Abstract
The Global Vascular Risk Management (GVRM) Study is a 5-year prospective observational study of 87,863 patients (61% females) with hypertension and associated cardiovascular risk factors began January 1, 2010. Data are gathered electronically and cardiovascular risk is evaluated using the Consortium for Southeastern Hypertension Control™ (COSEHC™)-11 risk score. Here, we report the results obtained at the completion of 33 months since study initiation. De-identified electronic medical records of enrolled patients were used to compare clinical indicators, antihypertensive medication usage, and COSEHC™ risk scores across sex and diabetic status subgroups. The results from each subgroup, assessed at baseline and at regular follow-up periods, are reported since the project initiation. Inference testing was performed to look for statistically significant differences between goal attainments rates between sexes. At-goal rates for systolic blood pressure (SBP) were improved during the 33 months of the study, with females achieving higher goal rates when compared to males. On the other hand, at-goal control rates for total and low-density lipoprotein (LDL) cholesterol (chol) were better in males compared to females. Diabetic patients had lower at-goal rates for SBP and triglycerides but higher rates for LDL-chol. The LDL-chol at-goal rates were higher for males, while high-density lipoprotein (HDL)-chol rates were higher for females. Utilization of antihypertensive medications was similar during and after the baseline period for both men and women. Patients taking two or more antihypertensive medications had higher mean COSEHC™-11 scores compared to those on monotherapy. With treatment, hypertensive patients can reach SBP and cholesterol goals; however, population-wide improvement in treatment goal adherence continues to be a challenge for physicians. The COSEHC™ GVRM Study shows, however, that continuous monitoring and feedback to physicians of accurate longitudinal data is an effective tool in achieving better control rates of cardiovascular risk factors.
Introduction
The metabolic syndrome comprises a constellation of cardiovascular risk factorsCitation1,Citation2 that include abdominal obesity; insulin resistance; glucose intolerance; elevated blood pressure or antihypertensive drug treatment; low levels of high-density lipoprotein (HDL) cholesterol (chol); and elevated triglyceride levels. Abundant evidence shows that the metabolic syndrome predicts the development of cardiovascular disease (CVD).Citation3–Citation5 In a previous article,Citation6 we presented the baseline demographic profile and risk factor prevalence among patients enrolled in an observational large scale assessment of cardiometabolic risk factors in the southeastern United States, a region in which the Consortium for Southeastern Hypertension Control™ (COSEHC™) was among the first to raise awareness of an excess in cardiovascular-related deaths.Citation6–Citation15
The Global Vascular Risk Management (GVRM) Study is a large-scale project to prospectively determine the sensitivity of the COSEHC-cardiovascular risk assessment tool (COSEHC–CRT) to provide benchmarking data on treatment patterns and outcomes to participating COSEHC-designated Cardiovascular Centers of Excellence™.Citation6 De-identified electronic medical records were used to compare clinical indicators, antihypertensive medication usage, and COSEHC™ risk scores across sex and diabetic status subgroups. In this article, we examined the changes in cardiovascular risk factors and rate of clinical events for those patients who continued care with COSEHC™ health care providers during the first 33 months follow-up period from the reported baseline.Citation6 The report documents the changes in systolic blood pressure (SBP), cholesterol measures, and body mass index (BMI) across sex and diabetic status subgroups. We also present results on the utilization of antihypertensive medications for treatment of hypertension, lipid management, and diabetes, along with an evaluation of the COSEHC–CRT score as a function of antihypertensive medication usage.Citation13 The information, gathered throughout the first 33 months of the study, provides new information as to how a cohort of subjects from a population with a documented high rate of cardiovascular events and the participating physicians respond to a structured longitudinal surveillance process based on assessment of clinical and therapeutic goals through the use of electronic medical records (EMRs). The information attained in this study may be used by physicians to understand the value of EMRs and have increased awareness of expected results in terms of sex, clustering of risk factors including diabetes, and therapeutic effectiveness.
Methods
Study design
The GVRM Study is a voluntary, observational, prospective quality-improvement initiative conducted at eight COSEHC™-designated Cardiovascular Centers of Excellence™ from patients who were at least 18 years of age and met one or more of the following criteria: (1) at least one predefined International Classification of Disease, 9th Revision (ICD-9) CVD risk factor code; (2) treatment at a participating COSEHC™-designated Center of Excellence; and (3) alternative treatment at a noncardiac-related outpatient clinic for a cardiac-related Current Procedural Terminology code condition. All patients were treated per the standard of care for their respective conditions, and no predefined visits, medical, laboratory tests, procedures, or interventions were required. Of the eleven variables in the modified COSEHC– CRT score,Citation6 age, sex, smoking status, and family history of coronary heart disease (CHD) were collected only once, all other data were updated on a quarterly basis.
Study duration and outcomes
The baseline measures for this 5-year prospective study are published elsewhere.Citation6 The follow-up time period analyzed in this article is 33 months (January 1, 2010, to September 30, 2012).
Data flow and management
The GVRM Study was approved on a central level by the Copernicus Group Institutional Review Board (IRB) (Research Triangle Park, Durham, NC, USA) and by the IRBs of each participating site. The selected numerical values from individual patient EMRs were submitted to the COSEHC™ Coordinating Center at the Wake Forest University School of Medicine on a quarterly basis. The following ICD-9 codes were used for clinical events: anterior myocardial infarction (AMI: 410); acute coronary syndrome (ACS: 411); stroke: 430–434; transient ischemic attack (TIA: 435); other cardiovascular disease (CVD: 436); and congestive heart failure (CHF: 428). Only patients with no evidence of the specified condition during the baseline period, and a subsequent diagnosis for that condition during follow-up are included in the subanalysis. Antihypertensive medication usage was defined as number of refills (see Statistical methods).
Statistical methods
The analysis focused on patients from the study baseline period who continued care with COSEHC™ health care providers during the follow-up period (at least one visit throughout each of the following years). We evaluated clinical measures and treatment goal attainment across patient subgroups defined by sex and diabetic status, as recorded in . At-goal rates, defined as achieving current clinical practice guidelinesCitation16,Citation17 were: hypertension (SBP <140 mmHg for nondiabetic subjects and <130 mmHg for those with an associated diagnosis of chronic kidney disease or diabetes); total cholesterol <200 mg/dL, HDL-chol >40 mg/dL, low-density lipoprotein (LDL)-chol <100 mg/dL; and plasma triglycerides >150 mg/dL. These goal attainment rates were also compared from the baseline period to the follow-up period. We used the Chi-square test to compare at-goal rates. In addition to these clinical measures, antihypertensive medication usage, BMI, and the COSEHC™ score were also compared across patient subgroups.
Table 1 Average differences in goal achievement at the end of 33 months follow-up
As this is an observational study not all patients received every lab test during the follow-up period, and some patients had more than one value for some risk factors. Thus, the sample size differed across all measures. When patients had more than one value, the most recent valid follow-up value was used. When patients had only a subset of the measures recorded during follow-up, the patient remained in the study. There was no carry-forward of values from the baseline period other than the patient’s height for computation of BMI.
A separate analysis for the diabetic subpopulation (by sex) was also performed. The percentage of patients at-goal for each measure was calculated. These percentages were calculated for both the baseline and follow-up period, with separate reporting for patients who were or were not at-goal at baseline. The total number of antihypertensive medications either prescribed during the follow-up period or continued from the baseline period was counted for each patient. All unique antihypertensive medication names were used as the basis for these counts. Since this is a count of prescriptions written, it does not distinguish between a patient switching medications during follow-up or concurrently adding a second medication.
As documented by us previously,Citation6,Citation13 the COSEHC–CRT score is an additive score calculated with separate algorithms for men and women. The individual scoring elements are: age (5-year ranges); smoking status (with differential scores for each age range); SBP ranges; laboratory test score ranges; and patient history variables. The final COSEHC™ score is the sum of each of these individual scoring elements. We also report treatment goal attainment rates for each CVD factor by sex and diabetic status. These at-goal rates were reported for baseline and follow-up (with separate reporting for patients not at-goal during baseline and patients at-goal during baseline). Inference testing was performed to look for statistically significant differences between goal attainment rates by sex. SBP at-goal status was modeled as a function of sex, clinical history, and follow-up at-goal status for the following measures (LDL-chol, HDL-chol, triglycerides, and BMI). Logistic regression using SAS/STAT software, (Version 9.2 of the SAS® System for Windows. Copyright 2002-2008 SAS Institute, Cary, NC, USA) was also performed, modeling the at-goal rate for SBP during follow-up. This model was developed to determine correlation between baseline risk factors and SBP at-goal rates. Independent variables included sex, risk factors such as smoking and diabetic status, and adherence to other treatment goals, including HDL-chol, LDL-chol, triglycerides, and BMI.
Results
Demographic and clinical history
Out of the 87,863 patients with at least one follow-up visit, 61% were female (mean age of 62 years). Of the 76,383 patients with known race/ethnicity, 15.1% were African-Americans; 77.7% were Caucasian; less than 1% were Hispanic, and 6.6% were listed as other. According to medical record review, 32.2% of the patients were current smokers, 38.4% were diabetics, 2.4% reported a history of premature CHD in their family, and 3.8% had left ventricular hypertrophy.
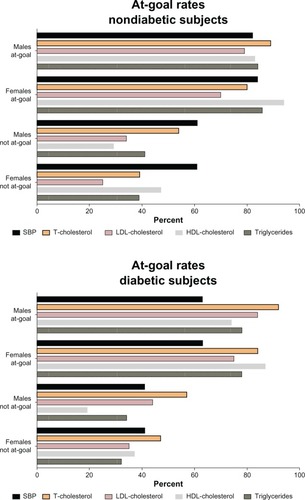
shows the group averages of systolic blood pressures and lipids at 33 months of follow-up and the changes in at-goal achievement rates for the entire patient cohort and the subgroups of subjects who did or did not have a diabetic diagnosis at the time of patient enrollment.
Systolic blood pressure
At the time of enrollment (baseline; ), goal-attainment rates for SBP in the entire population are above 60%. The highest goal attainment rates at baseline are found in the subgroup of subjects with a negative history of diabetes (78%; ), while the lowest at-goal rates are present in diabetic subjects (48%; ). Modest, albeit statistically significant, improvements in SBP at-goal rates were observed in all subjects at the completion of the follow-up period (). Improvement in the at-goal rates for diabetic subjects (51%; ) is significantly lower (P < 0.05) than those measured for the entire population (70%) or the subgroups of subjects with a negative diagnosis of diabetes at baseline (79%). also shows lower SBP values and higher at-goal rates for males compared to females for the whole patient population and nondiabetic subjects. In contrast, SBP at-goal rates in diabetic subjects are not different in males and females ().
Lipid profile
Baseline at-goal rates for total-chol, HDL-chol, and triglycerides are greater than 50% at the time of patient enrollment into the study (). Overall, LDL-cholesterol baseline at-goal rates are below 50% (). Over the course of the 33 months of follow-up, small improvements in lipid at-goal rates are present for both nondiabetic and diabetic patients (). In addition, at-goal rates for total-chol and LDL-chol are significantly higher in males compared to females whether comparisons are made for all subjects or those without diabetes. At-goal rates improved from baseline to follow-up for males and females in both subgroups (P < 0.0001), with an overall improvement in the total-chol rate from 62% to 66% for males and 79% to 83% for females (). These findings contrast with the concurrent demonstration of lower at-goal rates for LDL-chol when compared to total-chol for all subjects or the subgroup without diabetes (). The conversion rate for baseline not-at-goal patients and the retention rate for patients at-goal during baseline rate were also lower for diabetic patients.
Total-chol at-goal rates for subjects that were at-goal at baseline were higher for males than females (66% versus 83%; P < 0.0001) during the follow-up period (, all subjects). Goal-retention rates for patients at-goal for LDL-chol during baseline were higher for males compared to females, and this sex difference persisted throughout the follow-up period (). In terms of HDL measurements, lower values of HDL-chol at-goal rates for both sexes showed significantly lower at-goal rates in males, not at baseline goal. This difference, albeit attenuated in magnitude, is still present in males who were at-goal for HDL-chol (). For plasma triglycerides, diabetic females and males had similar goal rates for all cohorts. These rates were also similar when compared to those for all patients with the exception of the conversion rate among diabetic males that was higher than that for all male patients (). For the BMI measure, we observed lower rates across all cohorts, including the conversion rate for baseline at-goal patients and the retaining rate for patients at-goal during baseline, when compared to all patients.
Influence of diabetic status
shows the follow-up results for subjects who were or were not at-goal at baseline in diabetic and nondiabetic subjects. It is evident that being not at-goal at the initiation of the study is associated with similar, lesser attainments of goal rates during the follow-up period. Within the diabetic subgroup, both females and males had lower at-goal rates for SBP during the follow-up period, as well as lower conversion rates when compared to the entire cohort of patients ( and ). This is primarily due to the stricter goal criteria for diabetic patients (<130 mmHg for diabetics versus <140 mmHg for nondiabetics without chronic kidney disease).
COSEHC–CRT score
shows higher COSEHC–CRT scores in males at the initiation of the study, at follow-up, and even in the subset of subjects who were not at-goal during the baseline period and whether they are being treated with one or more antihypertensive drugs. Higher COSEHC–CRT score values in males, in part, refects the fact that the COSEHC™ score itself automatically assigns higher scores for males than females.Citation6,Citation13
Figure 2 Sex differences in COSEHC™ risk scores.
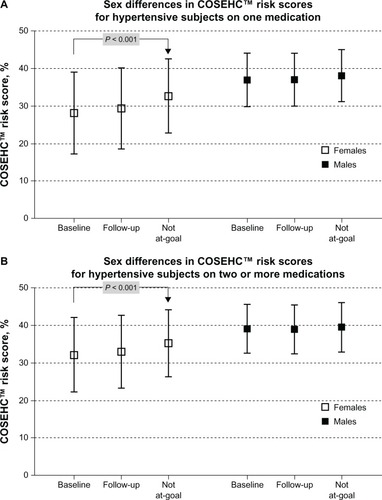
Utilization of antihypertensive medications was similar in the at-goal patient population for both men and women (P > 0.05); however, the not-at-goal female subgroup had slightly higher average prescription counts compared to the male subgroup (2.2 versus 2.1; P < 0.0001). As illustrated in , patients taking two or more antihypertensive medications had higher mean COSEHC–CRT scores for both females and males, compared to those taking a single antihypertensive medication.
A logistic regression analysis was performed to model the probability of SBP at-goal status during follow-up as a function of sex, diabetic status, and other measures. Diabetes, smoking status, and BMI were significant at P < 0.05. Diabetes status had the smallest odds ratio (OR = 0.36) and was significant at P < 0.0001, showing that diabetics were at least three times more likely to not be at-goal for SBP. In other words, the logistic regression analysis indicates that being at-goal for SBP is more likely if the subject is a nonsmoker who is at-goal for both BMI and LDL-cholesterol and is nondiabetic. The Hosmer–Lemeshow Goodness of Fit Test had a P-value of 0.2578. This high P-value indicates a good model fit.
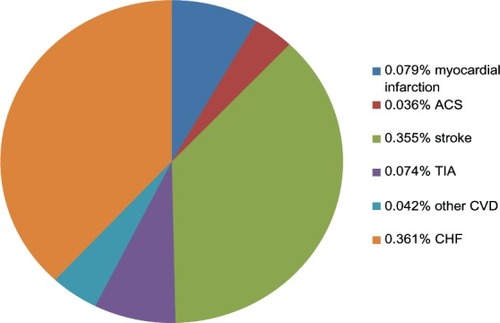
shows the number of clinical events occurring throughout the follow-up period (2010–2012). There were a total of 832 clinical events among 811 patients (0.92% of the total patient population). Strokes and CHF represented the highest event categories.
Figure 3 Clinical events documented during the 33 months for the overall patient population.
Abbreviation: ACS, acute coronary syndrome; TIA, transient ischemic attack; CHF, congestive heart failure; CVD, other cardiovascular events.
Discussion
Multiple theories have been suggested to address the factors contributing to poor medication compliance, quality of care, and patient outcomes. The results obtained during a 33 month follow-up of a large practice-based southeastern high cardiovascular risk patient population observational study show improvements in blood pressure control, as well as modest increases in the at-goal rates for total cholesterol, LDL-chol, and HDL-chol that are most evident in males compared to females. Presence of diabetes mellitus worsens the ability of subjects to reach or maintain cardiovascular risk factors’ at-goal control rates while the data also confirm the higher risk that males are exposed to compared to females in terms of cardiovascular events, as reflected in the higher COSEHC–CRT score rates.
The GVRM Study seeks to provide prospective benchmarking of recognized cardiovascular risk factors on treatment patterns and patient outcomes from eight of 33 COSEHC™-designated Cardiovascular Centers of Excellence™. The population served by the physicians enrolled in the study includes a large number of patients at high risk for cardiovascular events due to their location in the southeastern USA, a region with a recognized higher cardiovascular morbidity and mortality.Citation8,Citation10,Citation12,Citation14,Citation15,Citation18–Citation20 Data, appropriately de-identified, were collected electronically from each participating center, and the subjects included in the study met the inclusion criterions defined in our previous publication.Citation6 Importantly, the current report provides a glimpse of the study’s predefined main outcome measures of cardiovascular morbidity and mortality, including the development of CHD, CHF, stroke, transient ischemic attack, abdominal aortic aneurysm, myocardial infarction, renal failure, and diabetic retinopathy.
The overall number of clinical events, approximately 0.92% of the total monitored population over the 33 month observational period, is relatively low as the at-goal rates were generally higher than 50%, except in diabetic subjects. Since the COSEHC–CRT score estimates the 5-year absolute cardiovascular mortality risk, and individuals can be identified as high relative risk (>60th percentile) compared to the reference population, or identified as high absolute risk (5-year mortality risk exceeding 2.5%), the average 32%–36% score measured in our population indicates a higher than 0.85% risk of 5-year cardiovascular mortality.Citation13 This finding suggests a lower occurrence of events compared with other studies having similar commonalities in terms of patient characteristics and follow-up.Citation21–Citation24 In assessing the benefits of a healthy diet on the composite of cardiovascular events (cardiovascular death (CV); myocardial infarction (MI); stroke; or CHF) in the combined patient population enrolled in the Ongoing Telmisartan Alone in Combination with Ramipril Global End Point Trial (ONTARGET; 4,221 patients) and in the Telmisartan Randomized Assessment Study in ACEI Intolerant Subjects with Cardiovascular Disease (TRANSCEND; 969 patients) trials,Citation25–Citation30 Dehghan et alCitation23 showed that the rates of clinical events for myocardial infarction, stroke, and congestive heart failure in their population are higher than those found in our study. These data might suggest that physicians participating as COSEHC’s members have higher skills and motivational capabilities to address the greater risk for cardiovascular events than their patient population is exposed to. Whether these lower rates are related to a more aggressive treatment approach in terms of use of specific medications, greater physician time spent with the subjects, or more frequent follow-up were not assessed in this study. Nevertheless, the programmatic structured educational system that COSEHC™ members are exposed to might be a factor.Citation7,Citation10,Citation31
Table 2 Logistic regression results
The changes in goal attainment in terms of SBP and lipids were the primary outcome metric used in our study. Sex differences in at-goal rates were also included in the analysis since accumulating data suggest important differences in response rates, medication use, and clinical outcomes between men and women.Citation32–Citation34 Several studies showed that the reduction of arterial pressure, particularly SBP, to the goals recommended by current and past guidelines leads to robust reductions in the occurrence of cardiovascular events.Citation35–Citation37 The focus of our study on SBP was determined by the nature of the factors that enter in the calculation of the COSEHC™ risk score,Citation6,Citation13 which derives further strength from studies showing a higher association of cardiovascular risk with systolic rather than diastolic blood pressure in subjects older than 50 years.Citation31,Citation38–Citation41 A detailed analysis by BasileCitation42 documented the limitations of using response rates to a particular treatment rather than attainment of a predefined or recommended at-goal value. Our 33-month observational follow-up study in this large population demonstrates important sex differences in at-goal rates that become relatively less marked in diabetic subjects. Gueyffier et alCitation32 reported that while antihypertensive treatment in men prevented as many coronary events as stroke events, a similar benefit of therapy in women was primarily reflected in a reduction in stroke rates. In our study, we showed that nondiabetic men evidenced higher at-goal rates for total and LDL-chol while lower at-goal rates occurred for SBP, HDL-chol, and plasma triglycerides. Furthermore, the logistic regression results demonstrated that goal rates for BMI had a much larger effect than sex. These findings do not negate the role of sex in the progression of CVD since a recent report showed a higher risk for large myocardial infarction and heart failure in females with the cardiometabolic syndrome.Citation43
There were a number of limitations present in this study design. Since this was an observational study based on EMR data, patients have variable intervals between their baseline and follow-up visits. In some cases, data elements from multiple visits had to be combined to create a complete record. The pharmaceutical records did not include stop dates, making it difficult to discern between patients adding additional therapies and patients switching therapies. Limitations of the COSEHC™ risk score are detailed elsewhere.Citation6,Citation13 The COSEHC™ risk score provides a more accurate estimate of the absolute risk of cardiovascular events compared to the Framingham risk score, since the latter is based on a homogeneously white healthy individual population. Known limitations of the Framingham risk score include under (over) estimation of risk for non-Caucasian individuals (African-Americans, Hispanic-Americans, and Native Americans).Citation44,Citation45 The wider generalizability and discriminatory sensitivity of the COSEHC™ risk score is based on the model published by the INdividual Data ANalysis of Antihypertensive intervention trials (INDANA) project, which is based on a much larger patient population of multiple ethnic origin.Citation13,Citation32,Citation46–Citation51
The interim analysis, 33 months after completion of baseline measures, provides new evidence about the difficulties that physicians face in improving treatment outcomes, particularly in terms of lipid management in women. There is clear evidence that diabetes worsens the ability of physicians to treat to goal, particularly in those subjects whose SBP and lipids were not at-goal rates at the completion of the baseline observational period. On the other hand, the data show improved control rates for SBP and even lipids in diabetic subjects who were or were not at-goal levels at the time of enrollment in the program. Our data further confirm previous studies showing that higher use of medications is required to control arterial pressure and that higher use of medications is associated with worse COSEHC™-risk scores.
In summary, the GVRM Study documents the characteristics of risk factors’ prevalence and response to treatment in a population at higher risk of cardiovascular events. At the time when the Centers for Medicare and Medicaid Services (CMS), as well as both small and large health care plans, strive to develop approaches to improve the quality and patient-centeredness of care for its enrollees, the studies and methods embedded in the GVRM Study allow for a quantitative assessment of absolute risk, based on a longitudinal monitoring of cardiovascular variables. While the practices participating in this project exercised their own independent best-practice approaches to the care of their patients, the regular reporting of cardiometabolic data captured from their electronic medical record systems with regular feedback to the providers make it plausible that these tools can contribute significantly to improved patient care and outcomes.
Acknowledgments
Partial support for the conduction of this study was provided by grant 2PO1 HL-051952 from the National Heart, Lung and Blood Institute of the National Institutes of Health (CM Ferrario) and Daiichi Sankyo, Inc. We thank Alex Sheek of COSEHC™ for technical assistance and the following COSEHC Centers of Excellence™ investigators for participating in the GVRM Study: Bryan N Batson of the Hattiesburg Clinic, Hattiesburg, MS, USA; Michael Canfield, formerly of the Palmetto Primary Care Physicians, North Charleston, SC, USA; Alexander Cohen, of Pee Dee Healthcare, Darlington, SC, USA; Gary Goforth, formerly of Self Regional Healthcare, Greenwood, SC, USA; Mark Houston, of the Hypertension Institute at St Thomas Hospital, Nashville, TN, USA; and Steve Ross, of Internal Medicine Specialists, Florence, SC, USA.
Disclosure
The authors report no conflicts of interest in this work.
References
- AthyrosVGGanotakisESElisafMSLiberopoulosENGoudevenosIAKaragiannisAGREECE-METS Collaborative GroupPrevalence of vascular disease in metabolic syndrome using three proposed definitionsInt J Cardiol200711720421016854482
- DaskalopoulouSSAthyrosVGKolovouGDAnagnostopoulouKKMikhailidisDPDefinitions of metabolic syndrome: Where are we now?Curr Vasc Pharmacol20064318519716842136
- de SimoneGDevereuxRBChinaliMStrong Heart Study InvestigatorsPrognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the Strong Heart StudyDiabetes Care20073071851185617440172
- DekkerJMGirmanCRhodesTMetabolic syndrome and 10-year cardiovascular disease risk in the Hoorn StudyCirculation2005112566667316061755
- GamiASWittBJHowardDEMetabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studiesJ Am Coll Cardiol200749440341417258085
- FerrarioCMMooreMABestermannWJrCOSEHC global vascular risk management quality improvement program: rationale and designVasc Health Risk Manag201061135114521931496
- BestermannWHoustonMCBasileJAddressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States, part II: treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndromeAm J Med Sci2005329629230515958871
- EganBMLacklandDTIgho-PemuPCardiovascular risk factor control in communities – update from the ASH Carolinas-Georgia Chapter, the Hypertension Initiative, and the Community Physicians’ NetworkJ Clin Hypertens (Greenwich)200681287988617170614
- FerrarioCMHighlights of the Tenth Annual COSEHC National Scientific SessionAm J Med Sci2004327523423515166740
- FerrarioCMCOSEHC overviewAm J Med Sci2004327523315166739
- FerrarioCMHighlights of the Twelfth Annual COSEHC National Scientific SessionAm J Med Sci200633125916479176
- HallWDFerrarioCMMooreMAHypertension-related morbidity and mortality in the southeastern United StatesAm J Med Sci199731341952099099149
- HoustonMCBasileJBestermannWHAddressing the global cardiovascular risk of hypertension, dyslipidemia, and insulin resistance in the southeastern United StatesAm J Med Sci2005329627629115958870
- JonesDBasileJCushmanWManaging hypertension in the southeastern United States: applying the guidelines from the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI)Am J Med Sci1999318635736410616159
- LacklandDTBendallHEOsmondCEganBMBarkerDJLow birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United StatesArch Intern Med2000160101472147610826460
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final reportCirculation2002106253143342112485966
- ChobanianAVBakrisGLBlackHRNational Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureNational High Blood Pressure Education Program Coordinating CommitteeThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- GoASMozaffarianDRogerVLAmerican Heart Association Statistics Committee and Stroke Statistics SubcommitteeExecutive summary: heart disease and stroke statistics – 2013 update: a report from the American Heart AssociationCirculation2013127114315223283859
- HowardVJWoolsonRFEganBMPrevalence of hypertension by duration and age at exposure to the stroke beltJ Am Soc Hypertens201041324120374949
- LacklandDTEganBMThe dominant role of systolic hypertension as a vascular risk factor: evidence from the southeastern United StatesAm J Med Sci1999318636536810616160
- AIM-HIGH Investigators; BodenWEProbstfieldJLAndersonTThe role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes (AIM-HIGH)Am Heart J2011161347147721392600
- AIM-HIGH Investigators; BodenWEProbstfieldJLAndersonTNiacin in patients with low HDL cholesterol levels receiving intensive statin therapyN Engl J Med2011365242255226722085343
- DehghanMMenteATeoKKRelationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countriesCirculation2012126232705271223212996
- MichosEDSibleyCTBaerJTBlahaMJBlumenthalRSNiacin and statin combination therapy for atherosclerosis regression and prevention of cardiovascular disease events: reconciling the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial with previous surrogate endpoint trialsJ Am Coll Cardiol201259232058206422520249
- TeoKYusufSSleightPONTARGET/TRANSCEND InvestigatorsRationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trialsAm Heart J20041481526115215792
- TeoKKMitchellLBPogueJBoschJDagenaisGYusufSHOPE InvestigatorsEffect of ramipril in reducing sudden deaths and nonfatal cardiac arrests in high-risk individuals without heart failure or left ventricular dysfunctionCirculation20041101413141715353497
- McQueenMJKavsakPAXuLShestakovskaOYusufSPredicting myocardial infarction and other serious cardiac outcomes using high-sensitivity cardiac troponin T in a high-risk stable populationClin Biochem2013461–25923063983
- ONTARGET Investigators; YusufSTeoKKPogueJTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med2008358151547155918378520
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) InvestigatorsYusufSTeoKAndersonCEffects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trialLancet200837296441174118318757085
- YusufSDienerHCSaccoRLPRoFESS Study GroupTelmisartan to prevent recurrent stroke and cardiovascular eventsN Engl J Med2008359121225123718753639
- BasileJHoustonMFerrarioCIncremental risk-factor reduction improves overall cardiovascular benefit: is it time to abandon the silos?J Clin Hypertens (Greenwich)200681068668817028480
- GueyffierFBoutitieFBoisselJPEffect of antihypertensive drug treatment on cardiovascular outcomes in women and men. A meta-analysis of individual patient data from randomized, controlled trials. The INDANA InvestigatorsAnn Intern Med1997126107617679148648
- JonesCANagpalSAn update: women, hypertension and therapeutic efficacyCan J Cardiol200117121283128911773939
- QuanAKerlikowskeKGueyffierFBoisselJPINDANA investigatorsPharmacotherapy for hypertension in women of different racesCochrane Database Syst Rev20003CD00214610908526
- ManciaGGrassiGAntihypertensive treatment: past, present and futureJ Hypertens Suppl1998161S1S79534090
- ZanchettiAManciaGBlackHRFacts and fallacies of blood pressure control in recent trials: implications in the management of patients with hypertensionJ Hypertens200927467367919516168
- ZanchettiAGrassiGManciaGWhen should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisalJ Hypertens200927592393419381107
- BestermannWHLacklandDTRiehleJEEganBMA systematic approach to managing hypertension and the metabolic syndrome in primary careSouth Med J2004971093293815558916
- ManciaGOmboniSParatiGThe importance of blood pressure variability in hypertensionBlood Press Monit20005Suppl 1S9S1510904237
- ManciaGGrassiGSystolic and diastolic blood pressure control in antihypertensive drug trialsJ Hypertens20022081461146412172300
- ManciaGDefining blood pressure goals: is it enough to manage total cardiovascular risk?J Hypertens Suppl2009275S3S819587552
- BasileJBlood pressure responder rates versus goal rates: which metric matters?Ther Adv Cardiovasc Dis20093215717419299428
- KranjcecDAltabasVMetabolic syndrome influencing infarct size and heart failure in patients with acute coronary syndrome: does gender matter?Endocr J201259121065107622971940
- PocockSJMcCormackVGueyffierFBoutitieFFagardRHBoisselJPA score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trialsBMJ20013237304758111451781
- SchlendorfKHNasirKBlumenthalRSLimitations of the Framingham risk score are now much clearerPrev Med200948211511619124038
- GueyffierFBoutitieFBoisselJPINDANA: a meta-analysis on individual patient data in hypertension. Protocol and preliminary resultsTherapie19955043533627482389
- GueyffierFBoisselJPBoutitieFEffect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project CollaboratorsStroke19972812255725629412649
- GueyffierFBulpittCBoisselJPAntihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA GroupLancet1999353915579379610459960
- LiWGueyffierFBoisselJPGirardPBoutitieFCucheratM[Identification and prediction of responders to a therapy. A model and its preliminary application to hypertension.]Arch Mal Coeur Vaiss199891810591063 French [with English abstract]9749165
- LièvreMGueyffierFEkbomTEfficacy of diuretics and beta-blockers in diabetic hypertensive patients. Results from a meta-analysis. The INDANA Steering CommitteeDiabetes Care200023Suppl 2B65B7110860193
- MatsumotoMCerebrovascular disease: [Impact of INDANA meta-analysis and PROGRESS trial.]Nippon Rinsho200462Suppl 3605611 Japanese15179938