase) may contribute to the pathogenesis of AFCitation92 and its suppression by statins may reduce arrhythmias. Nonetheless, recent meta-analyses have provided conflicting reports about the effects of statin therapy in patients with AF.Citation88,Citation93 One such study showed that statin therapy is significantly associated with a decreased risk of incidence or recurrence of AF in patients with various cardiovascular conditions (sinus rhythm with a history of previous AF in those undergoing cardiac surgery or after acute coronary syndrome).Citation88 This meta-analysis provided robust evidence of the benefit of statins beyond their lipid lowering activity.Citation88 In a subsequent meta-analysis of published and unpublished studies of statins in various cardiovascular conditions, short term treatment with statins provided compelling evidence of AF prevention, however, long term treatment showed no protective effect of statins on AF.Citation93 Thus, prospective randomized clinical trials may still be needed to establish whether statins are beneficial and are an appropriate therapeutic option in all subgroups of patients for the treatment of AF, particularly HF.
In conclusion, statins exert pleiotropic effects in tandem with their lipid lowering activity to interfere with the pathophysiology of HF. Statins restore normal neurohormonal balance, prevent ventricular remodeling, prevent recurrent ischemia, reduce inflammation, and improve cardiac function in patients with HF. Statin treatment has been shown to favorably affect endothelial function, increase capillary density as well as circulating endothelial progenitor cells, and slow the progression of coronary atherosclerosis. These potential beneficial effects result from both cholesterol-dependent and cholesterol-independent actions of statins on the mevalonate pathway. Thus, statins may reverse or attenuate progression and reduce mortality in patients with ischemic and nonischemic HF.
Potential harmful effects of statins in HF
Statins are often not prescribed for patients with established HF. This is likely to be due to concerns about detrimental effects observed, largely, in retrospective studies. Three hypotheses have been suggested to explain the potential harmful effects of statins in HF.
First, the endotoxin–lipoprotein hypothesis, which postulates that higher levels of cholesterol might be beneficial in HF due to the ability of cholesterol to regulate inflammation.Citation94 HF is associated with increased inflammatory cytokines, which might be partly linked to elevated endotoxin levels. Endotoxins stimulate the release of cytokines in patients with HF. Circulating cholesterol and triglyceride-rich lipoproteins are natural nonspecific buffers of endotoxins, which are capable of binding and detoxifying bacterial lipopolysaccharides.Citation95 Thus, lowered circulating cholesterol may result in endotoxemia which is associated with poorer prognosis in HF.
Second, the ubiquinone hypothesis states that inhibition of ubiquinone synthesis in the mevalonate pathway possibly impairs mitochondrial energy production. Ubiquinone is present in all cells and is critical to mitochondrial respiration. Statins inhibit ubiquinone synthesis, which in turn impairs cellular energy production to adversely affect ventricular function and exercise tolerance in HF. Statins also inhibit ubiquinone synthesis, resulting in statin-induced myalgias and myopathy.Citation96
Third, the selenoprotein hypothesis postulates that statins interfere with the enzymatic isoprenylation of selenocysteine transfer RNA (tRNA) to inhibit its maturation to functional tRNA molecules, thereby decreasing selenoprotein levels. Statin-induced myopathies have been associated with severe selenoprotein deficiency.Citation97
The above three hypotheses, mechanisms, and possible harmful effects of statins in HF are summarized in . Despite these concerns, statin trials as well as systematic reviews and meta-analyses of statin treatment in heart failure have not established any detrimental effects, but suggest favorable effects in HF populations.Citation18,Citation19
Table 1 Hypothesis, mechanism, and effects of statins in heart failure
Clinical experience with statins in HF
Owing to potential unfavorable effects, earlier statin trials have excluded patients with symptomatic HF. But their pleiotropic actions suggest HF patients may benefit from statin treatment aside from cholesterol lowering effects. These claims have been partially confirmed, as post hoc analyses of statin trials in various cardiovascular conditions showed improved survival in patients with HF as summarized in .Citation4,Citation6,Citation10–Citation13,Citation60,Citation82,Citation100–Citation103 Likewise, retrospective and subgroup analyses of the effects of statins in HF trials which evaluated outcomes of other therapeutic agents showed reduced hospitalization and mortality.Citation104–Citation106
Table 2 Retrospective analyses of statin trials in various cardiovascular conditions
Evidence from non-randomized studies
Many non-randomized studies have provided evidence to support the use of statins in HF. These non-randomized studies evaluated the effects of statins on outcomes in patients with HF and various cardiovascular conditions. Generally, statin therapy has been associated with reduced mortality in HF, however, equivocal results have been reported.Citation103
A prospective study conducted at the Duke Heart Failure Clinic enrolled 96 consecutive outpatients with ejection fraction (EF) < 40% and NYHA class II to IV symptoms, and 14 healthy volunteers as a control.Citation107 Enrolled patients were on standard care appropriate to disease severity, were clinically observed directly and/or through family interactions during clinic visits, and had telephone follow-up over a 12-month period. They were observed for the occurrence of adverse events such as death, hospitalization for all causes, worsening heart failure, and angina. Combined therapy of ACE inhibitors and β-blockers was associated with lower CRP levels, improved survival, and reduced outcomes of hospitalization, worsening heart failure, and incidence of angina. Statin therapy had no effect on CRP levels and failed to improve outcome benefits, but did not worsen HF outcomes.Citation107 In another prospective cohort study, 6427 cardiologist-diagnosed HF patients (mean age of 69 ± 11 years) were followed for 12 months.Citation14 Enrolled patients had varying degrees of renal insufficiency. In 2545 of the patients observed, statin use was associated with significantly better survival, after adjustment for other medications, even in advanced renal insufficiency compared with non-statin users.Citation14 In the Kaiser Permanente congestive HF cohort of 24,598 patients, Go et al evaluated the association between initiating statin therapy and risk of death and hospitalization among adults who had HF after a median follow-up of 2.4 years.Citation108 Statin therapy was associated with a 24% (hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.72 to 0.80) lower risk for death and a 21% (HR 0.79, 95% CI 0.74 to 0.85) lower risk of hospitalization for HF. Statins were, however, more likely to be prescribed in younger patients and those known to have CAD, diabetes, or hypertension. The difference in mortality was independent of cholesterol level or coronary disease.Citation108
Foody et al evaluated the association between statin use and survival in a large retrospective observational study of 54,940 Medicare beneficiaries hospitalized for HF.Citation109 About 16.7% of the patients primarily diagnosed with HF without contraindications were discharged on statin therapy. Treatment with statin at the time of discharge was associated with significant reduction in mortality at 1 (HR 0.80, 95% CI 0.76 to 0.84) and 3 years (HR 0.82, 95% CI 0.79 to 0.85). It is worth noting that the significant reduction in mortality was independent of patient demographics, treatments, physician specialty, and hospital characteristics. There was a significant difference in mortality between statin users and nonusers independent of CAD status or total cholesterol levels.Citation109
In conclusion, previous studies have reported decreased hospital admissions, improved surrogate endpoint outcomes, and overall mortality with statin therapy as summarized in , thus appearing to support its beneficial role in HF.Citation14,Citation109–Citation112 The equivocal results reported by the Duke Heart Failure Clinic study may be due to the small sample size of the study, which might have introduced type 2 (β) errors, particularly when it assessed major clinical outcomes.Citation107 Further, overlap of treatment groups, particularly of patients that received β-blockers and statins created difficulty in detecting the effect of each medication on clinical outcomes. In addition, the assessment of CRP and medication use was determined at a single time point, which might have introduced potential intrapatient variability and made longitudinal analysis of the effect of medications on CRP levels impracticable. Statin therapy has been associated with reduced hospital admissions and mortality in non-randomized studies as shown in . This body of evidence comes from non-randomized studies which are susceptible to confounding and bias and should be interpreted with caution and regarded as “hypothesis-generating.”
Table 3 Major non-randomized studies evaluating effect of statins in heart failure outcomes
Randomized controlled trials (RCTs) of statins in HF
Secondary analyses of statin trials in various cardiovascular conditions show improved HF outcomes, though mechanisms are indistinct. RCTs have evaluated the effects of statin treatment on surrogate endpoints as well as major clinical outcomes (hospital admissions and mortality) in HF ().
Table 4 Randomized controlled trials of statins in heart failure
Many small scale RCTs show improved cardiac and endothelial function, reduced inflammation and oxidation markers,Citation116–Citation121 whereas in other trials, statins had shown neither favorable nor detrimental effects in HF.Citation61,Citation122,Citation123 Most of the studies appeared to be insufficiently powered to determine major outcomes such as hospital admissions and mortality, but significantly improved surrogate endpoints in HF. Sola et alCitation119 conducted one such study that randomized 108 nonischemic HF patients to atorvastatin 20 mg/day or a matching placebo and followed them up for 12 months. The study observed changes in left ventricular EF (LVEF) determined by transthoracic echocardiography as the primary endpoint and inflammatory and oxidation markers as the secondary endpoint. It observed a significant reduction in inflammatory and oxidation markers and improved LVEF. Interestingly, atorvastatin failed to reduce the frequency of hospital admissions or mortality in HF.
Laufs et alCitation124 corroborated the beneficial effect of cerivastatin with improved brachial artery flow mediated dilation, quality of life score, functional ability, and reduced levels of plasminogen activator inhibitor-1 (PAI-1), CRP, and TNF-α in patients with dilated cardiomyopathy in a 4-month follow-up period.
In another study, 60 HF patients of ischemic and nonischemic origin with NYHA class II to III and LVEF < 40% were randomized to atorvastatin 10 mg or a matching placebo for 4 weeks.Citation125 The efficacy was determined by measuring various biomarkers and flow-mediated vasodilatation at the baseline and at 4 weeks. Reactive hyperemia caused by flow mediated vasodilatation increased significantly and there was a consequent reduction in inflammatory markers such as vascular adhesion molecule-1 (VCAM-1), IL-6, and TNF-α.Citation125 These evidences suggest statins could be beneficial in both ischemic and nonischemic HF.
Conversely, neither favorable nor detrimental effects on cardiac function, endothelial function, and inflammatory markers were observed with studies that used high doses of statins in HF.Citation61,Citation123 In one such study, 15 patients with Non-Ischemic Cardiomyopathy (NICM) on optimal heart failure treatment were enrolled in a randomized, double-blind, placebo-controlled, cross over trial.Citation123 Patients were randomized to atorvastatin 80 mg/day or matching placebo for the 12-week treatment period with a minimum of an 8-week washout period. The study evaluated surrogate markers such as NT-proBNP, high-sensitivity CRP, oxidized LDL antibody, soluble receptor TNF-α, TNF-α, circulating levels of intercellular adhesion molecule-1 (ICAM-1), VCAM-1, and P-selectin; all are parameters of noninvasive endothelial function and heart rate variability.Citation123 Statin therapy reduced LDL but was inert to the surrogate endpoints. Statin was neither beneficial nor detrimental as determined by surrogate marker measures. Similarly, the UNIVERSE study randomized 95 ischemic and nonischemic HF patients to rosuvastatin 40 mg/day or a matching placebo in addition to standard optimal therapy for a period of 6 months in a double-blind fashion.Citation61 The highest dose of rosuvastatin markedly reduced LDL cholesterol, but failed to improve cardiac function among patients in the treatment group.
Generally, many small randomized studies show statins to improve surrogate markers and endpoints, but do not reduce the frequency of hospitalization and mortality in HF ().Citation116–Citation121,Citation125,Citation127,Citation128,Citation131 It appears the effect of statins shown in patients with HF varies among the different types of statins employed.
Evidence from systematic reviews and meta-analyses
Evidence for statin therapy in HF mainly comes from non-randomized studies which evaluated its effects on clinical outcomes in patients with HF and various cardiovascular conditions.Citation4,Citation6,Citation10–Citation14,Citation60,Citation82,Citation100–Citation103,Citation107,Citation108,Citation110–Citation112 Subgroup and post hoc analyses of statin trials in various cardiovascular conditions and HF trials that evaluated other pharmacological agents too provided substantial evidence for statin use in HF.Citation104–Citation106
Several systematic reviews and meta-analyses have been conducted to synthesize evidence for statin therapy in reducing major adverse events in HF. van der Harst et alCitation19 conducted the first systematic review mainly from retrospective, non-randomized trials and a few prospective randomized studies of statin treatment in HF. The researchers found that there is a paucity of prospective data required to determine the effect of statins on clinical outcomes in HF and concluded that available experimental, post hoc data, observational data, and theoretical considerations are inconsistent. The authors reported that: (1) lower cholesterol levels are associated with poorer outcomes in HF patients and may be related to the function of cholesterol as a scavenger for harmful endotoxins; (2) statins in HF may adversely affect mitochondrial function through inhibition of ubiquinone; and (3) statins may decrease selenoproteins, which could result in decreased myocardial function. The researchers concluded that statin treatment may favor HF and recommended a large randomized clinical trial.
Ramasubbu et al’sCitation18 meta-analysis from 13 studies – eleven retrospective studies and two prospective studies – reported that statin treatment favored HF with a significant 26% decrease in relative risk of mortality. Conversely, two recent meta-analyses performed on randomized clinical trials did not show improved survival with statin in HF.Citation132,Citation133 It appears that the majority of patient data came from CORONACitation15 and GISSI-HFCitation16 trials which randomized older patients to low dose rosuvastatin or matching placebo that may have skewed the summary statistic towards the results of these two large trials. From the various studies, low and moderate doses of statins seem to have better outcomes than high doses of statins in patients with HF. However, these claims were not confirmed when investigated with meta regression models and subgroup analysis. Similarly, the age and sex of patients did not influence the outcomes of HF with statin therapy in any of the meta-analyses.
Recent evidence for statin therapy in HF
To provide more conclusive data on whether or not statins confer survival and other outcome benefits in HF, CORONA (2007)Citation15 and GISSI-HF (2008)Citation16 trials were conducted and sufficiently powered to evaluate outcomes of HF with statin treatment.
The CORONA study was a large randomized, placebo controlled trial of rosuvastatin 10 mg versus a placebo in patients with chronic symptomatic systolic HF of ischemic etiology.Citation15 The study enrolled 5011 patients aged ≥ 60 years with NYHA class II symptoms and an EF of <35%, or NYHA class III to IV symptoms and an EF of <40% with an average of 3 years follow-up. Rosuvastatin did not confer survival benefits, but reduced the number of HF hospitalizations in older patients with systolic HF. The CORONA trial may have failed to improve the primary outcome due to enrollment of elderly patients who may have had many comorbidities which could have attenuated the benefits. Further, the drug may have potentially interacted with the complex medical therapy of the geriatric population.
Moreover, as the CORONA study recruited ischemic HF patients, a significant number of patients could have developed a statin tolerance due to the long period of exposure from treatment of CAD, and prevention and treatment of HF, which meant they may have required a higher dose or a different statin to elicit the desired response to bring about a significant survival benefit. However, it is reassuring to note that post hoc analyses of the CORONA trial show HF patients of ischemic origin with low levels of galectin-3Citation67 and NT-proBNPCitation55 may benefit from rosuvastatin treatment. The findings do not recommend the general use of statins in HF, but endorse their use in ischemic heart disease patients with plasma galectin-3 concentrations lower than 19.0 ng/mL and NT-proBNP less than 103 pmol/L (868 pg/mL). This observation complements that of the Heart Protection Study (HPS), where patients with low levels of BNP benefited from simvastatin treatment.Citation134 Nonetheless, the presence of HF was not recorded at baseline, and it was impossible to directly estimate the effect of simvastatin in patients with and without HF at randomization in the HPS. This evidence is from a retrospective analysis, which may thus be considered as hypothesis-generating and should be confirmed in a prospective study.
The GISSI-HF trial was a multicenter, randomized, double-blind study that assessed the effect of n-3 polyunsaturated fatty acids and rosuvastatin 10 mg versus placebo on the cardiovascular morbidity and mortality of patients with chronic symptomatic HF.Citation16 This study enrolled 4574 HF patients and employed broad eligibility criteria, requiring NYHA class II to IV symptoms of any etiology. There were no exclusions based on EF or baseline cholesterol levels. The GISSI-HF trial also did not show any significant effect of rosuvastatin on clinical outcomes in patients with chronic HF of ischemic and nonischemic etiologies after 3 years of follow-up. The GISSI-HF and CORONA studies reported minimal adverse drug events and statin therapy did not worsen HF outcomes, even in older patients with possibly compromised myocardium. Thus, it may be concluded that statins are safe in HF.
A recent study appraised the effect of statin on all-cause mortality in a large cohort of 10,510 consecutive patients (mean age 72 years) from the Veterans Affairs health system, with ischemic and nonischemic HF over 3 years.Citation113 The study also assessed the effect of incremental duration of statin therapy on mortality. Statin use was associated with significantly lower all-cause mortality among the veterans. Most of the enrolled patients used simvastatin and atorvastatin, which might have accounted for the marked improvement in survival.Citation113 Patients were comparable to those of the GISSI-HF study,Citation16 which recounted a nonsignificant 29% mortality compared with the placebo group, as both studies recruited elderly patients with ischemic and nonischemic HF. In this study, patients who used statins for <25% of the 3-year follow-up had no benefit, but survival was apparent when statin use was >25% of the follow-up duration, suggesting compliance as an important confounding factor. A major weakness of the GISSI-HF study was noncompliance, as about a third of the study population were not compliant with statin treatment for various reasons and may have influenced its result. Likewise the Veterans Affairs study was similar to the CORONA study (mean age: 73 years, mortality rate: 11.9%), but the reason for the disparity in mortality rate recounted remains unclear. Differences in revascularization rates, aggressive lipid control, and other comorbid conditionsCitation135 may have accounted for the disparity in mortality rates. The CORONA and GISSI-HF studies assessed the effect of one type of statin at a low dose, thus the insignificant results provide inconclusive evidence for the class effect of statins in HF. The majority of patients in the Veteran Affairs health system study used simvastatin and atorvastatin; therefore, an evaluation of the effects of various statins in patients with both ischemic and nonischemic HF may be required.
Similarly, a prospective study assessed the effects of statin therapy in 960 elderly HF patients of ischemic and nonischemic etiologies for a maximum follow-up of 9 years.Citation115 Most patients were prescribed atorvastatin or simvastatin and statin use generally was associated with improved survival (HR 0.45, 95% CI 0.37 to 0.54) for HF.Citation115 In contrast to the CORONA and GISSI-HF studies, statin was independently and significantly associated with lower mortality after adjusting for all confounders, such as concurrent medication, concurrent therapies, comorbid conditions, gender, HF etiology, HF duration, cholesterol level, LVEF, NYHA class, and sex.
Rosuvastatin, used in the CORONA and GISSI-HF studies, is hydrophilic and employs active transport into hepatocytes to exert its effect.Citation2,Citation3 It penetrates poorly into extra hepatic tissues; thus, it has less risk of adverse effects, but has a very low uptake by cardiac muscles to exert the pleiotropic effects believed to contribute greatly to attenuate HF symptoms.
Conversely, atorvastatin and other lipophilic statins commonly prescribed in clinics appear to have higher levels of exposure in extra hepatic tissues and very high uptake into cardiac muscles.Citation2,Citation3 It appears that the effect of statins should not be considered a class effect since small and large scale trials that employed rosuvastatin appeared not to have had a beneficial effect in HF. In addition, a recent meta-analysis of RCTs, which included the CORONA and GISSI-HF trials, suggests lipophilic statins have a significant outcome benefit which was not observed in patients randomized to rosuvastatin or placebo.Citation133 However, a class effect of statins at relatively low doses has been reported in elderly cohorts of congestive HF with atorvastatin, simvastatin, pravastatin, and lovastatin without rosuvastatin in a large population study.Citation136
Maison et alCitation114 recently followed a cohort of 281 chronic HF patients after hospital admission through a search of the health insurance and national mortality data base for 1 year and 8 years, respectively. The use of β-blockers and statins was associated with statistically significant survival ahead of ACE inhibitors, spironolactone, and diuretics after controlling for confounders using multivariate analysis. Statin therapy was associated with better survival at 8 years. The findings correspond with those of the prospective study that followed patients for about 9 years, suggesting that statins may reduce mortality after longer follow-ups.Citation115
Given that this recent evidence for statin therapy comes from non-randomized studies which suffer from threats of internal validity arising from risk of confounding and bias, the robust study design and statistical analyses employed by Thambidorai et alCitation113 and Gastelurrutia et alCitation115 and Maison et al,Citation114 eliminate or partly address the bias. Granted that, in these studies, the benefit of statins was assessed in real life healthcare settings without strict inclusion criteria questioning the external validity of RCTs, these findings complement previous evidence provided by small RCTs that employed lipophilic statins.Citation116,Citation117,Citation121 The two recent large trials – CORONACitation15 and GISSI-HFCitation16 – confirmed the surrogate effects exhibited by statins in small randomized trials but failed to ultimately confer survival benefit. The choice of statins, dose, as well as patient background, may possibly have accounted for the findings of the CORONA and GISSI-HF studies in contrast with earlier and recent studies. On the other hand, evidence from recent studies, though non-randomized, complements the findings of the small randomized trials but seems to suggest lipophilic statins provide better outcomes than hydrophilic statins in patients with HF.Citation113,Citation115
Statins and HF comorbid conditions
Comorbidities are common in patients with HF. While some of these contribute to the underlying pathogenesis, others may lead to the progression, associated poor prognosis, and consequently increase mortality in HF patients. Germane to appropriate management of these comorbidities exists the concern of polypharmacy in hitherto overburdened HF patients.
Hypertension causes or coexists with HF and its management in HF could be challenging. Statins possess many pleiotropic effects including improvement in endothelial function, reduction in inflammation and oxidative stress, and downregulation of angiotensin II receptors and endothelin, which would suggest that statins may reduce blood pressure in patients with hypertension. Indeed, evidence from various experimental models suggests antihypertensive actions, though clinical evidence has been inconclusive.Citation137 Statins may augment actions of ACE inhibitors, ARBs, and β-blockers, which double as antihypertensive and conventional pharmacological agents for HF.Citation138
Further, hyperuricemia is frequently present in chronic HF and has been attributed to increased production or decreased urinary excretion of uric acid (UA) or both in a compromised circulation.Citation135 An elevated plasma level of UA is linked with a wide variety of injurious processes comprising increased inflammatory markers, cell apoptosis, and ED,Citation139 which could cumulatively worsen HF. Serum UA is known to be a marker of HF prognosis and mortalityCitation140–Citation142 and statins have been shown to decrease UA levels by increasing urate excretion.Citation143,Citation144
Chronic renal failure (CRF) is a condition which often complicates pharmacotherapy in HF. CRF is also associated with hyperuricemia worsened by diuretic therapy, which is critical in the management of fluid retention, but increases UA levels. Statins have been shown to improve renal function partly through their modulation of the mevalonate pathway to reduce oxidative stress, inflammation, and hypercoagulability,Citation145 all of which are linked with renal dysfunction via increased atherosclerosis and ED.Citation144,Citation146
Chronic obstructive pulmonary disease (COPD) is a common comorbid condition with incidence varying from 20% to 30% in patients with HF.Citation147 COPD is an independent predictor of mortality in HF.Citation148 Beta-adrenergic agonists are commonly prescribed as mainstay therapy in COPD, but have been shown in a meta-analysis to significantly increase the risk of cardiovascular events such as tachycardia, AF, myocardial infarction, and HF.Citation149 The current therapy relieves symptoms and reduces hospitalization, but does not change disease progression or reduce mortality.Citation150 Statins inhibit isoprenoid production on the mevalonate pathway to reduce inflammation (systemic and pulmonary), thereby improving exercise tolerance and reducing mortality in patients with COPD.Citation151,Citation152
Statins exert various molecular mechanisms that may modulate the pathophysiology to an extent that may be identical to or even seem to overlap those of currently recommended HF therapies. Additionally, statins may favorably modulate the pathogenesis of HF comorbidities and possibly reverse and/or reduce progression as well as issues associated with polypharmacy in patients with HF. illustrates how statins influence the pathophysiology of HF and its comorbidities. Therefore, statins could merit second line treatment consideration in HF guidelines.
Further research is required to clarify whether statins are still beneficial in HF and warrants consideration into guidelines for the treatment of HF. A sufficiently powered randomized trial to evaluate the effect of other statins apart from rosuvastatin in HF is necessary. In countries where another statin trial in HF is unethical and unlikely, a comparative effectiveness research study is required to compare the efficacies of various statins in patients with HF using data from registries, hospital records, and insurance claims. Alternatively, a direct head to head comparison of the two potent statins (rosuvastatin and atorvastatin) may be ethical since no eligible patients are denied treatment or given a placebo. In addition, an indirect comparison meta-analysis of RCTs which compared statin versus placebo or no statin treatment in HF may be required in the absence of adequately powered head-to-head comparison studies to investigate whether statins are comparable or some types have superior efficacy over others.Citation153
Conclusion
Statin therapy for hypercholesterolemia and primary and secondary prevention of CAD has been established, however, their effects on HF survival remain unclear. The latest evidence from prospective but non-randomized studies complements that of small randomized statin trials in HF and suggests lipophilic statins provide better clinical outcomes than hydrophilic statins. We therefore recommend a randomized trial to evaluate the class effect of statins in HF, but until sufficient evidence is amassed, statin treatment should be based on recommendations from guidelines. Our evidence shows that statins modulate the pathophysiology of HF to an extent that may be identical or even overlap recommended HF therapies. Moreover, statins exert mechanisms on various pathways to reduce or reverse progression of many HF comorbidities beyond the therapeutic actions of some of the mainstay medical therapies and could, in the worse scenario, merit second line or adjuvant therapy consideration in treatment guidelines for HF.
Acknowledgments
This research was funded by Monash University Sunway Campus.
Disclosure
The authors report no conflicts of interest in this work.
References
- McMurrayJJAdamopoulosSAnkerSDESC Committee for Practice GuidelinesESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESCEur J Heart Fail201214880386922828712
- WierzbickiASPostonRFerroAThe lipid and non-lipid effects of statinsPharmacol Ther20039919511212804701
- SchachterMChemical, pharmacokinetic and pharmacodynamic properties of statins: an updateFundam Clin Pharmacol200519111712515660968
- Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S)Lancet19943448934138313897968073
- ShepherdJCobbeSMFordIPrevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study GroupN Engl J Med199533320130113077566020
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study GroupN Engl J Med199833919134913579841303
- LaufsUKilterHKonkolCWassmannSBöhmMNickenigGImpact of HMG CoA reductase inhibition on small GTPases in the heartCardiovasc Res200253491192011922901
- LaufsULiaoJKPost-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPaseJ Biol Chem19982733724266242719727051
- LaufsULiaoJKIsoprenoid metabolism and the pleotropic effects of statinsCurr Atheroscler Rep20035537237812911847
- SacksFMPfefferMAMoyeLAThe effect of pravastatin on coronary events after myocardial myocardial infarction in patients with average cholesterol levels.Cholesterol and Recurrent Events trial investigatorsN Engl J Med199633514100110098801446
- SchwartzGGOlssonAGEzekowitzMDMyocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study InvestigatorsEffects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomised controlled trialJAMA2001285131711171811277825
- de LemosJABlazingMAWiviottSDInvestigatorsEarly intensive versus a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trialJAMA2004292111307131615337732
- KorenMJHunninghakeDBALLIANCE InvestigatorsClinical outcomes in managed care patients with coronary heart diseas treated agressively in lipid-lowering disease management clinics: the alliance studyJ Am Coll Cardiol20044491772177915519006
- EzekowitzJMcAlisterFAHumphriesKHAPPROACH InvestigatorsThe association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery diseaseJ Am Coll Cardiol20044481587159215489090
- KjekshusJApetreiEBarriosVCORONA GroupRosuvastatin in older patients with systolic heart failureN Engl J Med2007357222248226117984166
- TavazziLMaggioniAPMarchioliRGissi-HF InvestigatorsEffect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trialLancet200837296451231123918757089
- RauchhausMCoatsAJAnkerSDThe endotoxin-lipoprotein hypothesisLancet2000356923393093311036910
- RamasubbuKEstepJWhiteDLDeswalAMannDLExperimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathyJ Am Coll Cardiol200851441542618222351
- van der HarstPVoorsAAvan GilstWHBöhmMvan VeldhuisenDStatins in the treatment of chronic heart failure: a systematic reviewPLoS Med200638e33316933967
- FurchgottRFZawadzkiJVThe obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholineNature198028857893733766253831
- RubanyiGMRomeroJCVanhouttePMFlow-induced release of endothelium-derived relaxing factorAm J Physiol19862506 Pt 2H1145H11493487253
- LibbyPAikawaMJainMKVascular endothelium and atherosclerosisHandb Exp Pharmacol2006176 Pt 228530616999230
- DavignonJGanzPRole of endothelial dysfunction in atherosclerosisCirculation200410923 Suppl 1III27III3215198963
- BauersachsJWidderJDEndothelial dysfunction in heart failurePharmacol Rep200860111912618276993
- RamseyMWGoodfellowJJonesCJLuddingtonLALewisMJHendersonAHEndothelial control of arterial distensibility is impaired in chronic heart failureCirculation19959211321232197586306
- BuusNHBøttcherMHermansenFSanderMNielsenTTMulvanyMJInfluence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemiaCirculation2001104192305231011696470
- Vásquez-VivarJKalyanaramanBMartásekPSuperoxide generation by endothelial nitric oxide synthase: the influence of cofactorsProc Natl Acad Sci USA19989516922092259689061
- BorlaugBAMelenovskyVRussellSDImpaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fractionCirculation2006114202138214717088459
- LaufsULa FataVPlutzkyJLiaoJKUpregulation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitorsCirculation19989712112911359537338
- von HaehlingSAnkerSDBassengeEStatins and the role of nitric oxide in chronic heart failureHeart Fail Rev2003819910612652163
- VaughanCJMurphyMBBuckleyBMStatins do more than just lower cholesterolLancet19963489034107910828874463
- Hernández-PereraOPérez-SalaDNavarro-AntolínJEffects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cellsJ Clin Invest199810112271127199637705
- KatzSKhanTZeballosGADecreased activity of the L-arginine-nitric oxide metabolic pathway in patients with congestive heart failureCirculation199999162113211710217650
- DattaSR BAGreenbergMECellular survival: a play in three AktsGenes Dev199913222905292710579998
- KureishiYLuoZShiojimaIThe HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animalsNat Med2000691004101010973320
- WilliamsJKSukhovaGKHerringtonDMLibbyPPravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeysJ Am Coll Cardiol19983136846919502654
- JonesSPGreerJJvan HaperenRDunckerDJde CromRLeferDJEndothelial nitric oxide synthase overexpression attenuates congestive heart failure in miceProc Natl Acad Sci USA200310084891489612676984
- AmudhaKChoyAMMustafaMRLangCCShort-term effect of atorvastatin on endothelial function in healthy offspring of parents with type 2 diabetes mellitusCardiovasc Ther200826425326119035876
- EgashiraKHirookaYKaiHReduction in serum cholesterol with pravastatin improves endothelium dependent coronary vasomotion in patients with hypercholesterolemiaCirculation1994896251925248205659
- StroesESKoomansHAde BruinTWRabelinkTJVascular function in the forearm of hypercholesterolaemic patient’s off and on lipid-lowering medicationLancet199534689734674717637480
- LevineBKalmanJMayerLFillitHMPackerMElevated circulating levels of tumor necrosis factor in severe chronic heart failureN Engl J Med199032342362412195340
- PackerMIs tumor necrosis factor an important neurohormonal mechanism in chronic heart failure?Circulation1995926137913827664414
- PinskyDJCaiBYangXRodriguezCSciaccaRRCannonPJThe lethal effects of cytokine-induced nitric oxide on cardiac myocytes are blocked by nitric oxide synthase antagonism or transforming growth factor betaJ Clin Invest19959526776857532189
- PahanKSheikhFGNamboodiriAMSinghILovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophagesJ Clin Invest199710011267126799389730
- RosensonRSTangneyCCCaseyLCInhibition of proinflammatory cytokine production by pravastatinLancet1999353915798398410459915
- GripOJanciauskieneSLindgrenSPravastatin down-regulates inflammatory mediators in human monocytes in vitroEur J Pharmacol20004101839211134659
- GoldsteinJLBrownMSRegulation of the mevalonate pathwayNature199034362574254301967820
- CaseyPJProtein lipidation in cell signalingScience199526852082212257716512
- DechendRFiebelerAParkJKAmelioration of angiotensin II-induced cardiac injury by a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitorCirculation2001104557658111479256
- RidkerPMDanielsonEFonsecaFAJUPITER Study GroupRosuvastatin to prevent vascular events in men and women with elevated C-reactive proteinN Engl J Med2008359212195220718997196
- ThomasCVCokerMLZellnerJLHandyJRCrumbleyAJ3rdSpinaleFGIncreased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathyCirculation19989717170817159591765
- BellostaSViaDCanavesiMHMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophagesArterioscler Thromb Vasc Biol19981811167116789812903
- AikawaMRabkinESugiyamaSAn HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitroCirculation2001103227628311208689
- ClelandJGMcMurrayJJKjekshusJCORONA Study GroupPlasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure)J Am Coll Cardiol200954201850185919892235
- KangPMIzumoSApoptosis and heart failure: A critical review of the literatureCirc Res200086111107111310850960
- ThunyakitpisalPDChaisuparatRSimvastatin, an HMG-CoA reductase inhibitor, reduced the expression of matrix metalloproteinase-9 (Gelatinase B) in osteoblastic cells and HT1080 fibrosarcoma cellsJournal of Pharmacological Sciences200494440340915107580
- MitaniHBandohTIshikawaJKimuraMTotsukaTHayashiSInhibitory effects of fluvastatin, a new HMG-CoA reductase inhibitor, on the increase in vascular ACE activity in cholesterol-fed rabbitsBr J Pharmacol19961196126912758937733
- LuoJDZhangWWZhangGPGuanJXChenXSimvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosisClin Exp Pharmacol Physiol1999261190390810561812
- TakemotoMNodeKNakagamiHStatins as antioxidant therapy for preventing cardiac myocyte hypertrophyJ Clin Invest2001108101429143711714734
- ShepherdJBlauwGJMurphyMBPROSPER study groupPROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trialLancet200236093461623163012457784
- KrumHAshtonEReidCDouble-Blind, randomized, placebo-controlled study of high-dose hmg coa reductase inhibitor therapy on ventricular remodeling, pro-inflammatory cytokines and neurohormonal parameters in patients with chronic systolic heart failureJ Card Fail20071311717338996
- CannonCPBraunwaldEMcCabeCHPravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 InvestigatorsIntensive versus moderate lipid lowering with statins after acute coronary syndromesN Engl J Med2004350151495150415007110
- SharmaUCPokharelSvan BrakelTJGalectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunctionCirculation2004110193121312815520318
- van KimmenadeRRJanuzziJLJrEllinorPTUtility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failureJ Am Coll Cardiol20064861217122416979009
- LokDJVan Der MeerPde la PortePWPrognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF studyClin Res Cardiol201099532332820130888
- de BoerRALokDJJaarsmaTPredictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fractionAnn Med2011431606821189092
- GullestadLUelandTKjekshusJCORONA Study GroupGalectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA)Eur Heart J201233182290229622513778
- van der HarstPVoorsAAvan GilstWHvan VeldhuisenDJStatins and autonomic function in chronic heart failureCardiovasc Drugs Ther200519316716816142591
- GoldmanSJohnsonGCohnJNCintronGSmithRFrancisGMechanism of death in heart failure. The Vasodilator-Heart Failure Trials. The V-HeFT VA Cooperative Studies GroupCirculation199387Suppl 6VI24VI318500236
- MudersFKromerEPGrieseDPEvaluation of plasma natriuretic peptides as markers for left ventricular dysfunctionAm Heart J199713434424499327700
- RidkerPMRifaiNPfefferMASacksFBraunwaldELong-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) InvestigatorsCirculation1999100323023510411845
- OiSHanedaTOsakiJLovastatin prevents angiotensin II-induced cardiac hypertrophy in cultured neonatal rat heart cellsEur J Pharmacol19993761–213914810440099
- PliquettRUCornishKGPeulerJDZuckerIHSimvastatin normalizes autonomic neural control in experimental heart failureCirculation2003107192493249812695293
- MassonSLatiniRAnandISVal-HeFT InvestigatorsDirect comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) dataClin Chem20065281528153816777915
- OmlandTSabatineMSJablonskiKAPEACE InvestigatorsPrognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE TrialJ Am Coll Cardiol200750320521417631211
- TulevskiIIGroeninkMvan Der WallEEIncreased brain and atrial natriuretic peptides in patients with chronic right ventricular pressure overload: correlation between plasma neurohormones and right ventricular dysfunctionHeart2001861273011410557
- KrügerSGrafJKunzDStickelTHanrathPJanssensUBrain natriuretic peptide levels predict functional capacity in patients with chronic heart failureJ Am Coll Cardiol200240471872212204502
- McDonaghTARobbSDMurdochDRBiochemical detection of left-ventricular systolic dysfunctionLancet199835190959139433422
- NakayaRUzuiHShimizuHPravastatin suppresses the increase in matrix metalloproteinase-2 levels after acute myocardial infarctionInt J Cardiol20051051677316207547
- HayashidaniSTsutsuiHShiomiTFluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarctionCirculation2002105786887311854129
- KeidarSAviramMMaorIOiknineJBrookJGPravastatin inhibits cellular cholesterol synthesis and increases low density lipoprotein receptor activity in macrophages: in vitro and in vivo studiesBr J Clin Pharmacol19943865135197888289
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trialLancet2002360932672212114036
- RemmeWJOverview of the relationship between ischemia and congestive heart failureClin Cardiol2000237 Suppl 4IV4IV810894449
- RosensonRSTangneyCCAntiatherothrombotic properties of statins: implications for cardiovascular event reductionJAMA199827920164316509613915
- KjekshusJPedersenTROlssonAGFaergemanOPyöräläKThe effects of simvastatin on the incidence of heart failure in patients with coronary heart diseaseJ Card Fail1997342492549547437
- JacksonGGibbsCRDaviesMKLipGYABC of heart failure. PathophysiologyBMJ2000320722816717010634740
- HannaIRHeekeBBushHLipid-lowering drug use is associated with reduced prevalence of atrial fibrillation in patients with left ventricular systolic dysfunctionHeart Rhythm20063888188616876733
- FauchierLPierreBde LabriolleAGrimardCZannadNBabutyDAntiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trialsJ Am Coll Cardiol200851882883518294568
- VyasAKGuoHMossAJMADIT-II Research GroupReduction in ventricular tachyarrhythmias with statins in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-IIJ Am Coll Cardiol200647476977316487843
- GoldbergerJJSubaciusHSchaechterADEFINITE InvestigatorsEffects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathyJ Am Coll Cardiol20064861228123316979011
- TamargoJCaballeroRGómezRNúñezLVaqueroMDelpónELipid-lowering therapy with statins, a new approach to antiarrhythmic therapyPharmacol Ther2007114110712617287023
- AdamOFrostGCustodisFRole of Rac1 GTPase activation in atrial fibrillationJ Am Coll Cardiol200750435936717659204
- RahimiKEmbersonJMcGalePPROSPER ExecutiveEffect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trialsBMJ2011342d125021411487
- RauchhausMClarkALDoehnerWThe relationship between cholesterol and survival in patients with chronic heart failureJ Am Coll Cardiol200342111933194014662255
- TreasureCBKleinJLWeintraubWSBeneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery diseaseN Engl J Med199533284814877830728
- MortensenSALethAAgnerERohdeMDose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitorsMol Aspects Med199718SupplS137S1449266515
- MoosmannBBehlCSelenoprotein synthesis and side-effects of statinsLancet2004363941289289415031036
- PoehlmanETScheffersJGottliebSSFisherMLVaitekeviciusPIncreased resting metabolic rate in patients with congestive heart failureAnn Intern Med1994121118608627772113
- RundekTNainiASaccoRCoatesKDiMauroSAtorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and strokeArch Neurol200461688989215210526
- AthyrosVGMikhailidisDPPapageorgiouAAEffect of atorvastatin on high density lipoprotein cholesterol and its relationship with coronary events: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) StudyCurrent medical research and opinion5200420562763715171226
- PedersenTRFaergemanOKasteleinJJIncremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study GroupHigh – dose atorvastatin vrs usual – dose simvastatin for secondary prevention after myocardial infarction: IDEAL study: a randomized-controlled trialJAMA2005294192437244516287954
- KhushKKWatersDDBittnerVEffect of high-dose atorvastatin on hospitalization for heart failure: subgroup analysis of the Treat to New Targets (TNT) studyCirculation2007115557658317261662
- KubotaTMiyagishimaMAlvarezRJExpression of proinflammatory cytokines in the failing human heart: comparison of recent-onset and end-stage congestive heart failureJ Heart Lung Transplant200019981982411008069
- KrumHLatiniRMaggioniAPStatins and symptomatic chronic systolic heart failure: a post-hoc analysis of 5010 patients enrolled in Val-HeFTInt J Cardiol20071191485317049646
- KrumHBaileyMMeyerWImpact of statin therapy on mortality in CHF patients according to beta-blocker use: results of CIBIS IICardiology20071081283416960445
- AnkerSDClarkALWinklerRStatin use and survival in patients with chronic heart failure: results from two observational studies with 5200 patientsInt J Cardiol2006112223424216846656
- JoyntKEGattisWAHasselbladVEffect of angiotensin-converting enzyme inhibitors, beta blockers, statins, and aspirin on C-reactive protein levels in outpatients with heart failureAm J Cardiol200493678378515019895
- GoASLeeWYYangJLoJCGurwitzJHStatin therapy and risks for death and hospitalization in chronic heart failureJAMA2006296172105211117077375
- FoodyJMShahRGalushaDMasoudiFAHavranekEPKrumholzHMStatin and mortality among elderly patients hospitalized with heart failureCirculation200611381086109216490817
- HognestadADicksteinKMyhreESnapinnSKjekshusJOPTIMAAL InvestigatorsEffect of combined statin and beta-blocker treatment on one-year morbidity and mortality after acute myocardial infarction associated with heart failureAm J Cardiol200493560360614996587
- RayJGGongYSykoraKTuJVStatin use and survival outcomes in elderly patients with heart failureArch Intern Med20051651626715642876
- SolaSMirMQSRajagopalanSHelmyTTandonNKhanBVStatin therapy is associated with improved cardiovascular outcomes and levels of inflammatory markers in patients with heart failureJ Card Fail200511860761216230264
- ThambidoraiSKDeshmukhARWaltersRWImpact of statin use on heart failure mortalityInt J Cardiol2011147343844320971517
- MaisonPDesamericqGHemeryFRelationship between recommended chronic heart failure treatments and mortality over 8 years in real-world conditions: a pharmacoepidemiological studyEur J Clin Pharmacol201369490190822993100
- GastelurrutiaPLupónJde AntonioMStatins in heart failure: the paradox between large randomized clinical trials and real lifeMayo Clin Proc201287655556022677075
- VrtovecBOkrajsekRGolicnikAAtorvastatin therapy may reduce the incidence of sudden cardiac death in patients with advanced chronic heart failureJ Card Fail200814214014418325461
- WojniczRWilczekKNowalany-KozielskaEUsefulness of atorvastatin in patients with heart failure due to inflammatory dilated cardiomyopathy and elevated cholesterol levelsAm J Cardiol200697689990416516598
- XieRQCuiWLiuFYangCPeiWNLuJCStatin therapy shortens QTc, QTcd, and improves cardiac function in patients with chronic heart failureInt J Cardiol2010140225525719042044
- SolaSMirMQLerakisSTandonNKhanBVAtorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failureJ Am Coll Cardiol200647233233716412856
- YamadaTNodeKMineTLong-term effect of atorvastatin on neurohumoral activation and cardiac function in patients with chronic heart failure: A prospective randomized controlled studyAm Heart J200715361055. e11055. e817540209
- NodeKFujitaMKitakazeMHoriMLiaoJKShort-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathyCirculation2003108783984312885745
- HamaadASosinMLipGYMacFadyenRJShort-term adjuvant atorvastatin improves frequency domain indices of heart rate variability in stable systolic heart failureCardiovasc Drugs Ther200519318318716142595
- BleskeBENicklasJMBardRLNeutral effect on markers of heart failure, inflammation, endothelial activation and function, and vagal tone after high-dose HMG-CoA reductase inhibition in non-diabetic patients with non-ischemic cardiomyopathy and average low-density lipoprotein levelJ Am Coll Cardiol200647233834116412857
- LaufsUWassmannSSchackmannSHeeschenCBöhmMNickenigGBeneficial effects of statins in patients with non-ischemic heart failureZ Kardiol200493210310814963675
- TousoulisDAndreouITentolourisCComparative effects of rosuvastatin and allopurinol on circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with chronic heart failureInt J Cardiol2010145343844319539384
- ErbsSBeckEBLinkeAHigh-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling – results from a randomized, double-blind, and placebo-controlled studyInt J Cardiol20111461566320236716
- TsutamotoTSakaiHIbeKEffect of atorvastatin vs rosuvastatin on cardiac sympathetic nerve activity in non-diabetic patients with dilated cardiomyopathyCirc J20117592160216621737951
- AndreouITousoulisDMiliouAEffects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: a randomized placebo-controlled studyAtherosclerosis2010210119419819962701
- Bielecka-DabrowaAGochJHMikhailidisDPRyszJMaciejewskiMBanachMThe influence of atorvastatin on parameters of inflammation and function of the left ventricle in patients with dilated cardiomyopathyMed Sci Monit20091512MS12MS2319946241
- TousoulisDAntoniadesCBosinakouEEffects of atorvastatin on reactive hyperemia and inflammatory process in patients with congestive heart failureAtherosclerosis2005178235936315694946
- HorwichTBMiddlekauffHRMaclellanWRFonarowGCStatins do not significantly affect muscle sympathetic nerve activity in humans with nonischemic heart failure: a double-blind placebo-controlled trialJ Card Fail2011171187988622041323
- ZhangSZhangLSunAJiangHQianJGeJEfficacy of statin therapy in chronic systolic cardiac insufficiency: a meta-analysisEur J Intern Med201122547848421925056
- LipinskiMJCauthenCABiondi-ZoccaiGGMeta-analysis of randomized controlled trials of statins versus placebo in patients with heart failureAm J Cardiol2009104121708171619962481
- EmbersonJRNgLLArmitageJBowmanLParishSCollinsRHeart Protection Study Collaborative GroupN-terminal Pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection studyJ Am Coll Cardiol200749331131917239712
- OchiaiMEBarrettoACOliveiraMTJrMunhozRTMorgadoPCRamiresJAUric acid renal excretion and renal insufficiency in decompensated severe heart failureEur J Heart Fail20057446847415921781
- RinfretSBehlouliHEisenbergMJHumphriesKTuJVPiloteLClass effects of statins in elderly patients with congestive heart failure: a population-based analysisAm Heart J2008155231632318215603
- JuncosLIJuncosLAGarcíaNHThe antihypertensive actions of statins: modulation by salt intakeAm J Hypertens201225111140114822833031
- PelatMBalligandJLStatins and hypertensionSemin Vasc Med20044436737515861317
- LeyvaFAnkerSSwanJWSerum uric acid as an index of impaired oxidative metabolism in chronic heart failureEur Heart J19971858588659152657
- AnkerSDDoehnerWRauchhausMUric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic stagingCirculation2003107151991199712707250
- CengelATürkoğluSTurfanMBoyaciBSerum uric acid levels as a predictor of in-hospital death in patients hospitalized for decompensated heart failureActa Cardiol200560548949216261779
- HareJMJohnsonRJUric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiologyCirculation2003107151951195312707249
- MilionisHJKakafkaAITsouliSGEffects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemiaAm Heart J2004148463564015459594
- AthyrosVGElisafMPapageorgiouAAGREACE Study Collaborative GroupEffect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) studyAm J Kidney Dis200443458959915042535
- LjungmanSLaraghJHCodyRJRole of the kidney in congestive heart failure. Relationship of cardiac index to kidney functionDrugs199039Suppl 41021 discussion 22–24.1462354670
- ElisafMMikhailidisDPStatins and renal functionAngiology200253549350212365855
- NagarajanVTangWHManagement of comorbid conditions in heart failure: a reviewMed Clin North Am201296597598522980059
- De BloisJSimardSAtarDAgewallSNorwegian Heart Failure RegistryCOPD predicts mortality in HF: the Norwegian Heart Failure RegistryJ Card Fail201016322522920206897
- SalpeterSROrmistonTMSalpeterEECardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysisChest200412562309232115189956
- RocheNWhere current pharmacological therapies fall short in COPD: symptom control is not enoughEur Respir Rev20071610598104
- JandaSParkKFitzGeraldJMEtminanMSwistonJStatins in COPD: a systematic reviewChest2009136373474319376844
- DoblerCCWongKKMarksGBAssociations between statins and COPD: a systematic reviewBMC Pulm Med200993219594891
- BonsuKOKadirveluAReidpathDDLipophilic versus hydrophilic statin therapy for heart failure: a protocol for an adjusted indirect comparison meta-analysisSystematic reviews201322223618535