Abstract
Background
Allowing patients to measure their blood pressure at home is recognized as being of clinical value. However, it is not known how often these measurements are taken correctly. Blood pressure monitors for home use fall into two types based on the position of the cuff, ie, at the upper arm or the wrist. The latter is particularly convenient, as measurements can be taken fully clothed. This study aimed to evaluate the performance of the wrist-type blood pressure monitors Omron RS8 (HEM-6310F-E), Omron RS6 (HEM-6221-E), and Omron RS3 (HEM-6130-E).
Methods
A team of three trained doctors validated the performance of these devices by comparing the measurements obtained from these devices with those taken using a standard mercury sphygmomanometer. All the devices met the validation requirements of the European Society of Hypertension International Protocol revision 2010.
Results
The difference in blood pressure readings between the tested device and the standard mercury sphygmomanometer was within 3 mmHg, which is acceptable according to the European Society of Hypertension guidelines.
Conclusion
All the home devices tested were found to be suitable for measuring blood pressure at home because their performance fulfilled the requirement of the guidelines.
Introduction
Enabling patients to measure their blood pressure (BP) at home can aid the prevention and early detection of hypertension and future cardiovascular disease. Recently, home BP measurement was found to be superior to clinical BP measurement for the detection and ongoing follow-up of hypertension.Citation1,Citation2 The important role of home BP measurement has been described in several guidelines, and many devices for home BP measurement portant to evaluate their accuracy. However, the fluctuating nature of BP makes the validation of these devices difficult in a clinical setting, and a number of international protocols have therefore been established for the validation of these devices.Citation5–Citation7
The standard location for BP measurement is the upper arm, but wrist devices have become popular for home use. The mechanism used by wrist and upper arm devices for measuring BP is essentially the same. The wrist devices have the advantage of being smaller and lighter than upper arm devices, are easier to fit, and do not require the patient to undress.
In this study, three devices were validated in three separate investigations in accordance with the international protocol of the European Society of Hypertension International Protocol revision 2010.Citation7
Materials and methods
Omron RS8
The Omron RS8 (also known as HEM-6310F-E; Omron Healthcare Co, Ltd, Kyoto, Japan) is an electronic oscillometric device used to measure BP at the wrist. The device measures BP during the inflation period of the cuff. It analyzes the pulse wave detected during inflation using an algorithm for determining systolic and diastolic BP. Immediately after determination of diastolic BP, it rapidly deflates the cuff to finish the measurement. The cuff is inflated automatically by an electric pump and then deflated by a mechanical valve. The cuff pressure ranges from 0 to 299 mmHg, and it can measure heart rate in the range of 40–180 beats per minute. The total weight of the device is approximately 80 g (without batteries), and the dimensions without the cuff are approximately 89 × 61 × 13 mm (W × H × D). The device can be powered by two type LR03 alkaline batteries. The time, date, systolic BP, diastolic BP, heart rate, and scale of the BP level are displayed on a liquid crystal display. The cuff can be used for wrist circumferences in the range of 135–215 mm. The device can store up to 100 measurements and calculates three readings to generate an average value within 10 minutes, for two users. The device calculates and displays the weekly average of measurements taken in the morning and evening over the previous eight weeks for each user. It also has a positioning sensor to set it at the level of the heart. The device can detect and display the wrapping state of the cuff. The device additionally has near-field communication technology to transfer data to a personal computer or mobile phone. The device detects and displays body motion and an irregular heartbeat during BP measurement.
Omron RS6
The Omron RS6 (also known as HEM-6221-E; Omron Healthcare Co, Ltd) is an electronic oscillometric device for BP measurement at the wrist. The performance of the device is the same as that of the Omron RS8. The total weight of the device is approximately 85 g (without batteries), and the dimensions without the cuff are approximately 87 × 64 × 14 mm (W × H × D). The device can be powered by two type LR03 alkaline batteries. The time, date, systolic BP, diastolic BP, heart rate, and scale of the BP level are displayed on a liquid crystal display. The cuff can be used for wrist circumferences in the range of 135–215 mm, and the device can store up to 90 measurements and calculates an average of three readings taken over 10 minutes. The device has the positioning sensor of the wrist like the Omron RS8, and also detects and displays the wrapping state of the cuff, body motion, and an irregular heartbeat during BP measurement.
Omron RS3
The Omron RS3 (also known as HEM-6130-E; Omron Healthcare Co, Ltd) is also an electronic oscillometric device for BP measurement at the wrist. The performance of the device is the same as that of the Omron RS8. The total weight of the device is approximately 101 g (without batteries), and the dimensions without the cuff are approximately 78 × 60 × 21 mm (W × H × D). The device can be powered by two type LR03 alkaline batteries. The time, date, systolic BP, diastolic BP, heart rate, and BP level are displayed on a liquid crystal display. The cuff can be used for wrist circumferences in the range of 135–215 mm, and the device can store up to 60 measurements and calculates an average of three readings taken over 10 minutes. The device detects and displays the wrapping state of the cuff, body motion, and an irregular heartbeat during BP measurement.
Blood pressure measurement
For each study, the manufacturer was asked to provide a complete device, which they described as the standard production model in each case. The validation team for each device consisted of three observers who were experienced in taking BP measurements. They were trained using information provided by the British Hypertension Society on its website (http://www.bhsoc.org). The control data were the measurements obtained by the observers using a standard mercury sphygmomanometer, ie, two of the three observers measured BP using a teaching stethoscope with two Y-tube headsets for simultaneous measurements with one standard mercury sphygmomanometer. The BP measurement components were carefully checked before the study; the third observer was the supervisor, who checked that the BP values obtained by the other two observers. The two observers performing the BP measurements were blinded to each other’s readings.
Participants
The participants were selected in accordance with the European Society of Hypertension International Protocol revision 2010. A total of 33 participants were recruited for the study, all of whom were at least 25 years old, and had systolic BP of 90–180 mmHg and diastolic BP of 40–130 mmHg at the beginning of the study. All the participants had a regular sinus rhythm and the systolic and diastolic BP at the beginning of the study were categorized. Participants were recruited from among outpatients and volunteers at the Kansai Medical University, Hirakata Hospital, Osaka, Japan. The protocol was approved by the internal review board at the hospital, and written informed consent was obtained from all participants.
Procedures
Each participant was allowed to sit in a quiet air-conditioned room for at least five minutes. The procedure then started with measurement of the arm and wrist circumferences. The measurement points were 8 cm above the left elbow joint and 4 cm above the left wrist joint, and all measurements were taken on the left arm and wrist. A total of nine BP measurements were repeated using the device and auscultation alternately in the same arm, in accordance with the European Society of Hypertension International Protocol revision 2010. Measurements with the Omron RS8 (HEM-6130F-E), Omron RS6 (HEM-6221-E), and Omron RS3 (HEM-6130-E) devices were taken in accordance with the manufacturer’s instructions.
Statistical analysis
Differences in measurements collected by the device and by the two observers using a sphygmomanometer were classified according to their magnitude. Differences were calculated by subtracting the mean value of the two observers’ readings from the value obtained by the device. The systolic and diastolic BP were analyzed separately. The data are expressed as the mean ± standard deviation. Bland–Altman plots were used to show deviations in the data.Citation8
Results
Omron RS8
A total of 43 participants were recruited for this study. Screening and recruiting details are shown in . The study included 33 participants (20 men, 13 women) with a mean age of 50 ± 12.3 (range 28–72) years. The mean arm circumference was 286 ± 338 (range 212–350) mm. The mean wrist circumference was 176 ± 20 (137–212) mm. The characteristics of these patients are shown in . Their mean BP values at entry were 140 ± 32.5 (range 88–219) mmHg for systolic BP and 85 ± 19.9 (range 50–118) mmHg for diastolic BP. The difference between the two observations was −0.2 ± 1.3 mmHg and 0.3 ± 1.4 mmHg for systolic and diastolic BP, respectively.
Table 1 Screening and recruitment details for the Omron RS8 (HEM-6310F-E) validation
Table 2 Participant characteristics for the Omron RS8 (HEM-6310F-E) validation
The mean differences between the device and the control sphygmomanometer BP measurements were 1.5 ± 5.8 (range −24 to 19) mmHg for systolic BP and −1.7 ± 4.6 (range −14 to 14) mmHg for diastolic BP. The similarities between the Omron RS8 and the control BP measurements, classified by magnitudes of 5, 10, and 15 mmHg, are shown in .
Table 3 Comparison of results obtained using the Omron RS8 and a sphygmomanometer
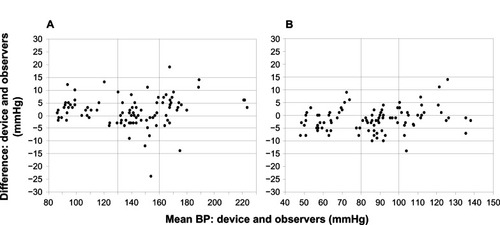
Bland–Altman plots of the differences between BP measurement obtained with the Omron RS8 and those obtained with the sphygmomanometer are shown in and for systolic and diastolic BP, respectively.
Omron RS6
A total of 41 participants were recruited for this study. Screening and recruiting details are shown in . This study included 33 participants (21 men, 12 women) with a mean age of 50 ± 11.6 (range 28–70) years. The mean arm circumference was 283 ± 42 (range 200–410) mm, and the mean wrist circumference was 178 ± 22 (range 135–212) mm. The characteristics of these patients are shown in . Their mean BP values at entry were 142 ± 32.0 (range 94–226) mmHg for systolic BP and 87 ± 21.7 (range 51–134) mmHg for diastolic BP. The difference between the two observations was 0.0 ± 1.4 mmHg and 0.2 ± 1.5 mmHg for systolic and diastolic BP, respectively.
Table 4 Screening and recruitment details for the Omron RS6 (HEM-6221-E) validation
Table 5 Participant characteristics for the Omron RS6 (HEM-6221-E) validation
The mean differences between the device and the control sphygmomanometer BP measurements were 1.3 ± 5.8 (range −11 to 19) mmHg for systolic BP and −0.3 ± 4.5 (range −13 to 11) mmHg for diastolic BP. The similarities between the Omron RS6 and the control BP measurements, classified by magnitudes of 5, 10, and 15 mmHg, are shown in . Bland–Altman plots of the differences between the BP measurements obtained with the Omron RS6 and the sphygmomanometer are shown in and for systolic and diastolic BP, respectively.
Figure 2 Bland–Altman plots of the differences between Omron RS6 (HEM-6221-E) and sphygmomanometer blood pressure (BP) measurements for systolic blood pressure (A) and diastolic blood pressure (B).
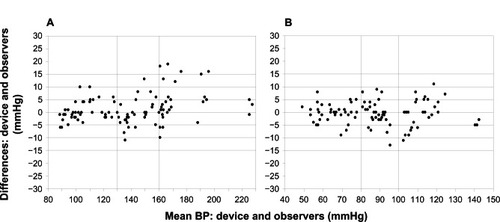
Table 6 Comparison of results obtained using the Omron RS6 and a sphygmomanometer
Omron RS3
A total of 81 participants were recruited for this study. Screening and recruiting details are shown in . This study included 33 participants (15 men and 18 women) with a mean age of 50 ± 11.8 (range 32–75) years. The mean arm circumference was 288 ± 48 (range 199–382) mm, and the mean wrist circumference was 170 ± 22 (range 136–208) mm. The characteristics of these patients are shown in . Their mean BP values at entry were 144 ± 31.1 (range 94–209) mmHg for systolic BP and 87 ± 19.9 (range 52–120) mmHg for diastolic BP. The difference between the two observations was −0.4 ± 1.6 mmHg and −0.3 ± 1.4 mmHg for systolic and diastolic BP, respectively.
Table 7 Screening and recruitment details for the Omron RS3 (HEM-6130-E) validation
Table 8 Participant characteristics for the Omron RS3 (HEM-6130-E) validation
The mean differences between the device and the control sphygmomanometer BP measurements were 1.8 ± 4.3 (range −7 to 18) mmHg for systolic BP and 1.7 ± 4.5 (range −9 to 11) mmHg for diastolic BP. The similarities between the Omron RS3 and the control BP measurements, classified by magnitudes of 5, 10, and 15 mmHg, are shown in . Bland–Altman plots of the differences between BP measurements obtained with the Omron RS3 and the sphygmomanometer are shown in and for systolic and diastolic BP, respectively.
Figure 3 Bland–Altman plots of the differences between Omron RS3 (HEM-6130-E) and sphygmomanometer blood pressure (BP) measurements for systolic blood pressure (A) and diastolic blood pressure (B).
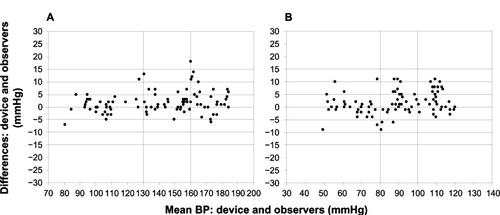
Table 9 Comparison of results obtained using the Omron RS3 and a sphygmomanometer
These results show that the Omron RS8, Omron RS6, and Omron RS3 all met the requirements of the International Protocol revision 2010. Thus, all devices fulfilled the validation criteria of the International Protocol revision 2010.
Discussion
In this validation study, all three Omron devices tested, ie, RS8, RS6, and RS3, met the validation requirements of the European Society of Hypertension International Protocol revision 2010, and may thus be useful for home BP measurement. It should be noted though that the magnitude of the measurement differences between each device and the control sphygmomanometer are greater at higher BP levels. This is because BP fluctuates more in hypertensive subjects due to their impaired baroreceptor sensitivity,Citation9 and differences in the alternately measured BP in the same arm become correspondingly greater. This is a limitation of this validation method.
The most innovative features of the devices are their ability to display the wrapping state of the cuff display (all three devices) and the positioning sensor of the wrist (the Omron RS8 and RS6). The dabl Educational Trust website (http://www.dableducational.org/) and the British Hypertension Society website provide lists of recommended BP monitors for home use that have been validated in accordance with the guidelines of the International Protocol, the British Hypertension Society, or the Association for the Advancement of Medical Instrumentation.Citation6 However, these devices were validated in studies where the BP monitors were used correctly. Unfortunately, it is not always possible to know whether a patient is using the BP measurement device properly at home. There are some important points that clinicians should be aware of when authorizing the use of home BP measurement. One is the possibility of the cuff being incorrectly wrapped and another is the importance of keeping the device at the level of the heart during BP measurement. Wrapping the cuff snugly around the arm allows an accurate reading to be taken.Citation10,Citation11 If the BP monitor is not used correctly, then the measurements will probably be incorrect, reducing the trust that users have in the monitor. Furthermore, correctly wrapping the cuff requires experience, and some users will not use a BP monitor because they find that wrapping the cuff is difficult. This is especially true of the wrist device, because its position substantially influences the BP reading, and it is necessary to keep the cuff at the heart level while measuring BP. The device senses the angle of the forearm, and let us realize the proper angle with blue or orange light. Only when the light is blue will it start to measure BP. A 10 cm difference between the heart level and the cuff position results in a 7 mmHg difference in BP reading; this difference is due to the changed hydrostatic pressure.Citation12,Citation13 The upper arm device is not influenced by hydrostatic pressure. As a result, the guidelines do not recommend the use of a wrist device for home BP measurement, except those with positioning sensors.Citation14 These issues have been addressed by the three devices validated in this study, which may make them useful for ensuring compliance in home BP measurement. A limitation of this study was that the participants were drawn from the general population. It would be desirable to repeat the study in specific populations, such as pregnant women and the elderly.
Conclusion
The results of this study show that the Omron RS8, RS6, and RS3 devices meet the requirements of the International Protocol revision 2010, and may be useful for BP measurement at home.
Acknowledgments
The authors thank the Omron Healthcare Company, Kyoto, Japan, for providing us with the home BP measurement monitors.
Disclosure
The authors report no conflicts of interest in this work.
References
- MenardJChatellierGDayMVaurLSelf-measurement of blood pressure at home to evaluate drug effects by the trough: peak ratioJ Hypertens Suppl199412S21S257707151
- OikawaTObaraTOhkuboTJ-HOME Study GroupCharacteristics of resistant hypertension determined by self-measured blood pressure at home and office blood pressure measurements: the JHOME studyJ Hypertens2006241737174316915022
- ManciaGDe BackerGDominiczakA2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens2007251105118717563527
- The Japanese Society of Hypertension Committee for Guidelines for the Management of HypertensionThe Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2009)Hypertens Res200932310719300436
- O’BrienEPetrieJLittlerWAThe British Hypertension Society protocol for the evaluation of blood pressure measuring devicesJ Hypertens199086076192168451
- Association for the Advancement of Medical InstrumentationAmerican national standards for electronic or automated sphygmomanometers Available from: http://marketplace.aami.org/eseries/scriptcontent/docs/preview%20files/sp100401preview.pdfAccessed April 12, 2013
- O’BrienEAtkinsNStergiouGEuropean Society of Hypertension International revision 2010 for the validation of blood pressure measuring devices in adultsBlood Press Monit201015233820110786
- AtkinsonGNevillAMStatistical methods for assessment error (reliability) in variables relevant to sports medicineSports Med1998262172389820922
- SevreKLefrandtJDNordbyGAutonomic function in hypertensive and normotensive subjects: the importance of genderHypertension2001371351135611408376
- RamseyMIIIBlood pressure monitoring: automated oscillometric devicesJ Clin Monit1991756671999699
- BannerTEGravensteinJSComparative effects of cuff size and tightness of fit on accuracy of blood pressure measurementsJ Clin Monit199172812841744670
- KikuyaMChonanKImaiYGotobEIshiiMAccuracy and reliability of wrist-cuff devices for self measurement of blood pressureJ Hypertens20022062963811910297
- YarowsSAComparison of the Omron HEM-637 wrist monitor to the auscultation method with the wrist position sensor on or disabledAm J Hypertens200417545814700513
- O’BrienEAsmarRBeilinLEuropean Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurementJ Hypertens20032182184812714851